1. Introduction
An imbalance in the generation of free radicals could induce oxidative stress in different mediums [
1]. An elevated concentration of free radicals is responsible for the degradation of biomolecules which could lead to different pathophysiological conditions in the human body, or accelerated deterioration of different products and reduction of shelf life. Plant-based products, like extracts and essential oils, are being thoroughly investigated as natural agents with antioxidant activity for use as substitutes for potentially toxic conventional synthetic antioxidants such as propyl galate, butylatedhydroxytoluene, and butylatedhydroxyanisole [
2].
Besides essential oils, some fatty oils obtained from plant seeds or berries have excellent antioxidant, anti-inflammatory, antimicrobial effects, among other effects [
3]. Pomegranate and its extracts from all fruit parts were used through history from ancient times, as it was well known to have good activity [
4,
5]. Nowadays it is the subject of many studies examining its chemical composition and mechanisms of activity. Pomegranate seed oil and fermented pomegranate juice extract are the most valuable products of pomegranate plants [
6]. Together with them, sea-buckthorn oil is becoming an important component of cosmetic products in recent days, because of its specific combination of active ingredients [
7]. Because of the high content of saturated and unsaturated fatty acids and many other valuable ingredients, they express antioxidant, anti-inflammatory, and regenerative effects to the skin [
8]. They are often used in products for mature skin, and for treatment of wounds and scars. The only disadvantage of these oils is their high sensitivity to oxidation, which decreases their shelf life and limits the way of application. It is often suggested that the oils should be encapsulated in some polymer, which will maintain its activity for a more extended time [
9].
Conventional techniques like extrusion, injection molding, or compression are not suitable for the production of cosmetic products containing natural compounds. Within all these techniques high temperature and/or pressure is applied, which has harmful effect on thermosensitive natural ingredients. Besides this, samples prepared this way are usually thick and lack flexibility, with low porosity and “plastic” feel on the skin. To ensure the activity of those materials, it is important to have a surface rich in active compounds, because the diffusion through the volume is very slow, and, in the short exposure time, often insufficient for getting an effect [
10]. Also, the loading of the polymeric matrix with natural compounds needs to be very high, for successful surface activity, which increases the price of the final products.
To avoid losses of valuable active compounds, encapsulation can be done by employing electrospinning technique. Electrospinning is a fiber-forming technique, where the process is running at room temperature and ambient pressure, and fiber production goes from highly viscous polymer-based solution influenced by the high voltage. On the way from the needle to the collector, two processes happened: elongation of the polymer solution and evaporation of the solvent, so at the collector nonwoven dry fibers are formed. Depending on the process parameters, it is possible to obtain different structures and morphologies of nanofibers and tailor materials’ properties according to application requirements. Sensitive components like essential oils and plant extracts are now often encapsulated in nanofibers [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21].
The main aim of this work was the examination of the antioxidant activity of functional materials containing pomegranate seed oil, sea-buckthorn oil, and extract of fermented pomegranate juice encapsulated into two different polymers: polylactide (PLA-based fibers) and poly(vinyl-pyrrolidone) (PVP-based fibers) using electrospinning technique. Beside excellent antioxidant activity achieved with low amounts of active compounds, mechanical, thermal, and chemical properties compared to basic polymers were sustained or even improved, which make them convenient for application in cosmetics. According to our knowledge, this is the first time that these combinations and active compounds are examined.
2. Materials and Methods
Semi-crystalline polylactide (PLA) was provided from Shenzhen Esun Industrial Co., Ltd. (Shenzhen, China), characterized by a number-average molecular weight Mn of 60,520 g/mol, a weight-average molecular weight Mw of 160,780 g/mol, and polydispersity index (PDI) of 2.66. Poly(vinyl-pyrrolidone) (PVP) with weight-average molecular weight Mw of 1,300,000 was purchased from Acros Organic. Dichloromethane (DHM) was purchased from Carlo Erba reagents, dimethylformamid (DMF) was purchased from Centrohem (StaraPazova, Serbia) and ethanol (96%) from Reahem (Novi Sad, Serbia). Pomegranate seed oil (PSO) and ethanolic extract of fermented pomegranate juice (EP) were purchased from Suncokret (Hajdukovo, Serbia) and cold-pressed sea-buckthorn oil (SB) was purchased from NaturaSiberica (Belgrade, Serbia). 1,1-Diphenyl-2-picryl-hydrazyl-hydrate (DPPH) was purchased from Sigma (Sigma-Aldrich GmbH, Steinheim, Germany). TPZT (2,4,6-tris (2-pyridil)-s-triazine), iron (III)-chloride, iron (II)-sulfatheptahydrate, and potassium persulfate and trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were purchased from Sigma–Aldrich (Milano, Italy). Sodium acetate and hydrochloric acid were obtained from Merck (Darmstadt, Germany). ABTS (2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) was purchased from J&K Scientific GmbH (Pforzheim, Germany). All chemicals were used as received.
Samples were prepared by electrospinning using Fluidnatek LE-10 setup by BioInicia. The feed rate was adjusted in the range of 0.5–2.5 mL, needle-to-collector distance was from 10–15 cm and voltage was from 10–15 kV. Detailed electrospinning process parameters for each sample are given in
Table 1.
The initial solution of pure PLA for electrospinning was prepared as 12 wt% in solvent mixture of dichloromethane and dimethylformamide in ratio 6:4. PVP was dissolved in ethanol in concentration of 9 wt%. The concentration of active compound was calculated on the weight of polymer and active compounds were added to those solutions prior to electrospinning. Polymer-based solutions were mixed on magnetic stirrer 24 h before electrospinning. All samples are summarized in
Table 1.
The morphology of the samples was examined under a few magnifications using Hitachi Tabletop TM3030 scanning electron microscope. Samples were placed on a holder and thin layer of gold was deposited on top by an evaporation method, after what they were exposed to electron beam of 15 kV. Using SEM micrographs, average fiber diameter was calculated using homemade software by measuring 50 fibers. Values of fiber diameters were expressed as mean ± standard deviation.
The chemical structure of samples was examined with ATR-FTIR, attenuated total reflectance Fourier transform infrared spectroscopy, using Shimadzu IRaffinity-1S instrument in the range of 400 to 4000 cm−1 and resolution of 4 cm−1.
Mechanical properties were measured using Shimadzu EZ Test machine. Samples were cut in a rectangular shape and stretched using the constant speed of 1 mm/min. Elongation at break and maximum stress were followed for all samples. The thickness of the samples was measured using byko-test 4500 Fe/NFe tester by BYK, Germany.
Thermal properties were examined by DSC TA Instruments Q20 using 5–10 mg of sample for each analysis. PLA-based samples were heated in one cycle from 20 °C to 200 °C, while PVP-based samples were heated from 20 °C to 180 °C, both series with heating rate of 10 °C/min.
Antioxidant Activity
DPPH Assay
Scavenging capacity of electrospun mats towards 2,2-diphenyl-1-picrylhydrazyl free radicals (DPPH
∙) was measured using a slightly modified method originally presented by Brand-Williams et al. [
22]. For that purpose, methanolic solution of the DPPH reagent (65 µM) was freshly prepared and adjusted with methanol to reach absorbance of 0.70 (±0.02). DPPH reagent (2.9 mL), methanol (0.1 mL) and electrospun mats (10 mg) were mixed in the 10 mm glass cuvettes and incubated at room temperature for 60 min. Absorbance was further measured at 517 nm in triplicates with UV-VIS spectrophotometer (6300 Spectrophotometer, Jenway, UK). DPPH
∙ inhibition was expressed according to following equation [
23]:
where
Acontrol and
Asample are the absorbance values of the DPPH solution without and with the presence of the nonwoven samples. The measurements were carried out in triplicate and results were expressed as mean ± standard deviation.
FRAP Assay
Reduction capacity of electrospun mats towards Fe
3+ was measured using slightly modified method firstly presented by Benzie and Strain [
24]. The FRAP reagent was freshly prepared from 300 mM acetate buffer (pH = 3.6), 10 mM 2,4,6-tris(2-pyridil)-s triazine (TPZT), 40 mM HCl solution and 20 mM FeCl
3 aqueous solution. Solutions were mixed in the ratio 10:1:1 (v/v/v). Electrospun mats (10 mg), FRAP reagent (2.8 mL) and acetate buffer (0.1 mL) were mixed and incubated in the dark at 37 °C for 10 min. Absorbance measurements were performed at 593 nm (in triplicates) with UV–VIS spectrophotometer (6300 Spectrophotometer, Jenway, UK). The measurements were carried out in triplicate and results were expressed as reduction percentage of Fe
3+ as mean ± standard deviation [%].
ABTS Assay
The ability of fibers towards scavenging of ABTS free radicals was measured using a modified method previously reported [
25]. ABTS stock solution was freshly prepared from a mixture (1:1, v/v) of 2.45 mM potassium persulfate aqueous solution and 7 mM ABTS (2,2′-azino-bis-(-3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) aqueous solution and left in the dark at room temperature for 16 h. A stock solution was diluted using 300 mM acetate buffer (pH = 3.6) to an absorbance of 0.70 (±0.02). Electrospun mats (10 mg), ABTS reagent (2.9 mL) and acetate buffer (0.1 mL) were mixed and stored in the dark at room temperature for 5 h. Absorbance was further measured at 734 nm in triplicates with UV–VIS spectrophotometer VIS spectrophotometer (6300 Spectrophotometer, Jenway, UK). Freshly prepared Trolox aqueous solutions (0–0.8 mM, R2 = 0.987) were used to obtain the calibration curve. The measurements were carried out in triplicate and results were expressed as percentage of inhibition (mean ± standard deviation).
All above mentioned tests were done on active ingredients (PSO, EP, and SB) using 1 mg of corresponding compound.
3. Results and Discussion
3.1. Morphology of the Samples
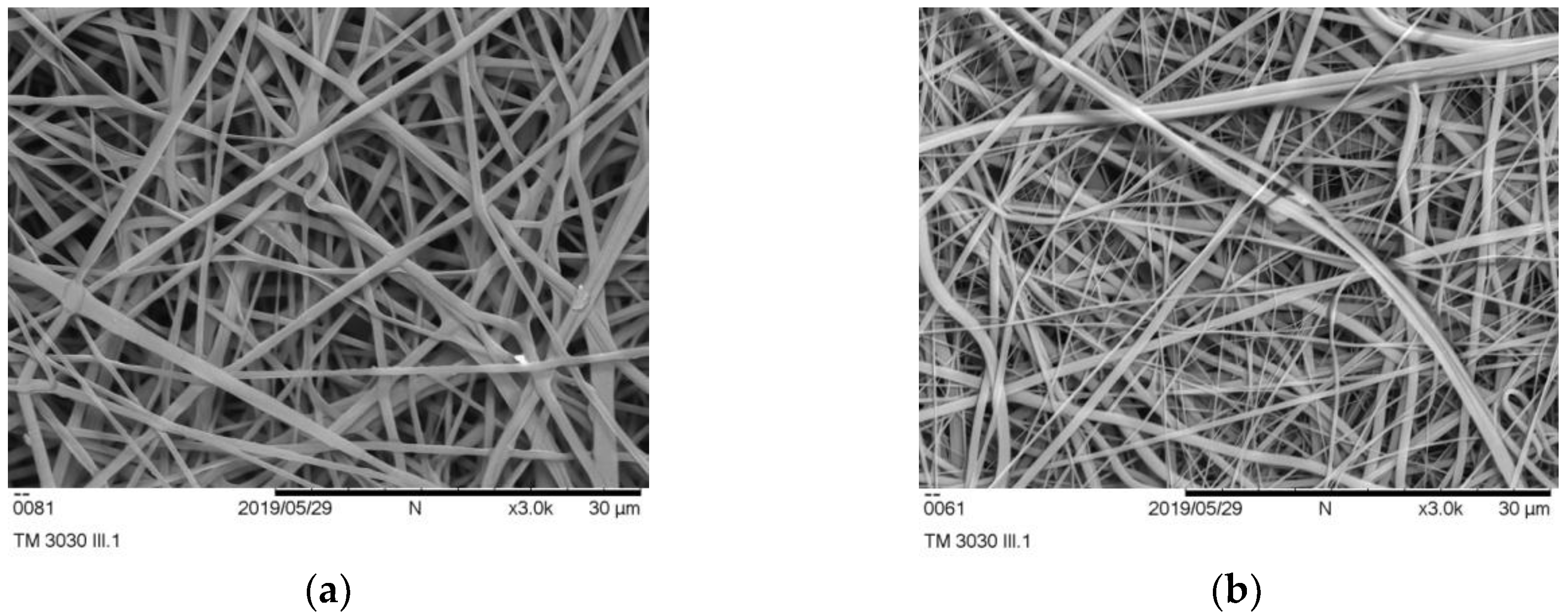
Prepared samples were first examined by visual inspection in order to determine the presence of visible defects like drops, and when it was confirmed that all of the samples had satisfying macro-characteristics, they were further examined using a scanning electron microscope.
In the beginning, it was necessary to confirm the fiber morphology of pure polymers, so PLA- and PVP-based nonwovens were examined by SEM (
Figure 1), and after that samples with active compounds were prepared.
As it can be seen in
Figure 1a,b, electrospinning parameters for pure polymers were well established as no irregularities like drops or beads along the fibers were not detected. Fibers were nicely shaped, smooth, and with regular morphology. PLA fibers had slightly higher diameters than pure PVP fibers.
The addition of active compounds changed the morphology compared to pure polymer fibers, especially in the case of PLA-based samples, where significant change in the shape of the fibers was observed. With the addition of PSO (
Figure 2a), fibers within the sample had high differences regarding fiber’s diameter (
Table 2), where very thin and very thick fibers were present in the structure, which increase standard deviation of the diameter values. It seems that one fiber was actually divided into two, which might be due to lower compatibility of PLA and PSO. In the case of the mixture of PLA with SB (
Figure 2b), fibers were flatter with thicker domains along them, which looked like reservoirs for oil. It seems that sea-buckthorn oil was not well dispersed within PLA matrix. Possible reason for this behavior within the PLA mixtures with PSO and SB can be the fact that PLA is approved for contact with food which contains fats and oils, pointing at good barrier properties of PLA against fatty matter. PSO and SB oils have high content of fatty acids, both saturated and unsaturated, which might be immiscible with PLA when added in higher concentrations.
The main effect of the addition of active compounds into PVP on the fiber morphology is the increase in the diameter of the fibers (
Table 2). Fibers look more voluminous and rounder in shape, whereby bead-less structure without drops was maintained (
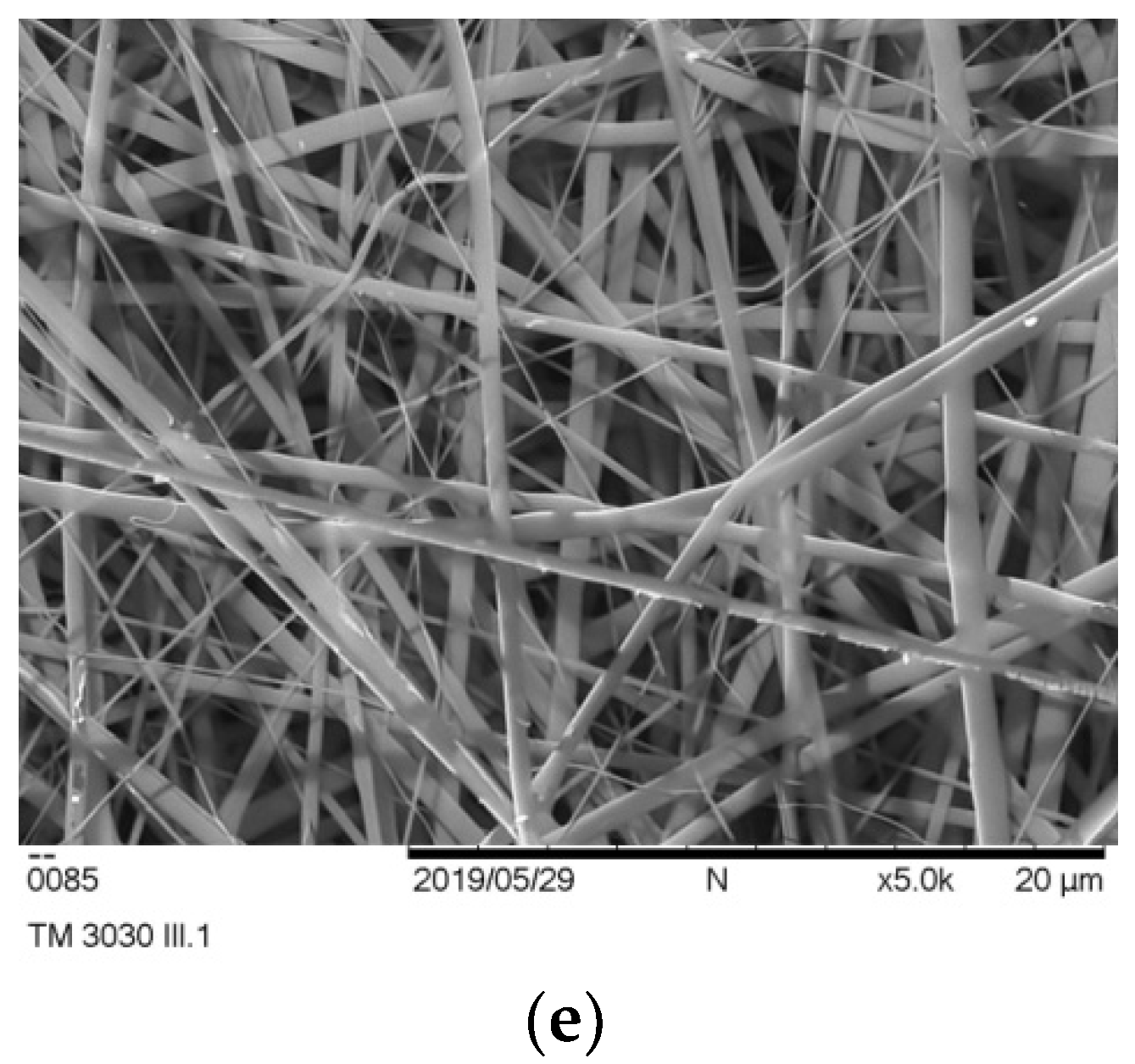
Figure 2c,d). In the case of the encapsulation of mixture of PSO and extract of fermented pomegranate juice (which is in the form of the powder), small particles of the extract were detected on the fibers’ surface as light dots (
Figure 2e). The addition of the EP did not affect regular morphology of the fibers.
3.2. Chemical Analysis
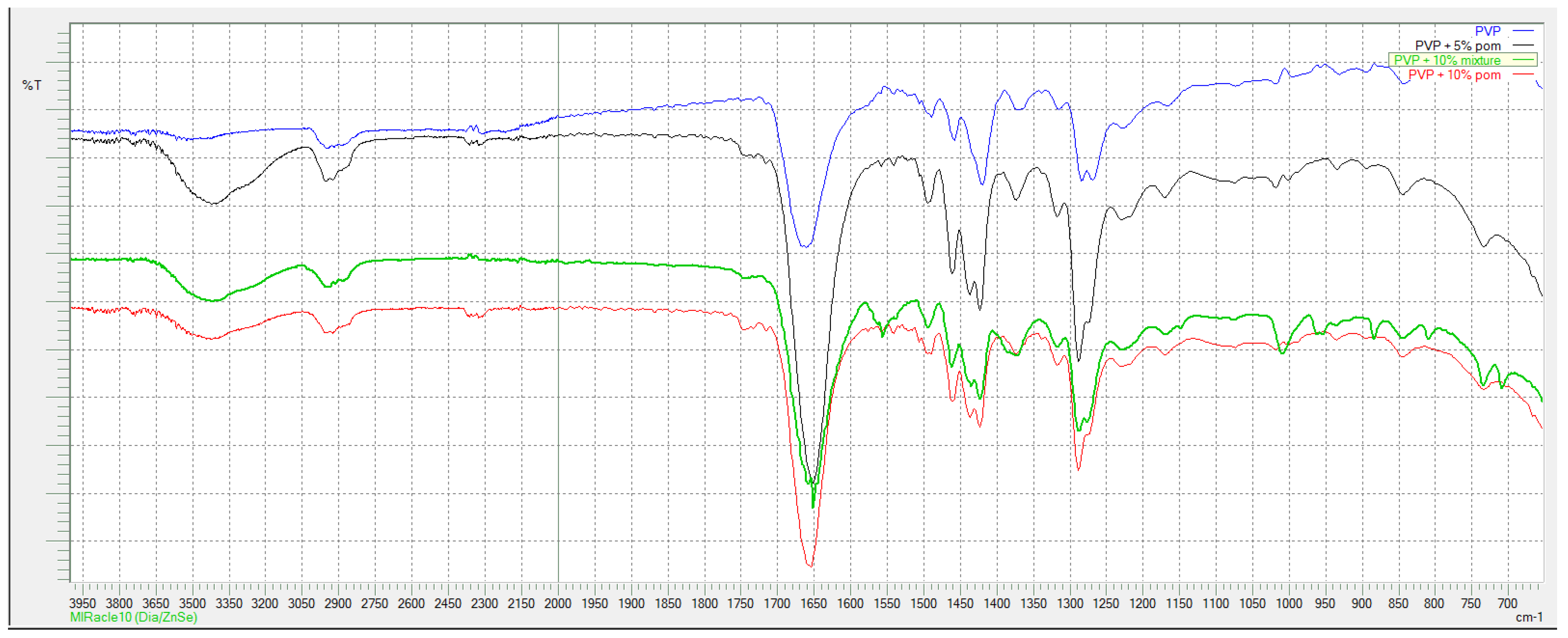
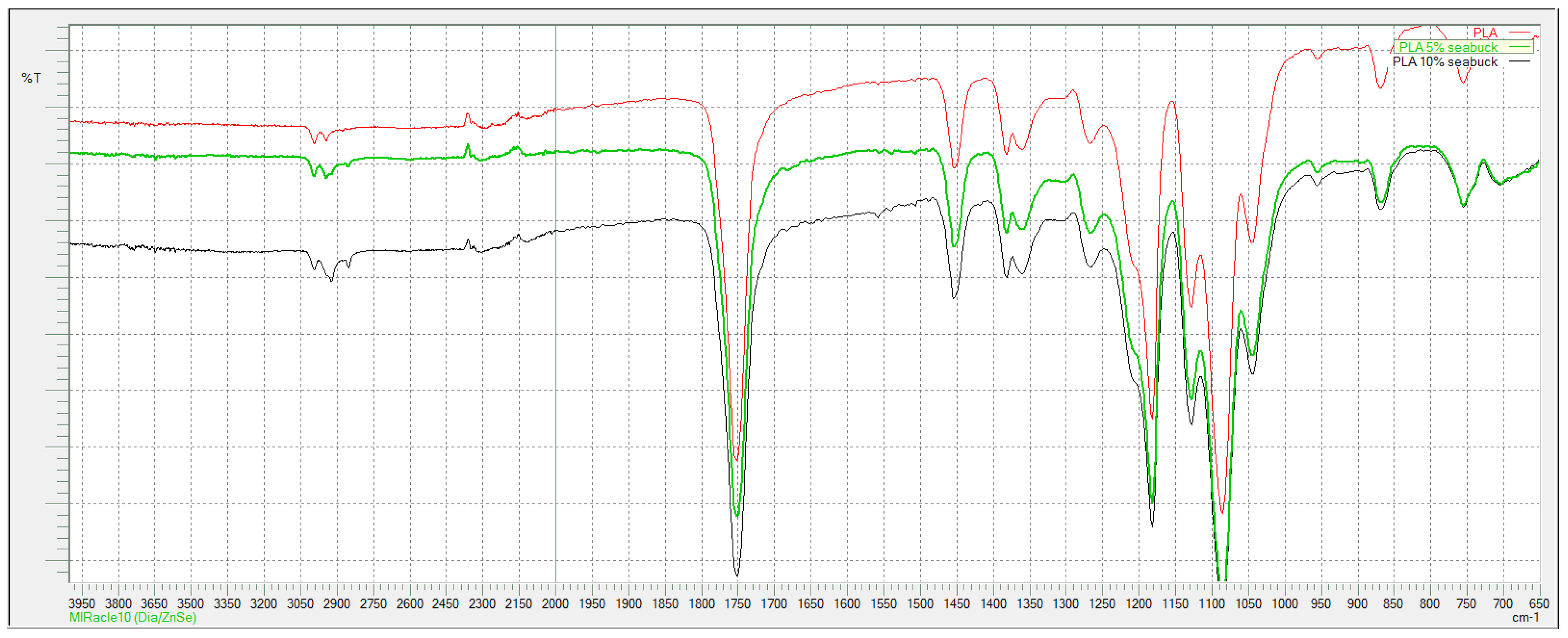
According to FTIR spectra shown in
Figure 3 and
Figure 4. It is clear that there is no chemical reaction between the active compound and particular polymer, which indicates that they are just physically mixed. It can be also concluded that the total amount of active compound added was encapsulated into the fibers or that the surface loading with active compounds is under the detection limit.
In
Figure 3. where spectra for PVP-based series are shown, typical peaks for PVP are present in all curves: wide peak at 3434 cm
−1 originates from O-H stretching vibration, small peak at 2955 cm
−1 is derived from C-H asymmetric stretching vibration, sharp peak at 1661 cm
−1 corresponds to C = O stretching vibrations. Peak at 1424 cm
−1 is from CH
2 bending vibration and peak at 1290 cm
−1 corresponds to C-N vibrations.
When comparing spectra of pure and composite PLA fibers, it can be seen that there is no difference between them (
Figure 4). Typical peaks for PLA are present, at 2997 and 2945 cm
−1 is peak from C-H stretching, C=O stretching peak is a sharp peak at 1746 cm
−1, C-H bending vibrations are presented with peaks at 1452 and 1365 cm
−1, while C-O stretching derived peaks at 1180 and 1080 cm
−1.
3.3. Mechanical Properties
Mechanical properties of nonwovens with potential application as beauty masks are important for easier manipulation by end-user. It needs to be consistent and to have certain mechanical strength to avoid breakage during the usage.
Pure PLA fibers have good tensile properties which were improved with the addition of both PSO and SB oil. Maximum stress was increased two times, while elongation at break increased from 2 to 4 times, by the addition of 5 and 10 wt% of SB, respectively (
Table 3). The encapsulation of the mixture composed of PSO and EP in PLA induced slight impairment of mechanical properties, which might be due to the presence of EP solid particles. The position of those solid particles might be the point of breakage of the fibers.
When lower concentrations of oils were incorporated in PVP fibers, both max. stress and elongation at break were improved compared to pure PVP fibers. The increase was not so high, compared to the improvement of the properties of PLA-based fibers, but it is still significant because PVP fibers were more brittle (
Table 3). The higher concentration of oils decreased the maximum stress together with the increase of elongation at break, which indicates on plasticizing effect on PVP. Presence of EP into the PVP fibers together with PSO increased both stress and elongation compared to pure PVP fibers; it had low influence on mechanical properties probably because it was located mainly on the surface of the fibers. In the case when only EP was embedded into PVP fibers, mechanical properties were significantly worse, which was assumed to be due to high concentration of solid particles present both on the surface and within fibers’ structure.
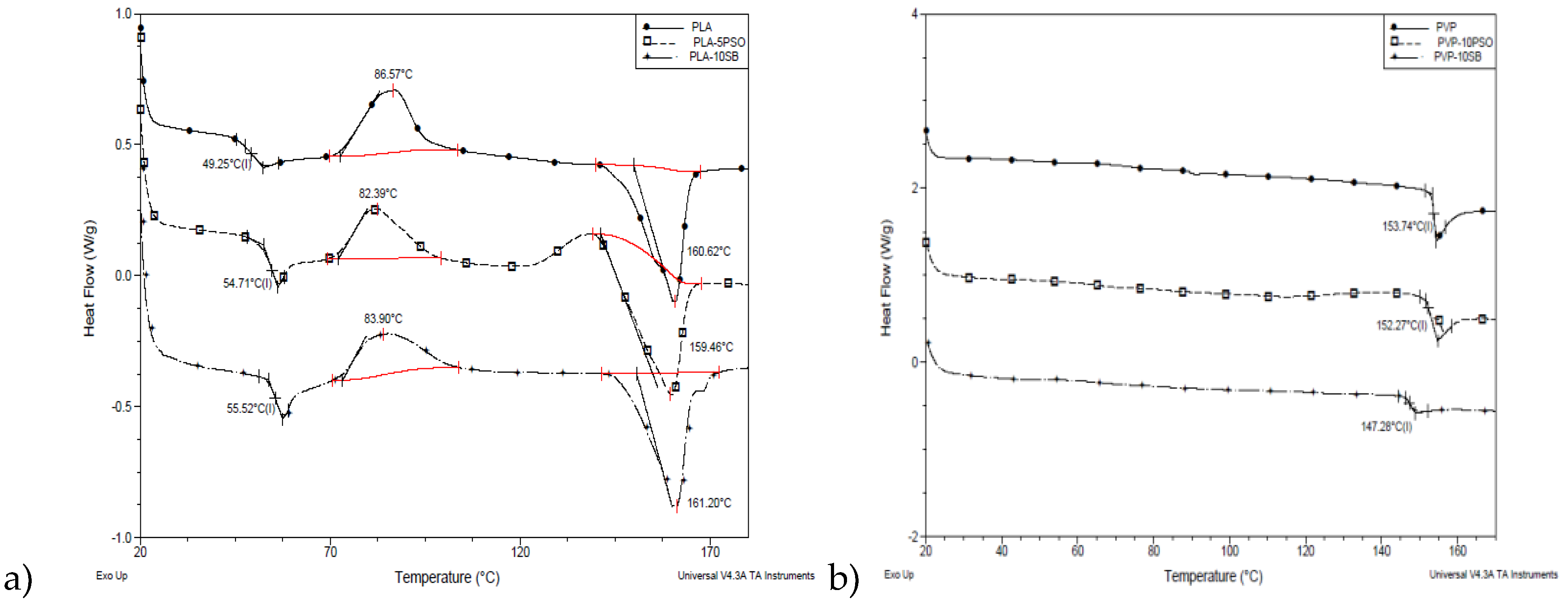
3.4. Thermal Properties
Figure 5 illustrates DSC thermograms of different pure and composite nanofibers. In
Figure 5a thermograms of PLA series of samples are shown. It can be seen that the addition of oils has an influence on Tg compared to pure PLA fibers, increasing it for 4–6 °C. For crystallization temperature, a slight decrease was recorded in the range of 2–3 °C, while melting temperatures were not affected by the addition of oils into the PLA fibers. This behavior is in the correspondence with the morphological characteristics of the samples and phenomenon of immiscibility of PLA with oils added. Phase separation of amorphous and crystalline phase occurred within PLA-based fibers, which had a stronger effect on the amorphous domains.
On thermal properties of PVP fibers (
Figure 5b), the addition of oils had plasticizing effect, which is evidenced by the decrease of Tg values from 2 to 5 °C. Because of the properties of PVP, which is water soluble polymer, fibers absorbed some water during the time of the storage. Endo peak with low intensity was detected at around 80 °C which corresponds to water evaporation.
Thermal properties for all prepared samples are given in the
Table 4.
3.5. Antioxidant Properties
Because of the high content of saturated and unsaturated fatty acids of both PSO and SB, they are extremely sensitive to oxidation and it is usually suggested that they be encapsulated. In
Table 5, results of the antioxidant tests performed are summarized.
Fibers obtained from pure PLA and PVP also had some antioxidant effect, probably because of specific morphology and surface properties. The trend noticed in antioxidant properties is that high concentration of oil loading manifested lower antioxidant activity. Within PLA-series of samples, the decrease of the activity can be explained as the effect of immiscibility of higher loadings of oils with PLA, inducing orientation of oil contents more to the surface of the fibers. As the electrospinning process was done at room conditions in the presence of the air, oxidation of the oil available on the surface might occur.
Antioxidant activity of pure components was also examined by using 1 mg instead of 10 mg of pure component, like it is usually done, to achieve more comparable results (
Table 5). The reason for this is the assumption that ex. 10 mg of PLA-10PSO contain 1 mg of PSO. Comparing the antioxidant activity of pure fatty oils and appropriate nonwoven mats, it can be seen that encapsulated forms have a few times higher antioxidant capacity. As electrospinning is a nano-technique and by forming nanofibers finer dispersion of active compound is achieved, this might be the reason for the enhanced antioxidant activity. It is already demonstrated that compounds on micro and nano level behave in a totally different manner, with the more potent activity of nanosized components, which is one of the many advantages of using nanotechnology. With lower amounts of oils encapsulated into polymer matrices, dispersion is better, while with the increased concentration aggregate-like structures are formed which is in correspondence with morphological examination, and this can also be one of the reasons of decreasing the antioxidant activity with the increasing of concentration of the active compound.
PVP-based fibers loaded with SB showed very low antioxidant activity, there was small difference between pure and oil-loaded fibers. The situation with incorporation of PSO was slightly better, but overall capacity of encapsulation and preservation of oil activity into PVP was lower compared to PLA samples. As PVP is hygroscopic, presence of water in the structure, which was confirmed by DSC, might trigger other processes like oxidation.
As expected, solid particles of EP were less sensitive to oxidation than oils, and with the addition of EP antioxidant activity was significantly improved. When EP was present together with PSO, higher antioxidant activity was detected, derived from the synergistic effect of two components. PVP fibers containing 10 wt% of EP reached 86% of inhibition of DPPH radicals, which is very close to the upper limit of antioxidant capacity of pure component.
4. Conclusions
PLA and PVP were successfully used for the encapsulation of pomegranate seed oil, sea-buckthorn oil, and extract of fermented pomegranate juice. The obtained fibers have good morphology and from all solutions it was possible to obtain a continuous network. The immiscibility of the oils with PLA was observed, but it did not have a significant influence on properties important for the foreseen application. The mechanical properties of all samples were satisfying for the potential application in cosmetics; in most of the cases, active compound improved mechanical properties compared with pure polymer fibers. Tg values of PLA increased with the addition of active compounds, which might be due to phase separation within fibers, while the presence of oils had a plasticizing effect on PVP properties by decreasing its Tg values. In the end, PLA fibers better preserved antioxidant properties of oils compared to PVP, which might be the result of hydrophobicity/hydrophilicity of basic polymers. Considering all presented results of in vitro testing, which are very promising, these materials have high potential to be used in the cosmetic industry.
Author Contributions
Conceptualization, A.M. and B.P. (Branka Pilić); methodology, A.M., B.P. (Branimir Pavlić), I.R., Z.Z., and B.P. (Branka Pilić); validation, B.P. (Branka Pilić) and Z.Z.; formal analysis, A.M., I.R., and B.P. (Branimir Pavlić); writing—original draft preparation, A.M.; writing—review and editing, B.P. (Branka Pilić) and I.R.; supervision, B.P. (Branka Pilić).
Funding
This project has received funding from the Bio Based Industries Joint Undertaking under the European Union’s Horizon 2020 research and innovation program under grant agreement No. 745839.
Acknowledgments
The authors would like to thank BioSense Institute, University of Novi Sad, Serbia, for the use of measurement equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Surai, P.; Deans, S.G. In Vitro Antioxidant Activity of a Number of Plant Essential Oils and Phytoconstituents. J. Essent. Oil Res 2000, 12, 241–248. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Fatty acids in vegetable oils and their importance in cosmetic industry. Chem. Aust. 2014, 68, 103–110. [Google Scholar]
- Aslam, M.N.; Lansky, E.P.; Varani, J. Pomegranate as a cosmeceutical source: Pomegranate fractions promote proliferation and procollagen synthesis and inhibit matrix metalloproteinase-1 production in human skin cells. J. Ethnopharmacol. 2006, 103, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Boroushaki, M.T.; Mollazadeh, H.; Afshari, A.R. Pomegranate seed oil: A comprehensive review on its therapeutic effects. Int. J. Pharm. Sci. Res. 2016, 7, 430–442. [Google Scholar]
- Gumienna, M.; Szwengiel, A.; Górna, B. Bioactive components of pomegranate fruit and their transformation by fermentation processes. Eur. Food Res. Technol. 2016, 242, 631–640. [Google Scholar] [CrossRef]
- Koskovac, M.; Cupara, S.; Kipic, M.; Barjaktarevic, A.; Milovanovic, O.; Kojicic, K.; Markovic, M. Sea buckthorn oil—A valuable source for cosmeceuticals. Cosmetics 2017, 4, 40. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 2017, 16, 95–105. [Google Scholar] [CrossRef]
- Isayev, J.I.; Karimov, Y.B.; Kazimov, H.A. New technology of sea-buckthorn oil extraction. Azerbaijan Med. J. 2005, 2, 7–9. [Google Scholar]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Güler, H.K.; Çallıoğlu, F.C.; Çetin, E.S. Antibacterial PVP/cinnamon essential oil nanofibers by emulsion electrospinning. J. Text. Inst. 2019, 110, 302–310. [Google Scholar] [CrossRef]
- Rieger, K.A.; Schiffman, J.D. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibers. Carbohydr. Polym. 2014, 113, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.L.; Holban, A.M.; Carzino, R.; Lauciello, S.; Grumezescu, A.M. Electrospun Fiber Pads of Cellulose Acetate and Essential Oils with Antimicrobial Activity. Nanomaterials 2017, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Rahimi, A. Poly(ε-caprolactone) electrospun nanofibers containing cinnamon essential oil nanocapsules: A promising technique for controlled release and high solubility. J. Ind. Text. 2019, 48, 1527–1544. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Vicente, A.A.; Reis, M.A.M.; Torres-Giner, S.; Lagaron, J.M. Antimicrobial and Antioxidant Performance of Various Essential Oils and Natural Extracts and Their Incorporation into Biowaste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Layers Made from Electrospun Ultrathin Fibers. Nanomaterials 2019, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Radusin, T.; Torres-Giner, S.; Stupar, A.; Ristic, I.; Miletic, A.; Novakovic, A.; Lagaron, J.M. Preparation, characterization and antimicrobial properties of electrospun polylactide films containing Allium ursinum L. extract. Food Packag. Shelf Life 2019, 21, 100357. [Google Scholar] [CrossRef]
- Altan, A.; Aytac, Z.; Uyar, T. Carvacrol loaded electrospun fibrous films from zein and poly(lactic acid) for active food packaging. Food Hydrocoll. 2018, 81, 48–59. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.H.; Feng, K.; Liu, F.J.; Lou, W.Y.; Li, N.; Zong, M.H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, C.; Kusmartseva, O.; Thomas, N.L.; Mele, E. Electrospinning of polylactic acid fibres containing tea tree and manuka oil. React Funct. Polym. 2017, 117, 106–111. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F. Processing, structure, property relationships and release kinetics of electrospun PLA/Carvacrol membranes. Eur. Polym. J. 2018, 100, 165–171. [Google Scholar] [CrossRef]
- Alipilakkotte, S.; Kumar, S.; Sreejith, L. Fabrication of PLA/Ag nanofibers by green synthesis method using Momordica charantia fruit extract for wound dressing applications. Colloids Surf. A 2017, 529, 771–782. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein-gallic acid system. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1995, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).