Acquisition and Repeatability of High-Frequency Distortion Product Otoacoustic Emissions Using Two Different Calibration Methods in Newborns
Abstract
Featured Application
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Equipment, Calibration, and Software
2.3. Procedures
2.4. Data Analysis
3. Results
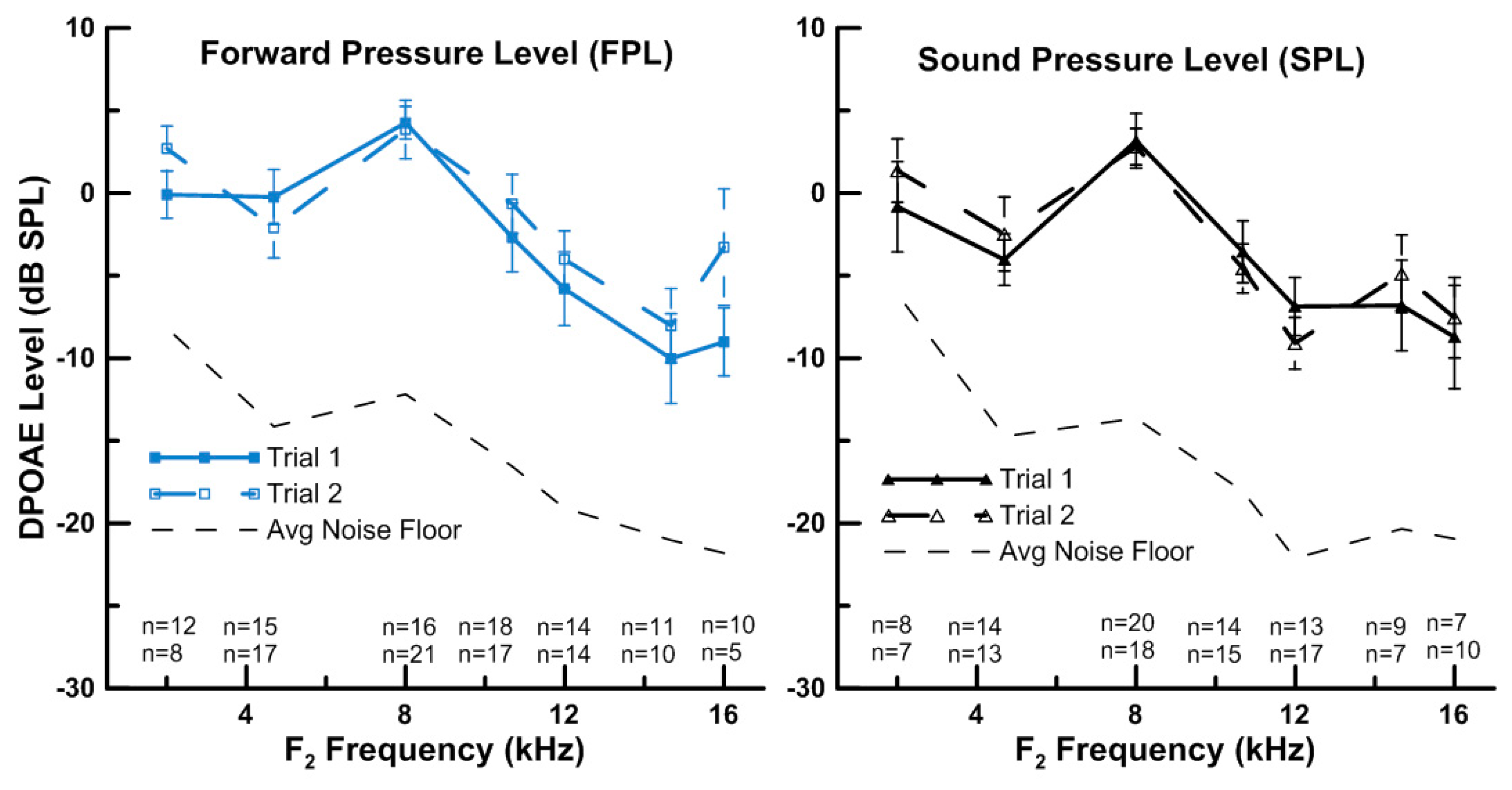
3.1. Measurement of HF DPOAEs (up to 16 kHz)
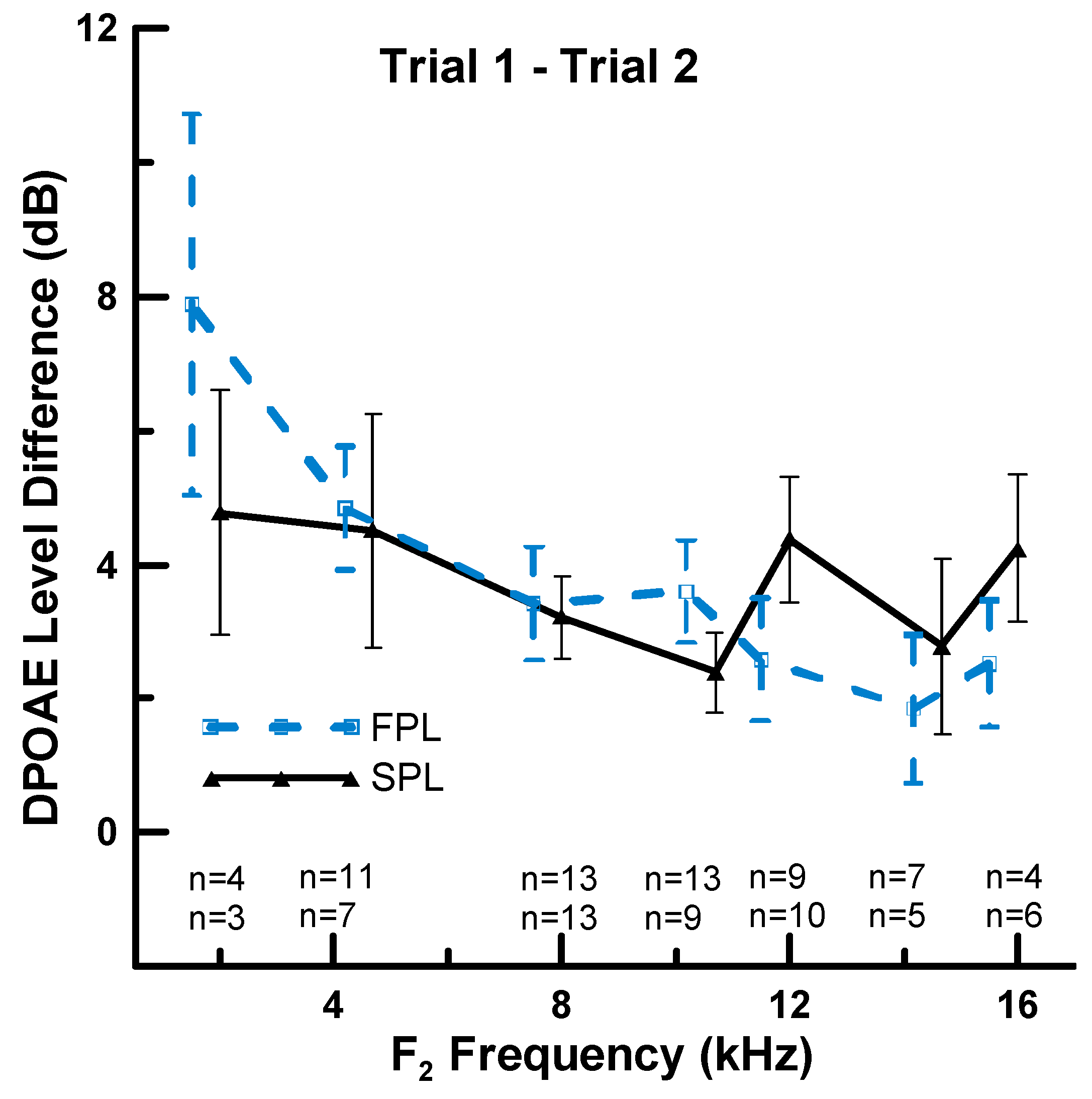
3.2. Effect of Calibration Method on Repeatability of HF DPOAEs
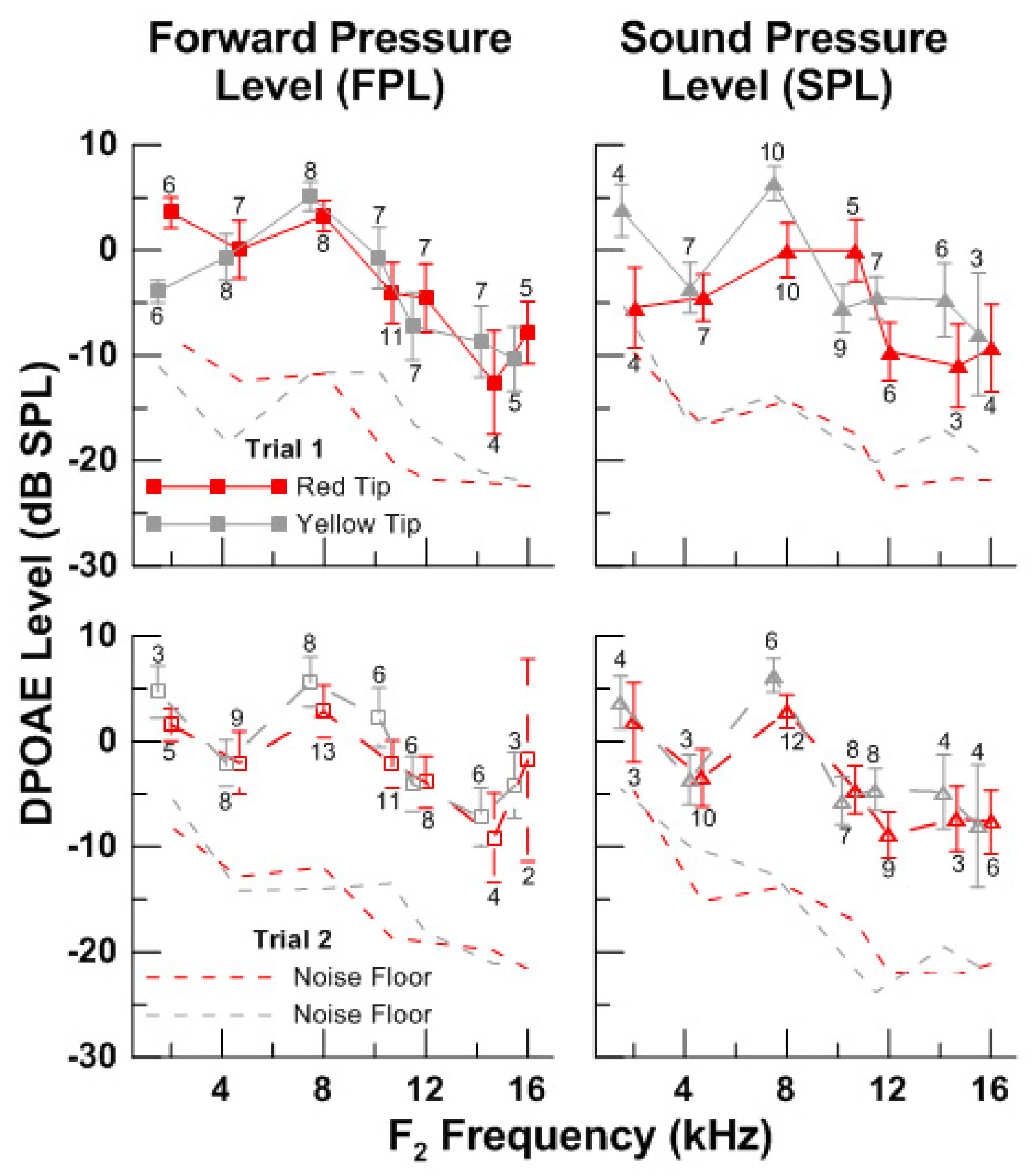
3.3. Effect of Eartip Fit on the Measurement of HF DPOAEs
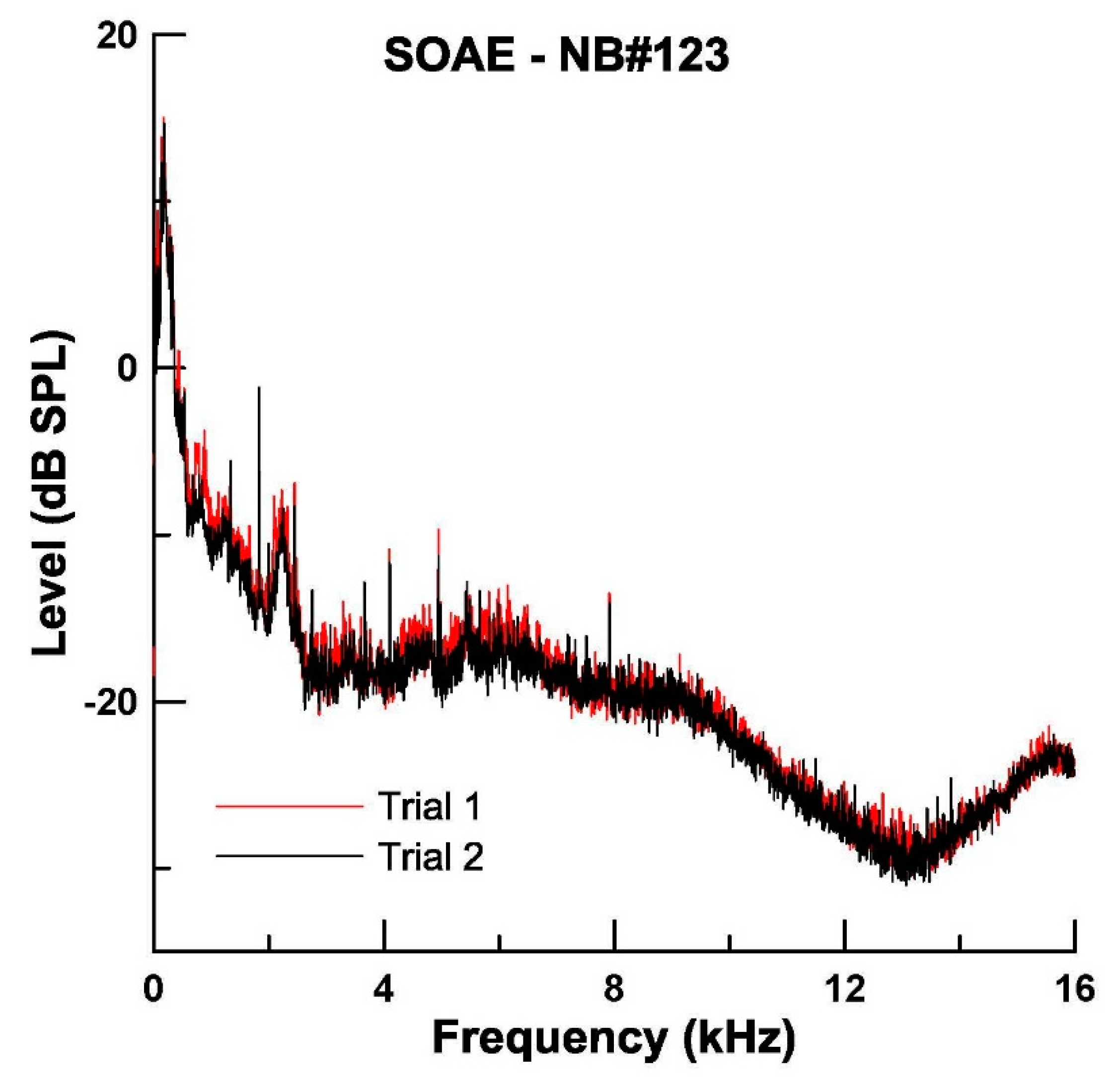
3.4. Measurement of SOAEs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kemp, D.T. Stimulated acoustic emissions from within the human auditory system. J. Acoust. Soc. Am. 1978, 64, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Kemp, D.T.; Ryan, S.; Bray, P. A guide to the effective use of otoacoustic emissions. Ear Hear. 1990, 11, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.P.; Lonsbury-Martin, B.L.; Stagner, B.B.; Coats, A.C.; Martin, G.K. Acoustic distortion products in humans: systematic changes in amplitudes as a function of f2/f1 ratio. J. Acoust. Soc. Am. 1989, 85, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Long, G.R.; Talmadge, C.L.; Tubis, A. The effect of stimulus-frequency ratio on distortion product otoacoustic emission components. J. Acoust. Soc. Am. 2005, 117, 3766–3776. [Google Scholar] [CrossRef] [PubMed]
- Dreisbach, L.E.; Siegel, J.H. Distortion-product otoacoustic emissions measured at high frequencies in humans. J. Acoust. Soc. Am. 2001, 110, 2456–2469. [Google Scholar] [CrossRef] [PubMed]
- Abdala, C.; Sininger, Y.S. The development of cochlear frequency resolution in the human auditory system. Ear Hear. 1996, 17, 374–385. [Google Scholar] [CrossRef]
- Abdala, C.; Oba, S.I.; Ramanathan, R. Changes in the DP-gram during the preterm and early postnatal period. Ear Hear. 2008, 29, 512–523. [Google Scholar] [CrossRef]
- Abdala, C.; Dhar, S. Maturation and Aging of the Human Cochlea: A View through the DPOAE Looking Glass. J. Assoc. Res. Otolaryngol. 2012, 13, 403–421. [Google Scholar] [CrossRef]
- Gorga, M.P.; Neely, S.T.; Bergman, B.; Beauchaine, K.L.; Kaminski, J.R.; Peters, J.; Jesteadt, W. Otoacoustic emissions from normal-hearing and hearing-impaired subjects: distortion product responses. J. Acoust. Soc. Am. 1993, 93, 2050–2060. [Google Scholar] [CrossRef]
- Lonsbury-Martin, B.L.; Harris, F.P.; Stagner, B.B.; Hawkins, M.D.; Martin, G.K. Distortion product emissions in humans. I. Basic properties in normally hearing subjects. Ann. Otol. Rhinol. Laryngol. Suppl. 1990, 147, 3–14. [Google Scholar]
- Lonsbury-Martin, B.L.; Martin, G.K. The clinical utility of distortion-product otoacoustic emissions. Ear Hear. 1990, 11, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Lonsbury-Martin, B.L.; Cutler, W.M.; Martin, G.K. Evidence for the influence of aging on distortion-product otoacoustic emissions in humans. J. Acoust. Soc. Am. 1991, 89, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.K.; Ohlms, L.A.; Franklin, D.J.; Harris, F.P.; Lonsbury-Martin, B.L. Distortion product emissions in humans. III. Influence of sensorineural hearing loss. Ann. Otol. Rhinol. Laryngol. Suppl. 1990, 147, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Abdala, C.; Visser-Dumont, L. Distortion Product Otoacoustic Emissions: A Tool for Hearing Assessment and Scientific Study. Volta Rev. 2001, 103, 281–302. [Google Scholar] [PubMed]
- Abdala, C.; Keefe, D.H. Effects of middle-ear immaturity on distortion product otoacoustic emission suppression tuning in infant ears. J. Acoust. Soc. Am. 2006, 120, 3832–3842. [Google Scholar] [CrossRef] [PubMed]
- Prieve, B.A.; Fitzgerald, T.S.; Schulte, L.E.; Kemp, D.T. Basic characteristics of distortion product otoacoustic emissions in infants and children. J. Acoust. Soc. Am. 1997, 102, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Kruger, B. An update on the external ear resonance in infants and young children. Ear Hear. 1987, 8, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Ikui, A.; Sando, I.; Sudo, M.; Fujita, S. Postnatal change in angle between the tympanic annulus and surrounding structures. Computer-aided three-dimensional reconstruction study. Ann. Otol. Rhinol. Laryngol. 1997, 106, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Ruah, C.B.; Schachern, P.A.; Zelterman, D.; Paparella, M.M.; Yoon, T.H. Age-related morphologic changes in the human tympanic membrane. A light and electron microscopic study. Arch. Otolaryngol. Head Neck Surg. 1991, 117, 627–634. [Google Scholar] [CrossRef]
- Richany, S.F.; Bast, T.H.; Anson, B.J. The development and adult structure of the malleus, incus and stapes. Ann. Otol. Rhinol. Laryngol. 1954, 63, 394–434. [Google Scholar] [CrossRef]
- Stinson, M.R. The spatial distribution of sound pressure within scaled replicas of the human ear canal. J. Acoust. Soc. Am. 1985, 78, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.H. Ear-canal standing waves and high-frequency sound calibration using otoacoustic emission probes. J. Acoust. Soc. Am. 1994, 95, 2589–2597. [Google Scholar] [CrossRef]
- Siegel, J.H.; Hirohata, E.T. Sound calibration and distortion product otoacoustic emissions at high frequencies. Hear. Res. 1994, 80, 146–152. [Google Scholar] [CrossRef]
- Souza, N.N.; Dhar, S.; Neely, S.T.; Siegel, J.H. Comparison of nine methods to estimate ear-canal stimulus levels. J. Acoust. Soc. Am. 2014, 136, 1768. [Google Scholar] [CrossRef] [PubMed]
- Scheperle, R.A.; Neely, S.T.; Kopun, J.G.; Gorga, M.P. Influence of in situ, sound-level calibration on distortion-product otoacoustic emission variability. J. Acoust. Soc. Am. 2008, 124, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Scheperle, R.A.; Goodman, S.S.; Neely, S.T. Further assessment of forward pressure level for in situ calibration. J. Acoust. Soc. Am. 2011, 130, 3882–3892. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.H. Otoreflectance of the cochlea and middle ear. J. Acoust. Soc. Am. 1997, 102, 2849–2859. [Google Scholar] [CrossRef]
- Neely, S.T.; Gorga, M.P. Comparison between intensity and pressure as measures of sound level in the ear canal. J. Acoust. Soc. Am. 1998, 104, 2925–2934. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.E.; Allen, J.B. Measurement of acoustic impedance and reflectance in the human ear canal. J. Acoust. Soc. Am. 1994, 95, 372–384. [Google Scholar] [CrossRef]
- Withnell, R.H.; Jeng, P.S.; Waldvogel, K.; Morgenstein, K.; Allen, J.B. An in situ calibration for hearing thresholds. J. Acoust. Soc. Am. 2009, 125, 1605–1611. [Google Scholar] [CrossRef]
- Keefe, D.H.; Bulen, J.C.; Arehart, K.H.; Burns, E.M. Ear-canal impedance and reflection coefficient in human infants and adults. J. Acoust. Soc. Am. 1993, 94, 2617–2638. [Google Scholar] [CrossRef] [PubMed]
- Kruger, B.; Rubin, R.J. The acoustic properties of the infant ear. Acta Otolaryngol. 1987, 103, 578–585. [Google Scholar]
- Chan, J.C.; Geisler, C.D. Estimation of eardrum acoustic pressure and of ear canal length from remote points in the canal. J. Acoust. Soc. Am. 1990, 87, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Zemplenyi, J.; Gilman, S.; Dirks, D. Optical method for measurement of ear canal length. J. Acoust. Soc. Am. 1985, 78, 2146–2148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Stephens, D. Test-retest variability of distortion-product otoacoustic emissions in human ears with normal hearing. Scand. Audiol. 1999, 28, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Beattie, R.C.; Bleech, J. Effects of sample size on the reliability of noise floor and DPOAE. Br. J. Audiol. 2000, 34, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Beattie, R.C.; Kenworthy, O.T.; Luna, C.A. Immediate and short-term reliability of distortion-product otoacoustic emissions. Int. J. Audiol. 2003, 42, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Heppelmann, G.; Vonthein, R.; Zenner, H.P. Test-retest repeatability of distortion product otoacoustic emissions. Ear Hear. 2008, 29, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Ng, I.-Y.; McPerson, B. Test-Retest Reliability of Distortion Product Otoacoustic Emissions in the 1 to 7 kHz Range. Audiol. Med. 2005, 108–115. [Google Scholar] [CrossRef]
- Konrad-Martin, D.; Poling, G.L.; Dreisbach, L.E.; Reavis, K.M.; McMillan, G.P.; Lapsley Miller, J.A.; Marshall, L. Serial Monitoring of Otoacoustic Emissions in Clinical Trials. Otol Neurotol 2016, 37, e286–294. [Google Scholar] [CrossRef]
- Reavis, K.M.; McMillan, G.P.; Dille, M.F.; Konrad-Martin, D. Meta-Analysis of Distortion Product Otoacoustic Emission Retest Variability for Serial Monitoring of Cochlear Function in Adults. Ear Hear. 2015, 36, e251–e260. [Google Scholar] [CrossRef] [PubMed]
- Roland, P.S. New developments in our understanding of ototoxicity. Ear Nose Throat J. 2004, 83, 15–16; discussion 16–17. [Google Scholar] [CrossRef] [PubMed]
- Dille, M.; McMillan, G.; Reavis, K.; Jacobs, P.; Fausti, S.; Konrad-Martin, D. Ototoxicity risk assessment combining distortion product otoacoustic emissions with a cisplatin dose model. J. Acoust. Soc. Am. 2010, 128, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Konrad-Martin, D.; Knight, K.; McMillan, G.P.; Dreisbach, L.E.; Nelson, E.; Dille, M. Long-Term Variability of Distortion-Product Otoacoustic Emissions in Infants and Children and Its Relation to Pediatric Ototoxicity Monitoring. Ear Hear. 2017. [Google Scholar] [CrossRef] [PubMed]
- Reavis, K.M.; Phillips, D.S.; Fausti, S.A.; Gordon, J.S.; Helt, W.J.; Wilmington, D.; Bratt, G.W.; Konrad-Martin, D. Factors affecting sensitivity of distortion-product otoacoustic emissions to ototoxic hearing loss. Ear Hear. 2008, 29, 875–893. [Google Scholar] [CrossRef]
- Reavis, K.M.; McMillan, G.; Austin, D.; Gallun, F.; Fausti, S.A.; Gordon, J.S.; Helt, W.J.; Konrad-Martin, D. Distortion-product otoacoustic emission test performance for ototoxicity monitoring. Ear Hear. 2011, 32, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.H.; Taylor, R.; Forge, A.; Schacht, J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear. Res. 2001, 155, 1–8. [Google Scholar] [CrossRef]
- Poling, G.L.; Siegel, J.H.; Lee, J.; Dhar, S. Characteristics of the 2f(1)-f(2) distortion product otoacoustic emission in a normal hearing population. J. Acoust. Soc. Am. 2014, 135, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Conrad, A.; Dreisbach, L. Repeatability of high-frequency DPOAE measures in normal-hearing children. Am. Audit. Soc. Abstr. 2011, 36, 42. [Google Scholar]
- Dreisbach, L.E.; Long, K.M.; Lees, S.E. Repeatability of high-frequency distortion-product otoacoustic emissions in normal-hearing adults. Ear Hear. 2006, 27, 466–479. [Google Scholar] [CrossRef]
- Dreisbach, L.; Zettner, E.; Chang Liu, M.; Meuel Fernhoff, C.; MacPhee, I.; Boothroyd, A. High-Frequency Distortion-Product Otoacoustic Emission Repeatability in a Patient Population. Ear Hear. 2018, 39, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.; Dreisbach, L. Repeatability of high-frequency behavioral and DPOAE measures in normal-hearing children. Am. Audit. Soc. Abstr. 2012, 37, 57. [Google Scholar]
- Dreisbach, L.; Ho, M.; Reid, E.; Siegel, J. Effects of Oxaliplatin, Carboplatin, and Cisplatin Across Treatment on High-Frequency Objective and Subjective Auditory Measures in Adults. Perspect. ASHA Spec. Interest Gr. 2017, 2, 17–36. [Google Scholar] [CrossRef]
- Fausti, S.A.; Henry, J.A.; Helt, W.J.; Phillips, D.S.; Frey, R.H.; Noffsinger, P.D.; Larson, V.D.; Fowler, C.G. An individualized, sensitive frequency range for early detection of ototoxicity. Ear Hear. 1999, 20, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Cheng, X.; En, H.; Huang, L.; Zhang, L. Characterization of spontaneous otoacoustic emissions in full-term newborns. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.M.; Arehart, K.H.; Campbell, S.L. Prevalence of spontaneous otoacoustic emissions in neonates. J. Acoust. Soc. Am. 1992, 91, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- McFadden, D.; Plattsmier, H.S. Aspirin Abolishes Spontaneous Otoacoustic Emissions. J. Acoust. Soc. Am. 1984, 16, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T.; Fukuda, S.; Chida, E.; Kashiwamura, M.; Matsumura, M.; Ohwatari, R.; Inuyama, Y. Effects of spontaneous otoacoustic emissions on distortion product otoacoustic emission. Auris Nasus Larynx 2001, 28, S33–S38. [Google Scholar] [CrossRef]
- Penner, M.J.; Glotzbach, L.; Huang, T. Spontaneous otoacoustic emissions: measurement and data. Hear. Res. 1993, 68, 229–237. [Google Scholar] [CrossRef]
- Burns, E.M.; Campbell, S.L.; Arehart, K.H. Longitudinal measurements of spontaneous otoacoustic emissions in infants. J. Acoust. Soc. Am. 1994, 95, 385–394. [Google Scholar] [CrossRef]
- Lamprecht-Dinnesen, A.; Pohl, M.; Hartmann, S.; Heinecke, A.; Ahrens, S.; Müller, E.; Riebandt, M. Effects of age, gender and ear side on SOAE parameters in infancy and childhood. Audiol. Neurootol. 1998, 3, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Strickland, E.A.; Burns, E.M.; Tubis, A. Incidence of spontaneous otoacoustic emissions in children and infants. J. Acoust. Soc. Am. 1985, 78, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Bonfils, P. Spontaneous otoacoustic emissions: clinical interest. Laryngoscope 1989, 99, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Groh, D.; Pelanova, J.; Jilek, M.; Popelar, J.; Kabelka, Z.; Syka, J. Changes in otoacoustic emissions and high-frequency hearing thresholds in children and adolescents. Hear. Res. 2006, 212, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T. Clinical investigation on spontaneous otoacoustic emission (SOAE) in 447 ears. Auris Nasus Larynx 2007, 34, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kok, M.R.; van Zanten, G.A.; Brocaar, M.P. Aspects of spontaneous otoacoustic emissions in healthy newborns. Hear. Res. 1993, 69, 115–123. [Google Scholar] [CrossRef]
- Burns, E.M. Long-term stability of spontaneous otoacoustic emissions. J. Acoust. Soc. Am. 2009, 125, 3166–3176. [Google Scholar] [CrossRef]
- Moulin, A.; Collet, L.; Veuillet, E.; Morgon, A. Interrelations between transiently evoked otoacoustic emissions, spontaneous otoacoustic emissions and acoustic distortion products in normally hearing subjects. Hear. Res. 1993, 65, 216–233. [Google Scholar] [CrossRef]
- Hall, J.W. Handb. Otoacoust. Emiss.; Singular Thomson Learning: San Diego, CA, USA, 2000; pp. 67–93. [Google Scholar]
- McFadden, D.; Mishra, R. On the relation between hearing sensitivity and otoacoustic emissions. Hear. Res. 1993, 71, 208–213. [Google Scholar] [CrossRef]
- Snihur, A.W.; Hampson, E. Sex and ear differences in spontaneous and click-evoked otoacoustic emissions in young adults. Brain Cognit. 2011, 77, 40–47. [Google Scholar] [CrossRef]
- Zurek, P.M. Spontaneous Narrowband Acoustic Signals Emitted by Human Ears. J. Acoust. Soc. Am. 1981, 69, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Collet, L.; Gartner, M.; Veuillet, E.; Moulin, A.; Morgon, A. Evoked and spontaneous otoacoustic emissions. A comparison of neonates and adults. Brain Dev. 1993, 15, 249–252. [Google Scholar] [CrossRef]
- McFadden, D. Sex differences in the auditory system. Dev. Neuropsychol. 1998, 14, 261–298. [Google Scholar] [CrossRef]
- Abdala, C.; Luo, P.; Shera, C.A. Characterizing spontaneous otoacoustic emissions across the human lifespan. J. Acoust. Soc. Am. 2017, 141, 1874. [Google Scholar] [CrossRef] [PubMed]
- Neely, S.T.; Liu, Z. EMAV: Otoacoustic Emission Averager. Tech. Memo No. 17 1993, 1–26. [Google Scholar]
- Neely, S.; Stevenson, R. SysRes. Tech. Memo No. 1 2002, 1–10. [Google Scholar]
- Dreisbach, L.E.; Siegel, J.H. Level dependence of distortion-product otoacoustic emissions measured at high frequencies in humans. J. Acoust. Soc. Am. 2005, 117, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Charaziak, K.K.; Shera, C.A. Compensating for ear-canal acoustics when measuring otoacoustic emissions. J. Acoust. Soc. Am. 2017, 141, 515. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dreisbach Hawe, L.; Portugal, N.; Aguilar, E.; Hansen, W.; Kite, D.; McIntyre, S.; Minasian, C. Acquisition and Repeatability of High-Frequency Distortion Product Otoacoustic Emissions Using Two Different Calibration Methods in Newborns. Appl. Sci. 2019, 9, 2947. https://doi.org/10.3390/app9152947
Dreisbach Hawe L, Portugal N, Aguilar E, Hansen W, Kite D, McIntyre S, Minasian C. Acquisition and Repeatability of High-Frequency Distortion Product Otoacoustic Emissions Using Two Different Calibration Methods in Newborns. Applied Sciences. 2019; 9(15):2947. https://doi.org/10.3390/app9152947
Chicago/Turabian StyleDreisbach Hawe, Laura, Nicholas Portugal, Eliza Aguilar, William Hansen, Daniela Kite, Sky McIntyre, and Celine Minasian. 2019. "Acquisition and Repeatability of High-Frequency Distortion Product Otoacoustic Emissions Using Two Different Calibration Methods in Newborns" Applied Sciences 9, no. 15: 2947. https://doi.org/10.3390/app9152947
APA StyleDreisbach Hawe, L., Portugal, N., Aguilar, E., Hansen, W., Kite, D., McIntyre, S., & Minasian, C. (2019). Acquisition and Repeatability of High-Frequency Distortion Product Otoacoustic Emissions Using Two Different Calibration Methods in Newborns. Applied Sciences, 9(15), 2947. https://doi.org/10.3390/app9152947



