Graphene-Based Membranes for CO2/CH4 Separation: Key Challenges and Perspectives
Abstract
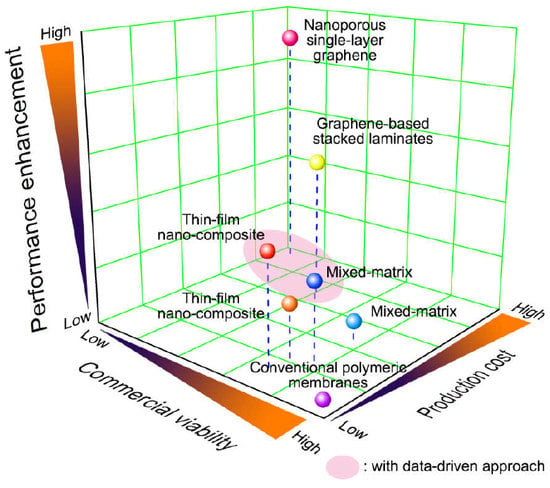
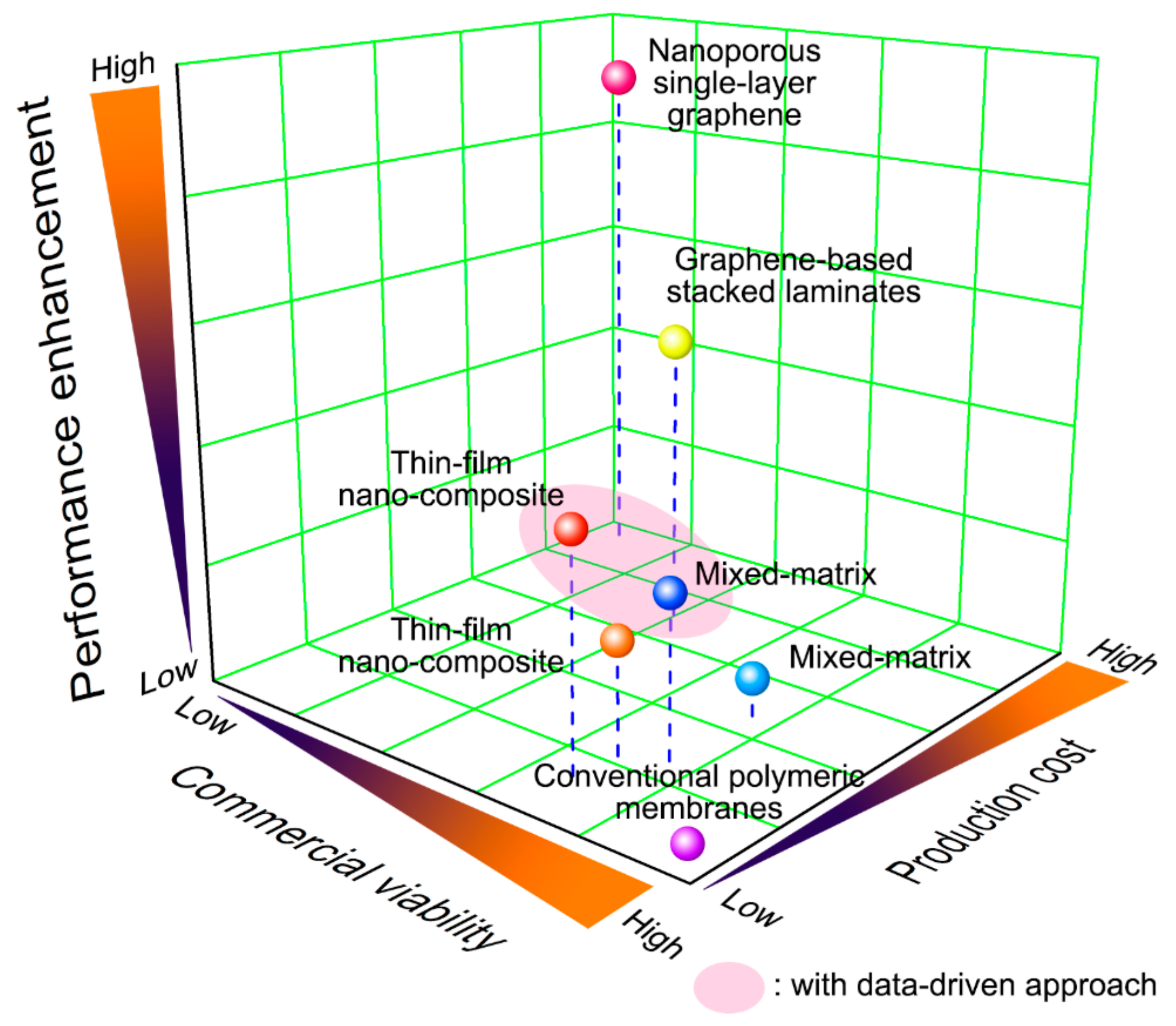
1. Introduction
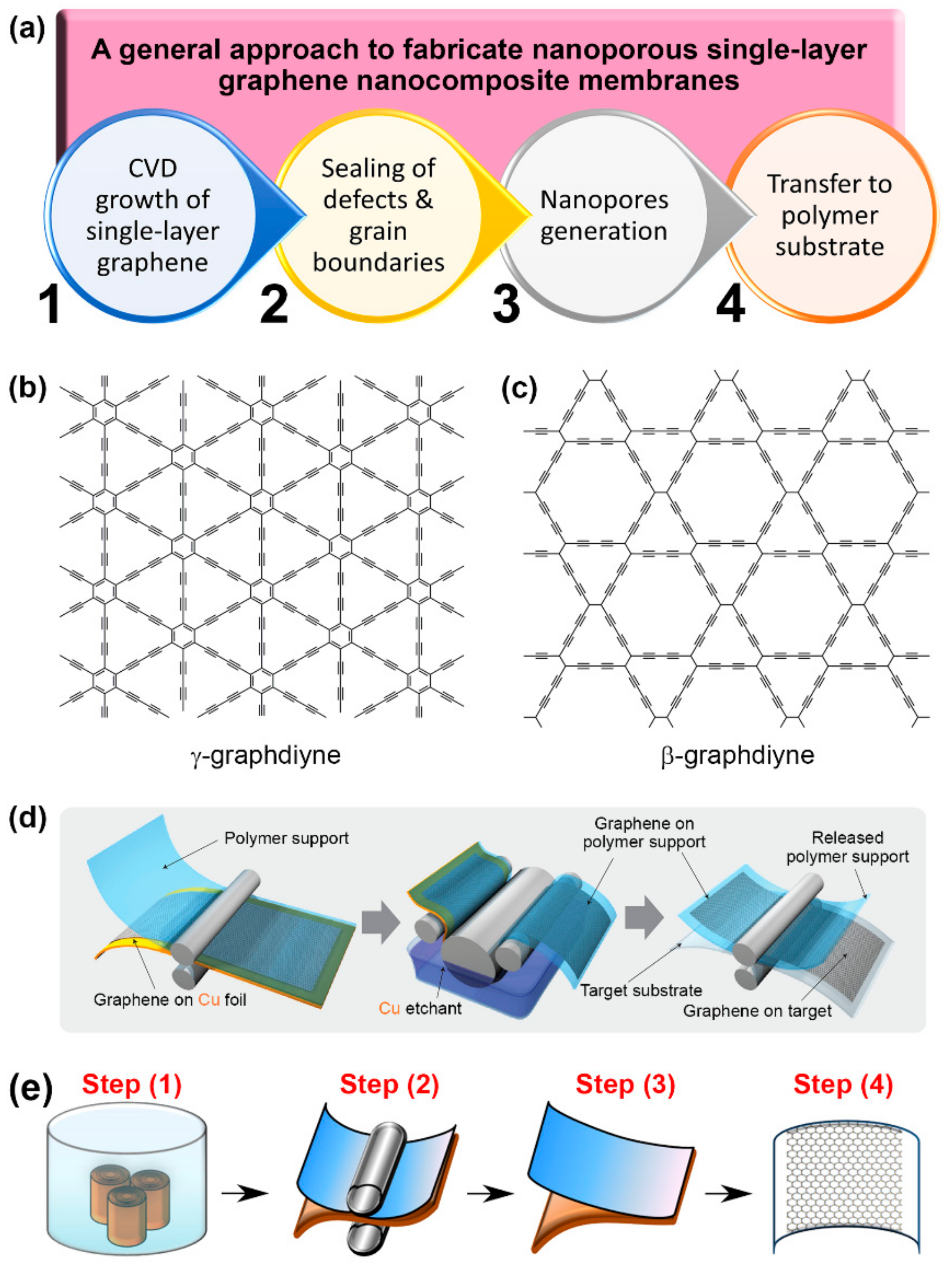
2. Nanoporous Singe-Layer Graphene
2.1. Challenges
2.2. Perspectives
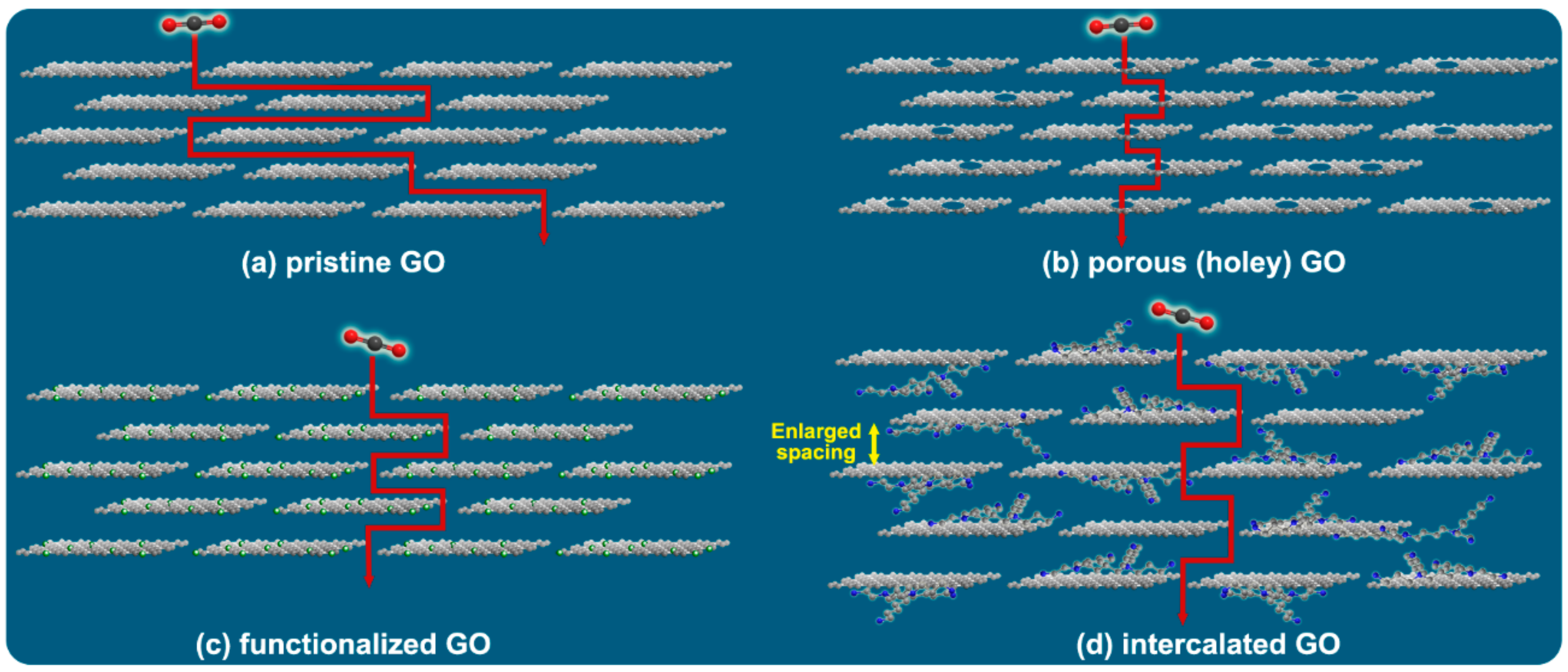
3. Few- to Multi-Layer Graphene-Based Stacked Laminates
3.1. Challenges
3.2. Perspectives
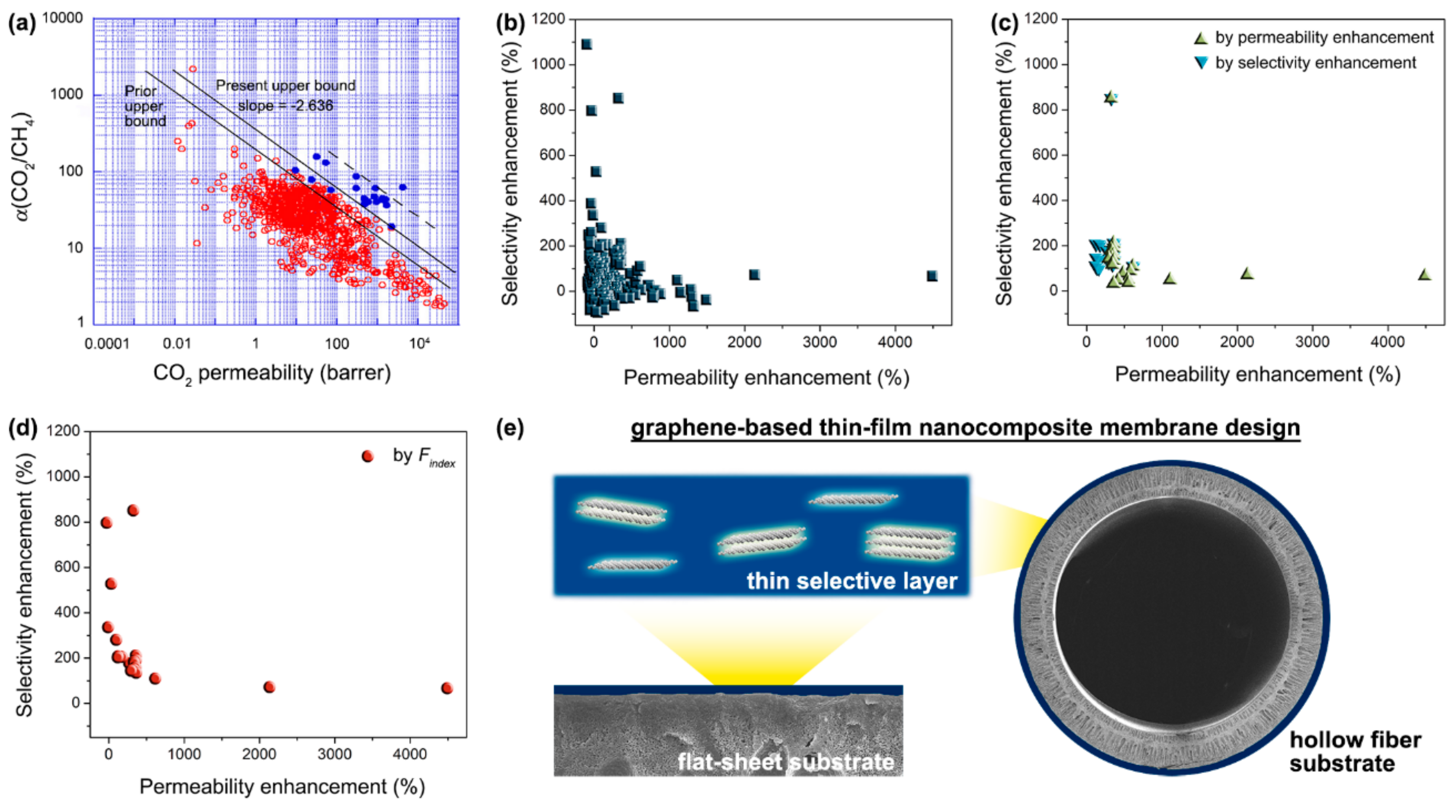
4. Mixed-Matrix Membranes
4.1. Challenges
4.2. Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Connor, P.; Cleveland, C.U.S. Energy Transitions 1780–2010. Energies 2014, 7, 7955–7993. [Google Scholar] [CrossRef]
- Holmes, D.E.; Smith, J.A. Chapter One—Biologically Produced Methane as a Renewable Energy Source. Adv. Appl. Microbiol. 2016, 97, 1–61. [Google Scholar] [PubMed]
- Geppert, F.; Liu, D.; van Eerten-Jansen, M.; Weidner, E.; Buisman, C.; Ter Heijne, A. Bioelectrochemical Power-to-Gas: State of the Art and Future Perspectives. Trends Biotechnol. 2016, 34, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Goh, K.; Yang, Y.; Gong, H.; Li, W.; Karahan, H.E.; Guiver, M.D.; Wang, R.; Bae, T.-H. Harnessing Filler Materials for Enhancing Biogas Separation Membranes. Chem. Rev. 2018, 118, 8655–8769. [Google Scholar] [CrossRef] [PubMed]
- Dumee, L.; Scholes, C.; Stevens, G.; Kentish, S. Purification of aqueous amine solvents used in post combustion CO2 capture: A review. Int. J. Greenh. Gas Control 2012, 10, 443–455. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas Control 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, H.; Chen, J.; Lv, Y.; Dong, C.; Sreeprasad, T.S. Graphene and graphene oxide: Advanced membranes for gas separation and water purification. Inorg. Chem. Front. 2015, 2, 417–424. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef]
- Celebi, K.; Buchheim, J.; Wyss, R.M.; Droudian, A.; Gasser, P.; Shorubalko, I.; Kye, J.-I.; Lee, C.; Park, H.G. Ultimate Permeation Across Atomically Thin Porous Graphene. Science 2014, 344, 289–292. [Google Scholar] [CrossRef]
- Goh, K.; Karahan, H.E.; Wei, L.; Bae, T.H.; Fane, A.G.; Wang, R.; Chen, Y. Carbon Nanomaterials for Advancing Separation Membranes: A Strategic Perspective. Carbon 2016, 109, 694–710. [Google Scholar] [CrossRef]
- Goh, K.; Setiawan, L.; Wei, L.; Si, R.; Fane, A.G.; Wang, R.; Chen, Y. Graphene oxide as effective selective barriers on a hollow fiber membrane for water treatment process. J. Membr. Sci. 2015, 474, 244–253. [Google Scholar] [CrossRef]
- Goh, K.; Heising, J.K.; Yuan, Y.; Karahan, H.E.; Wei, L.; Zhai, S.; Koh, J.X.; Htin, N.M.; Zhang, F.; Wang, R.; et al. Sandwich-Architectured Poly(lactic acid)-Graphene Composite Food Packaging Films. ACS Appl. Mater. Interfaces 2016, 8, 9994–10004. [Google Scholar] [CrossRef] [PubMed]
- Norahim, N.; Kajornsak, F.; Quitain, A.T.; Klaysom, C. Composite membranes of graphene oxide for CO2/CH4 separation. J. Chem. Technol. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Ali, A.; Pothu, R.; Siyal, S.H.; Phulpoto, S.; Sajjad, M.; Thebo, K.H. Graphene-based membranes for CO2 separation. Mater. Sci. Energy Technol. 2019, 2, 83–88. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Hegab, H.M.; Zou, L. Graphene oxide-assisted membranes: Fabrication and potential applications in desalination and water purification. J. Membr. Sci. 2015, 484, 95–106. [Google Scholar] [CrossRef]
- Yoo, B.M.; Shin, H.-J.; Yoon, H.W.; Park, H.B. Graphene and graphene oxide and their uses in barrier polymers. J. Appl. Polym. Sci. 2014, 131, 39628. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; van der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable Atomic Membranes from Graphene Sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef]
- O’Hern, S.C.; Boutilier, M.S.H.; Idrobo, J.-C.; Song, Y.; Kong, J.; Laoui, T.; Atieh, M.; Karnik, R. Selective Ionic Transport through Tunable Subnanometer Pores in Single-Layer Graphene Membranes. Nano Lett. 2014, 14, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Gethers, M.L.; Thomas, J.C.; Jiang, S.; Weiss, N.O.; Duan, X.; Goddard, W.A. III.; Weiss, P.S. Holey Graphene as a Weed Barrier for Molecules. ACS Nano 2015, 9, 10909–10915. [Google Scholar] [CrossRef] [PubMed]
- Surwade, S.P.; Smirnov, S.N.; Vlassiouk, I.V.; Unocic, R.R.; Veith, G.M.; Dai, S.; Mahurin, S.M. Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol. 2015, 10, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, G.; Huang, S.; Villalobos, L.F.; Dakhchoune, M.; Bassas, H.; Agrawal, K.V. Etching gas-sieving nanopores in single-layer graphene with an angstrom precision for high-performance gas mixture separation. Sci. Adv. 2019, 5, eaav1851. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.P.; Wang, L.D.; Pellegrino, J.; Bunch, J.S. Selective molecular sieving through porous graphene. Nat. Nanotechnol. 2012, 7, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, M.; Carmona-Novillo, E.; Hernández, M.I.; Campos-Martínez, J.; Pirani, F.; Giorgi, G. Graphdiyne Pores: “Ad Hoc” Openings for Helium Separation Applications. J. Phys. Chem. C 2014, 118, 29966–29972. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Liu, H.; Li, Y. Graphdiyne and graphyne: From theoretical predictions to practical construction. Chem. Soc. Rev. 2014, 43, 2572–2586. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Wang, D.; Zhang, J. Graphdiyne: Synthesis, properties, and applications. Chem. Soc. Rev. 2019, 48, 908–936. [Google Scholar] [CrossRef]
- Jiao, Y.; Du, A.; Hankel, M.; Zhu, Z.; Rudolph, V.; Smith, S.C. Graphdiyne: A versatile nanomaterial for electronics and hydrogen purification. Chem. Commun. 2011, 47, 11843–11845. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574. [Google Scholar] [CrossRef]
- Shivayogimath, A.; Whelan, P.R.; Mackenzie, D.M.A.; Luo, B.; Huang, D.; Luo, D.; Wang, M.; Gammelgaard, L.; Shi, H.; Ruoff, R.S.; et al. Do-It-Yourself Transfer of Large-Area Graphene Using an Office Laminator and Water. Chem. Mater. 2019, 31, 2328–2336. [Google Scholar] [CrossRef]
- Li, H.; Song, Z.; Huang, Y.; Li, S.; Mao, Y.; Ploehn, H.J.; Bao, Y.; Yu, M. Ultrathin, Molecular-Sieving Graphene Oxide Membranes for Selective Hydrogen Separation. Science 2013, 342, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Wu, Y.Z.; Zhang, N.; He, G.W.; Xin, Q.P.; Wu, X.Y.; Wu, H.; Cao, X.Z.; Guiver, M.D.; Jiang, Z.Y. A highly permeable graphene oxide membrane with fast and selective transport nanochannels for efficient carbon capture. Energy Environ. Sci. 2016, 9, 3107–3112. [Google Scholar] [CrossRef]
- Goh, K.; Jiang, W.C.; Karahan, H.E.; Zhai, S.L.; Wei, L.; Yu, D.S.; Fane, A.G.; Wang, R.; Chen, Y. All-Carbon Nanoarchitectures as High-Performance Separation Membranes with Superior Stability. Adv. Funct. Mater. 2015, 25, 7348–7359. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Chu, Z.; Jin, W.; Xu, N. Subnanometer Two-Dimensional Graphene Oxide Channels for Ultrafast Gas Sieving. ACS Nano 2016, 10, 3398–3409. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Yoon, H.W.; Yoon, S.-M.; Yoo, B.M.; Ahn, B.K.; Cho, Y.H.; Shin, H.J.; Yang, H.; Paik, U.; Kwon, S.; et al. Selective Gas Transport Through Few-Layered Graphene and Graphene Oxide Membranes. Science 2013, 342, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Moon, J.-H.; Ma, X.; Zhang, L.; Chen, Q.; Chen, L.; Peng, R.; Si, P.; Feng, J.; Li, Y.; et al. High performance graphene oxide nanofiltration membrane prepared by electrospraying for wastewater purification. Carbon 2018, 130, 487–494. [Google Scholar] [CrossRef]
- Akbari, A.; Sheath, P.; Martin, S.T.; Shinde, D.B.; Shaibani, M.; Banerjee, P.C.; Tkacz, R.; Bhattacharyya, D.; Majumder, M. Large-area graphene-based nanofiltration membranes by shear alignment of discotic nematic liquid crystals of graphene oxide. Nat. Commun. 2016, 7, 10891. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Bolling, L.; Priolo, M.A.; Grunlan, J.C. Super Gas Barrier and Selectivity of Graphene Oxide-Polymer Multilayer Thin Films. Adv. Mater. 2013, 25, 503–508. [Google Scholar] [CrossRef]
- Gadipelli, S.; Guo, Z.X. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2015, 69, 1–60. [Google Scholar] [CrossRef]
- Khakpay, A.; Rahmani, F.; Nouranian, S.; Scovazzo, P. Molecular Insights on the CH4/CO2 Separation in Nanoporous Graphene and Graphene Oxide Separation Platforms: Adsorbents versus Membranes. J. Phys. Chem. C 2017, 121, 12308–12320. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y.; He, G.; Xin, Q.; Zhang, J.; Yang, L.; Li, Y.; Wu, H.; Zhang, Y.; Guiver, M.D.; et al. Graphene Oxide Membranes with Heterogeneous Nanodomains for Efficient CO2 Separations. Angew. Chem. Int. Ed. 2017, 56, 14246–14251. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Peng, D.; Wu, H.; Yang, L.; Wu, X.; Wu, Y.; Wang, S.; Jiang, Z. Enhanced carbon dioxide flux by catechol–Zn2+ synergistic manipulation of graphene oxide membranes. Chem. Eng. Sci. 2019, 195, 230–238. [Google Scholar] [CrossRef]
- Fam, W.; Mansouri, J.; Li, H.; Hou, J.; Chen, V. Gelled Graphene Oxide–Ionic Liquid Composite Membranes with Enriched Ionic Liquid Surfaces for Improved CO2 Separation. ACS Appl. Mater. Interfaces 2018, 10, 7389–7400. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Greer, D.W.; O’Leary, B.W. Advanced Materials and Membranes for Gas Separations: The UOP Approach. In Nanotechnology: Delivering on the Promise Volume 2; American Chemical Society: Washington, WA, USA, 2016; pp. 119–135. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 1–538. [Google Scholar]
- Chen, L.; Li, Y.; Chen, L.; Li, N.; Dong, C.; Chen, Q.; Liu, B.; Ai, Q.; Si, P.; Feng, J.; et al. A large-area free-standing graphene oxide multilayer membrane with high stability for nanofiltration applications. Chem. Eng. J. 2018, 345, 536–544. [Google Scholar] [CrossRef]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane gas separation technologies for biogas upgrading. Rsc. Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Hydrogen Sulfide Capture: From Absorption in Polar Liquids to Oxide, Zeolite, and Metal-Organic Framework Adsorbents and Membranes. Chem. Rev. 2017, 117, 9755–9803. [Google Scholar] [CrossRef]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Scholz, M. Membrane based Biogas Upgrading Processes. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2013. [Google Scholar]
- Cheng, Y.; Wang, Z.; Zhao, D. Mixed Matrix Membranes for Natural Gas Upgrading: Current Status and Opportunities. Ind. Eng. Chem. Res. 2018, 57, 4139–4169. [Google Scholar] [CrossRef]
- Noble, R.D. Perspectives on mixed matrix membranes. J. Membr. Sci. 2011, 378, 393–397. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Jin, W.; Lee, K.R.; Xu, N. Membranes with Fast and Selective Gas-Transport Channels of Laminar Graphene Oxide for Efficient CO2 Capture. Angew. Chem. Int. Ed. 2015, 54, 578–582. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H. Efficient CO2 Capture By Functionalized Graphene Oxide Nanosheets as Fillers to Fabricate Multi-Permselective Mixed Matrix Membranes. ACS Appl. Mater. Interfaces 2015, 7, 5528–5537. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Li, Z.; Li, C.; Wang, S.; Jiang, Z.; Wu, H.; Zhang, Y.; Yang, J.; Cao, X. Enhancing the CO2 separation performance of composite membranes by the incorporation of amino acid-functionalized graphene oxide. J. Mater. Chem. A 2015, 3, 6629–6641. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Li, X.Q.; Ma, L.; Zhang, H.Y.; Wang, S.F.; Jiang, Z.Y.; Guo, R.L.; Wu, H.; Cao, X.Z.; Yang, J.; Wang, B.Y. Synergistic effect of combining carbon nanotubes and graphene oxide in mixed matrix membranes for efficient CO2 separation. J. Membr. Sci. 2015, 479, 1–10. [Google Scholar] [CrossRef]
- Li, W.; Samarasinghe, S.A.S.C.; Bae, T.-H. Enhancing CO2/CH4 separation performance and mechanical strength of mixed-matrix membrane via combined use of graphene oxide and ZIF-8. J. Ind. Eng. Chem. 2018, 67, 156–163. [Google Scholar] [CrossRef]
- García-Cruz, L.; Casado-Coterillo, C.; Irabien, Á.; Montiel, V.; Iniesta, J. High Performance of Alkaline Anion-Exchange Membranes Based on Chitosan/Poly (vinyl) Alcohol Doped with Graphene Oxide for the Electrooxidation of Primary Alcohols. C 2016, 2, 10. [Google Scholar] [CrossRef]
- Sánchez-González, S.; Diban, N.; Urtiaga, A. Hydrolytic Degradation and Mechanical Stability of Poly(ε-Caprolactone)/Reduced Graphene Oxide Membranes as Scaffolds for In Vitro Neural Tissue Regeneration. Membranes 2018, 8, 12. [Google Scholar] [CrossRef]
- Marcos-Madrazo, A.; Casado-Coterillo, C.; García-Cruz, L.; Iniesta, J.; Simonelli, L.; Sebastián, V.; Encabo-Berzosa, M.D.M.; Arruebo, M.; Irabien, Á. Preparation and Identification of Optimal Synthesis Conditions for a Novel Alkaline Anion-Exchange Membrane. Polymers 2018, 10, 913. [Google Scholar] [CrossRef]
- Zahri, K.; Goh, P.; Ismail, A. The incorporation of graphene oxide into polysulfone mixed matrix membrane for CO2/CH4 separation. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012007. [Google Scholar] [CrossRef]
- Castarlenas, S.; Gorgojo, P.; Casado-Coterillo, C.; Masheshwari, S.; Tsapatsis, M.; Téllez, C.; Coronas, J. Melt compounding of swollen titanosilicate JDF-L1 with polysulfone to obtain mixed matrix membranes for H2/CH4 separation. Ind. Eng. Chem. Res. 2013, 52, 1901–1907. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Pinnau, I. Fine-Tuned Intrinsically Ultramicroporous Polymers Redefine the Permeability/Selectivity Upper Bounds of Membrane-Based Air and Hydrogen Separations. ACS Macro Lett. 2015, 4, 947–951. [Google Scholar] [CrossRef]
- Berean, K.J.; Ou, J.Z.; Nour, M.; Field, M.R.; Alsaif, M.M.Y.A.; Wang, Y.C.; Ramanathan, R.; Bansal, V.; Kentish, S.; Doherty, C.M.; et al. Enhanced Gas Permeation through Graphene Nanocomposites. J. Phys. Chem. C 2015, 119, 13700–13712. [Google Scholar] [CrossRef]
- Ha, H.; Park, J.; Ha, K.; Freeman, B.D.; Ellison, C.J. Synthesis and gas permeability of highly elastic poly(dimethylsiloxane)/graphene oxide composite elastomers using telechelic polymers. Polymer 2016, 93, 53–60. [Google Scholar] [CrossRef]
- Ha, H.; Park, J.; Ando, S.; Kim, C.B.; Nagai, K.; Freeman, B.D.; Ellison, C.J. Gas Permeation and Selectivity of Poly(dimethylsiloxane)/Graphene Oxide Composite Elastomer Membranes. J. Membr. Sci. 2016, 518, 131–140. [Google Scholar] [CrossRef]
- Dai, Y.; Ruan, X.H.; Yan, Z.J.; Yang, K.; Yu, M.; Li, H.; Zhao, W.; He, G.H. Imidazole functionalized graphene oxide/PEBAX mixed matrix membranes for efficient CO2 capture. Sep. Purif. Technol. 2016, 166, 171–180. [Google Scholar] [CrossRef]
- Althumayri, K.; Harrison, W.J.; Shin, Y.; Gardiner, J.M.; Casiraghi, C.; Budd, P.M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. The influence of few-layer graphene on the gas permeability of the high-free-volume polymer PIM-1. Phil. Trans. R. Soc. A 2016, 374, 1–9. [Google Scholar] [CrossRef]
- Karunakaran, M.; Shevate, R.; Kumar, M.; Peinemann, K.V. CO2-Selective PEO-PBT (PolyActive)/Graphene Oxide Composite Membranes. Chem. Commun. 2015, 51, 14187–14190. [Google Scholar] [CrossRef]
- Castarlenas, S.; Tellez, C.; Coronas, J. Gas separation with mixed matrix membranes obtained from MOF UiO-66-graphite oxide hybrids. J. Membr. Sci. 2017, 526, 205–211. [Google Scholar] [CrossRef]
- Xue, Q.; Pan, X.; Li, X.; Zhang, J.; Guo, Q. Effective enhancement of gas separation performance in mixed matrix membranes using core/shell structured multi-walled carbon nanotube/graphene oxide nanoribbons. Nanotechnology 2017, 28, 065702. [Google Scholar] [CrossRef]
- Li, W.; Chuah, C.Y.; Nie, L.; Bae, T.-H. Enhanced CO2/CH4 selectivity and mechanical strength of mixed-matrix membrane incorporated with NiDOBDC/GO composite. J. Ind. Eng. Chem. 2019, 74, 118–125. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Del Mar López-Guerrero, M.; Irabien, Á. Synthesis and Characterisation of ETS-10/Acetate-based Ionic Liquid/Chitosan Mixed Matrix Membranes for CO2/N2 Permeation. Membranes 2014, 4, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Casado-Coterillo, C.; Fernández-Barquín, A.; Valencia, S.; Irabien, Á. Estimating CO2/N2 Permselectivity through Si/Al = 5 Small-Pore Zeolites/PTMSP Mixed Matrix Membranes: Influence of Temperature and Topology. Membranes 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Goh, K.; Weerachanchai, P.; Bae, T.-H. 3D covalent organic framework for morphologically induced high-performance membranes with strong resistance toward physical aging. J. Membr. Sci. 2019, 574, 235–242. [Google Scholar] [CrossRef]
- Wang, S.F.; Li, X.Q.; Wu, H.; Tian, Z.Z.; Xin, Q.P.; He, G.W.; Peng, D.D.; Chen, S.L.; Yin, Y.; Jiang, Z.Y.; et al. Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Pinnau, I. (Novel Ultra-Microporous Polymers and Thin-film Composite Membranes with Unprecedented Properties for Energy-Efficient Gas Separations. Invited Lecture Singapore Membrane Technology Centre, Singapore). Private communication, 2017.

| Filler | Polymer Matrix | Filler Loading (wt%) | Separation Performance | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Testing Condition | P(CO2) (barrer) | Permeability Enhancement (%) a | α(CO2/CH4) | Selectivity Enhancement (%) a | Findex value | |||||
| Pres. (bar) | Temp. (°C) | |||||||||
| Nonfunctionalized graphene | PDMS | 0.5 | 1.1 | 37 | 4460 | 47.68 | 4.2 | 16.67 | 0.80 | [66] |
| GO | PDMS | 1 | 10 | 35 | 2429.76 | −40.69 | 3.58 | 17.6 | −0.10 | [67] |
| GO | PDMS | 8 | 10.1 | 35 | 142.22 | −60.00 | 9.40 | 209.90 | 2.07 | [68] |
| GO | Pebax 1657 | 1 | 3 | 25 | 100.00 | 102.06 | 24.66 | 14.42 | 1.06 | [54] |
| PEI-PEG-GO | Pebax 1657 | 10 | 2 | 30 | 145.00 | 76.83 | 24.0 | 26.32 | 1.19 | [55] |
| PEI-PEG-GO | Pebax 1657 | 10 | 2 | 30 | 1330.00 | 170.87 | 45.0 | 136.84 | 3.27 | [55] |
| Im-GO | Pebax 1657 | 0.8 | 4 | 25 | 64.00 | 3.56 | 25.10 | −3.83 | −0.07 | [69] |
| Few-layer graphene | PIM-1 | 0.00096 | 1 | 25 | 12700 | 148.05 | 8.76 | −41.84 | −0.52 | [70] |
| Few-layer graphene | PIM-1 | 0.0071 | 1 | 25 | 3410 | 33.40 | 21.31 | 41.53 | 1.20 | [70] |
| Few-layer graphene | PIM-1 (200-day aging) | 0.00096 | 1 | 25 | 9240 | 151.77 | 9.43 | −48.62 | −0.83 | [70] |
| GO | Matrimid® | 0.5 | 0.5 | 26 | 76.00 | −49.33 | 21.71 | 21.6 | −0.16 | [71] |
| GO | Matrimid® | 2 | 2 | 30 | 11.73 | 32.69 | 48.83 | 43.62 | 1.24 | [58] |
| GO | Matrimid® | 10 | 2 | 30 | 6.46 | −26.92 | 70.30 | 106.76 | 1.60 | [58] |
| MWCNT/GO (1:1) | Matrimid® | 10 | 2 | 30 | 38.07 | 330.66 | 84.60 | 148.82 | 3.86 | [58] |
| Graphene | Matrimid® | 10 | 2 | 30 | 9.89 b | 20.76 | 20.16 | −28.6 | −0.70 | [58] |
| MWCNT/GO (1:1) | Matrimid® | 10 | 2 | 30 | 35.43 b | 332.60 | 78.57 | 178.12 | 4.16 | [58] |
| GO | PEO-PBTP | 0.075 | 0.5 | 25 | 130.00 | −13.33 | 21.70 | 21.5 | 0.37 | [71] |
| GO | PEO-PBTP/PAN | 0.075 | 0.5 | 25 | 603.07 c | −25.00 | 21.71 | 18.4 | 0.16 | [71] |
| GO | PEO-PBTP/PAN | 0.5 | 0.5 | 25 | 241.23 c | −70.00 | 21.29 | 16.1 | −0.81 | [71] |
| GO | Matrimid® | 8 | 3.4 | 35 | 3.89 | −47.07 | 20.50 | −36.34 | −1.83 | [72] |
| GO | PSF | 8 | 3.4 | 35 | 2.98 | −51.70 | 16.67 | −45.34 | −2.32 | [72] |
| MWCNT/GO nanoribbons | PI | 1 | 1 | 35 | 17 | 13.3 | 25.0 | 92.3 | 1.85 | [73] |
| Da-Cys-GO | SPEEK | 8 | 1.5 | 25 | 22.26 | 43.87 | 48.8 | 82.77 | 1.95 | [56] |
| Da-Cys-GO | SPEEK | 8 | 1 | 25 | 1247.60 | 120.70 | 81.8 | 207.52 | 3.75 | [56] |
| Da-Cys-GO | SPEEK | 8 | 1 | 25 | 1227.00 b | 117.05 | 80.7 | 203.38 | 3.70 | [56] |
| GO | ODPA-TMPDA | 5 | 1 | 25 | 98.51 d | 7.49 | 41.10 | 29.58 | 0.76 | [59] |
| ZIF-8/GO (1:2) | ODPA-TMPDA | 5 | 1 | 25 | 146.57 d | 60.00 | 40.92 | 29.01 | 1.14 | [59] |
| GO | Matrimid® | 10 | 1 | 25 | 7.28 d | –27.38 | 45.61 | 24.20 | 0.25 | [74] |
| NiDOBDC/GO (1:1) | Matrimid® | 10 | 1 | 25 | 10.34 d | 3.57 | 49.93 | 35.97 | 0.85 | [74] |
| Graphene | PSF | 0 | 5 | 25 | 86.80 c | 34.64 | 25.98 | 35.31 | 1.09 | [63] |
| GO | Pebax | 0.25 | 5 | 35 | 8.44 c | –21.92 | 13.89 | 83.00 | 1.35 | [14] |
| GO | Pebax/PEG (1/1) | 0.25 | 5 | 35 | 11.98 c | 10.82 | 14.02 | 84.72 | 1.72 | [14] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, K.; Karahan, H.E.; Yang, E.; Bae, T.-H. Graphene-Based Membranes for CO2/CH4 Separation: Key Challenges and Perspectives. Appl. Sci. 2019, 9, 2784. https://doi.org/10.3390/app9142784
Goh K, Karahan HE, Yang E, Bae T-H. Graphene-Based Membranes for CO2/CH4 Separation: Key Challenges and Perspectives. Applied Sciences. 2019; 9(14):2784. https://doi.org/10.3390/app9142784
Chicago/Turabian StyleGoh, Kunli, H. Enis Karahan, Euntae Yang, and Tae-Hyun Bae. 2019. "Graphene-Based Membranes for CO2/CH4 Separation: Key Challenges and Perspectives" Applied Sciences 9, no. 14: 2784. https://doi.org/10.3390/app9142784
APA StyleGoh, K., Karahan, H. E., Yang, E., & Bae, T.-H. (2019). Graphene-Based Membranes for CO2/CH4 Separation: Key Challenges and Perspectives. Applied Sciences, 9(14), 2784. https://doi.org/10.3390/app9142784