Supplementation of Non-Dairy Creamer-Enriched High-Fat Diet with D-Allulose Ameliorated Blood Glucose and Body Fat Accumulation in C57BL/6J Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of the General and Fatty Acid Compositions of the Non-Dairy Creamer
2.2. Animals and Diets
2.3. Plasma, Hepatic, and Fecal Lipid Profile Analysis
2.4. Lipid-Regulating Enzyme Activity Measurement
2.5. Plasma Cytokine and Adipokine Measurement
2.6. Real-Time qPCR Analysis
2.7. Histopathology Analysis
2.8. Statistical Analysis
3. Results
3.1. Components of Fatty Acids in Non-Dairy Creamer used for Animal Diet
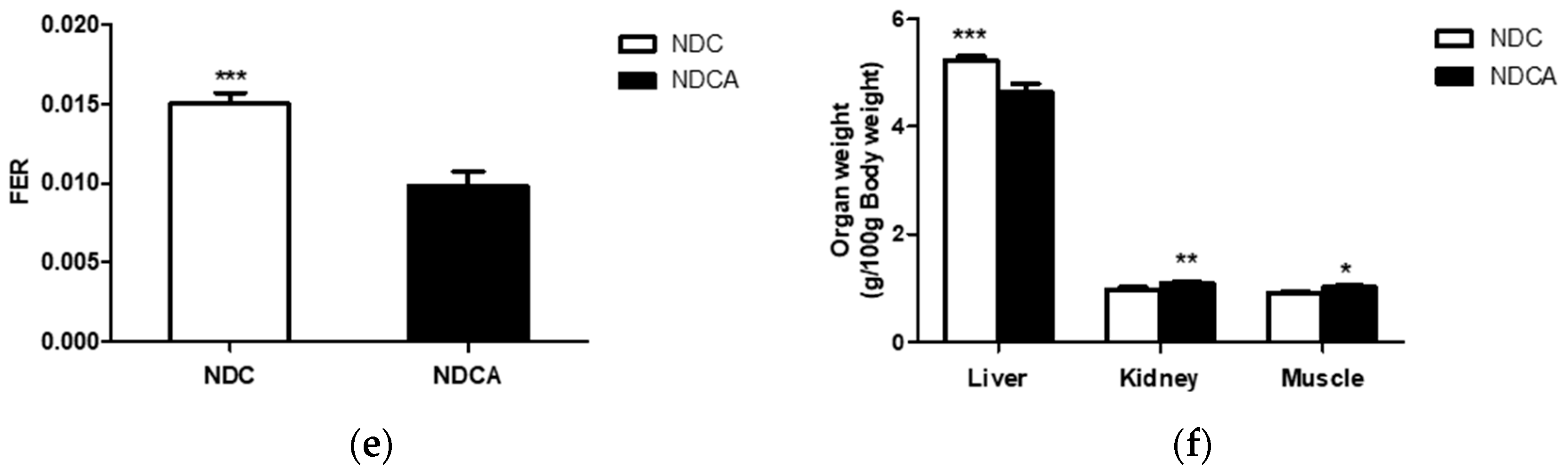
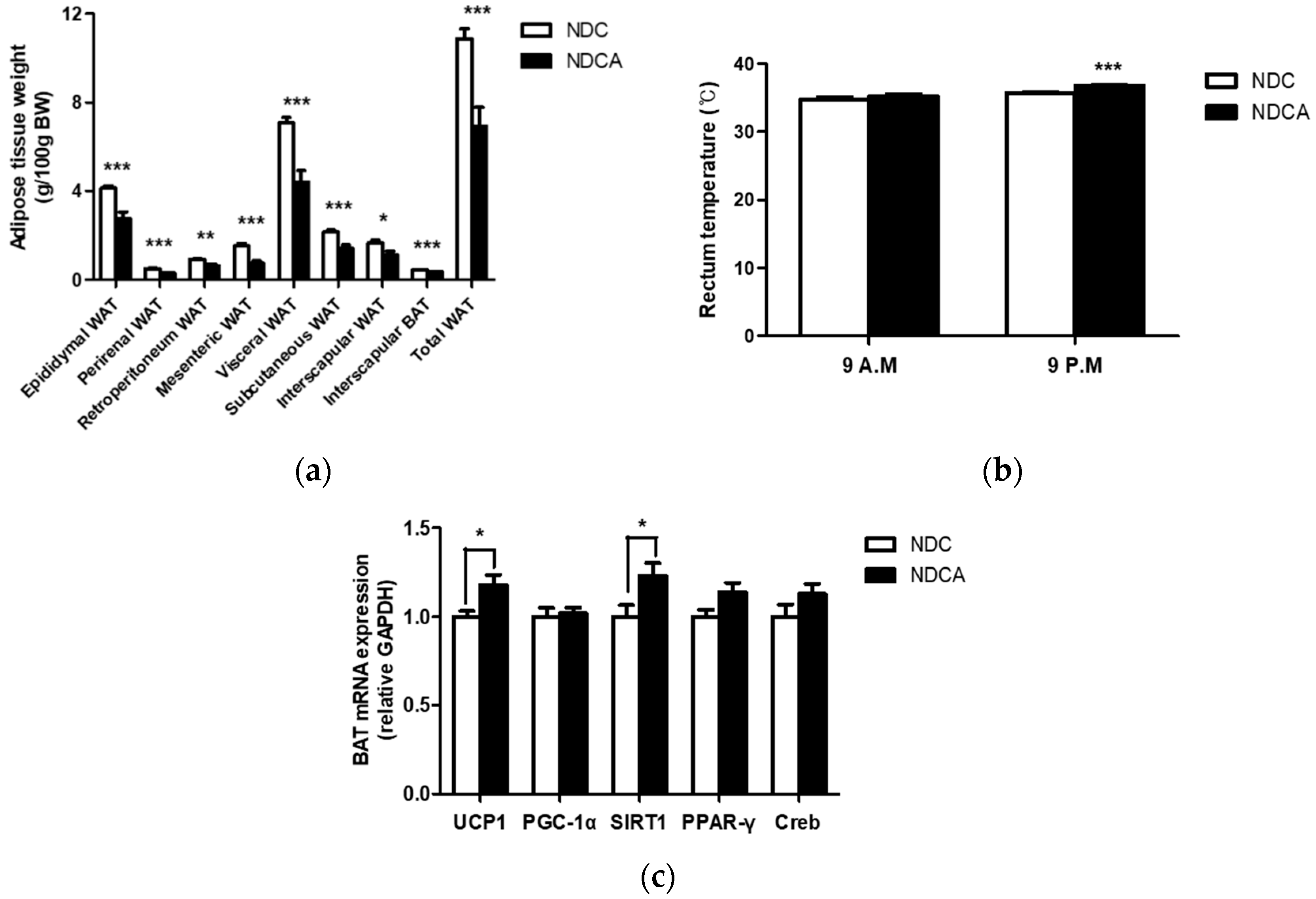
3.2. Effect of Consumption of D-Allulose with Non-Dairy creamer as Part of a Dietary Fat Source on Body and Organ Weights
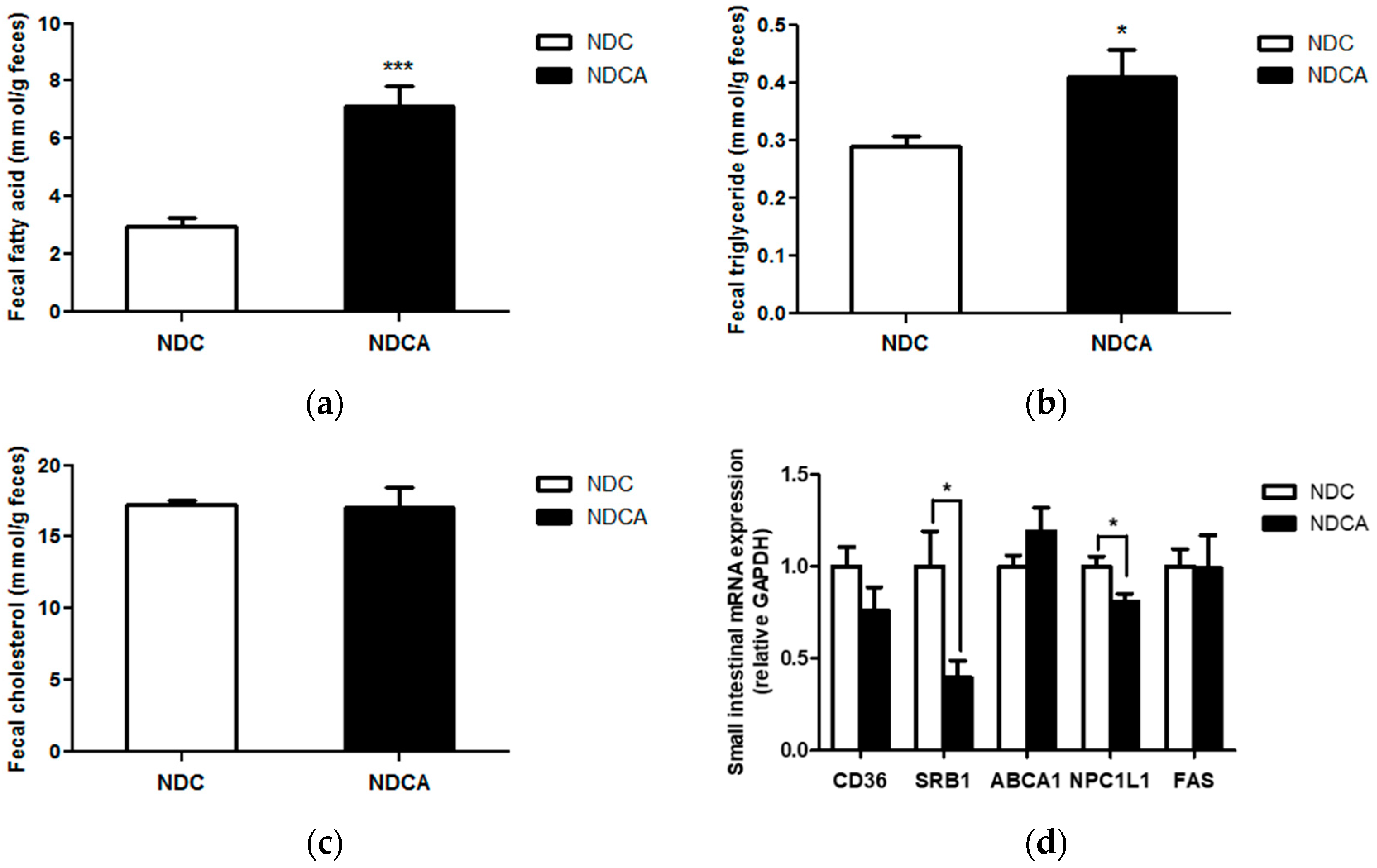
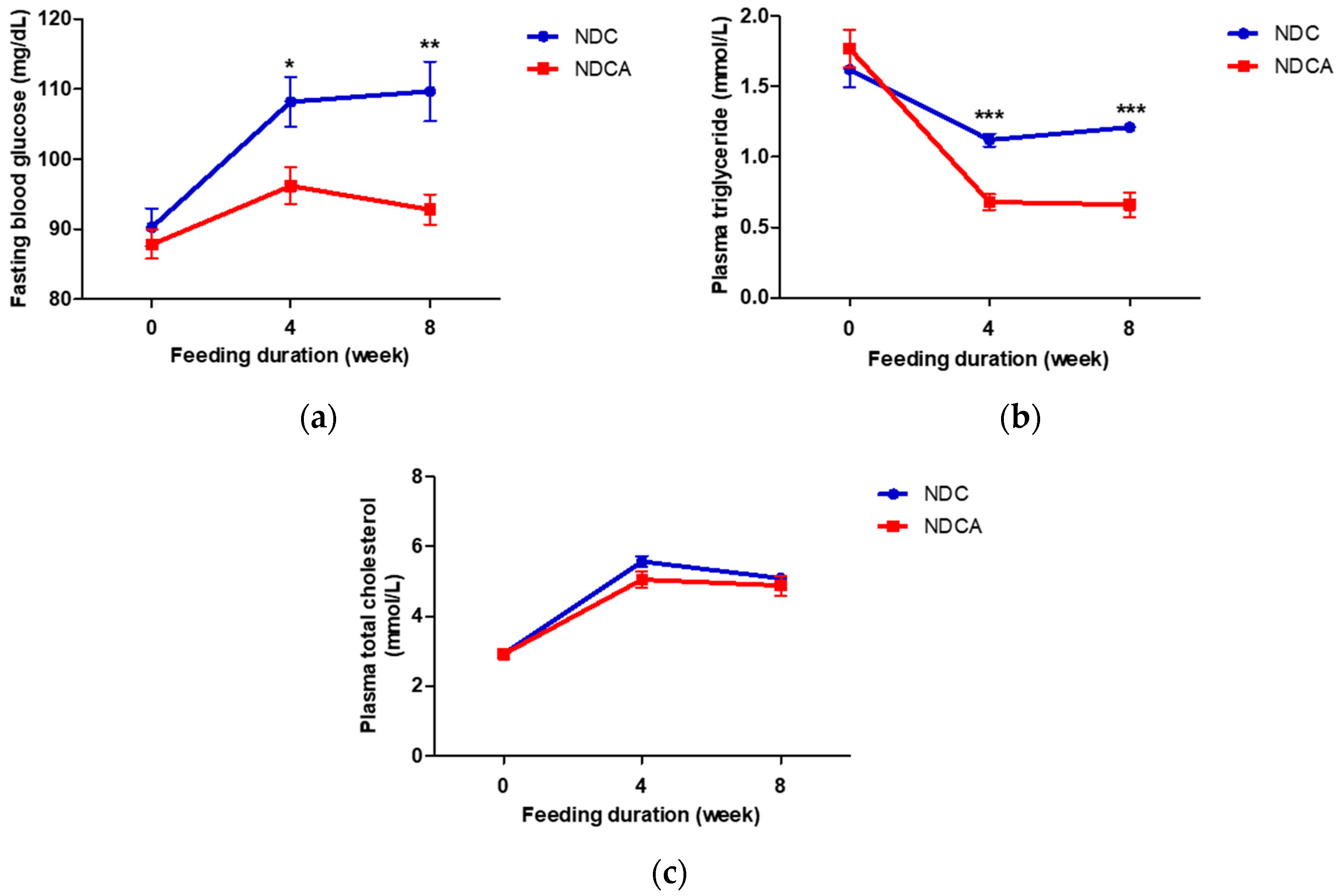
3.3. Effect of Consumption of D-Allulose with Non-Dairy Creamer on Fecal Lipid Excretion and Plasma Lipid Profiles
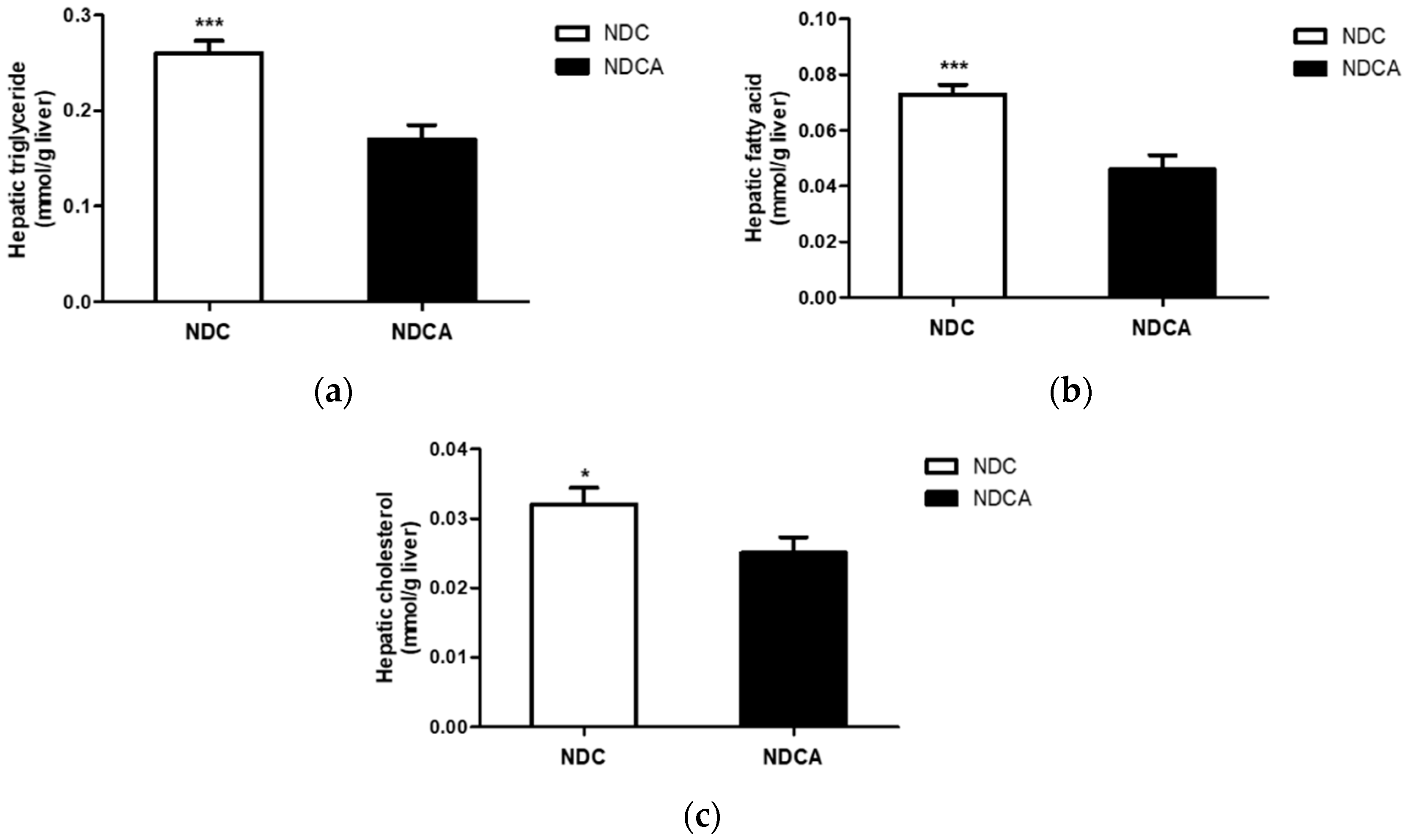
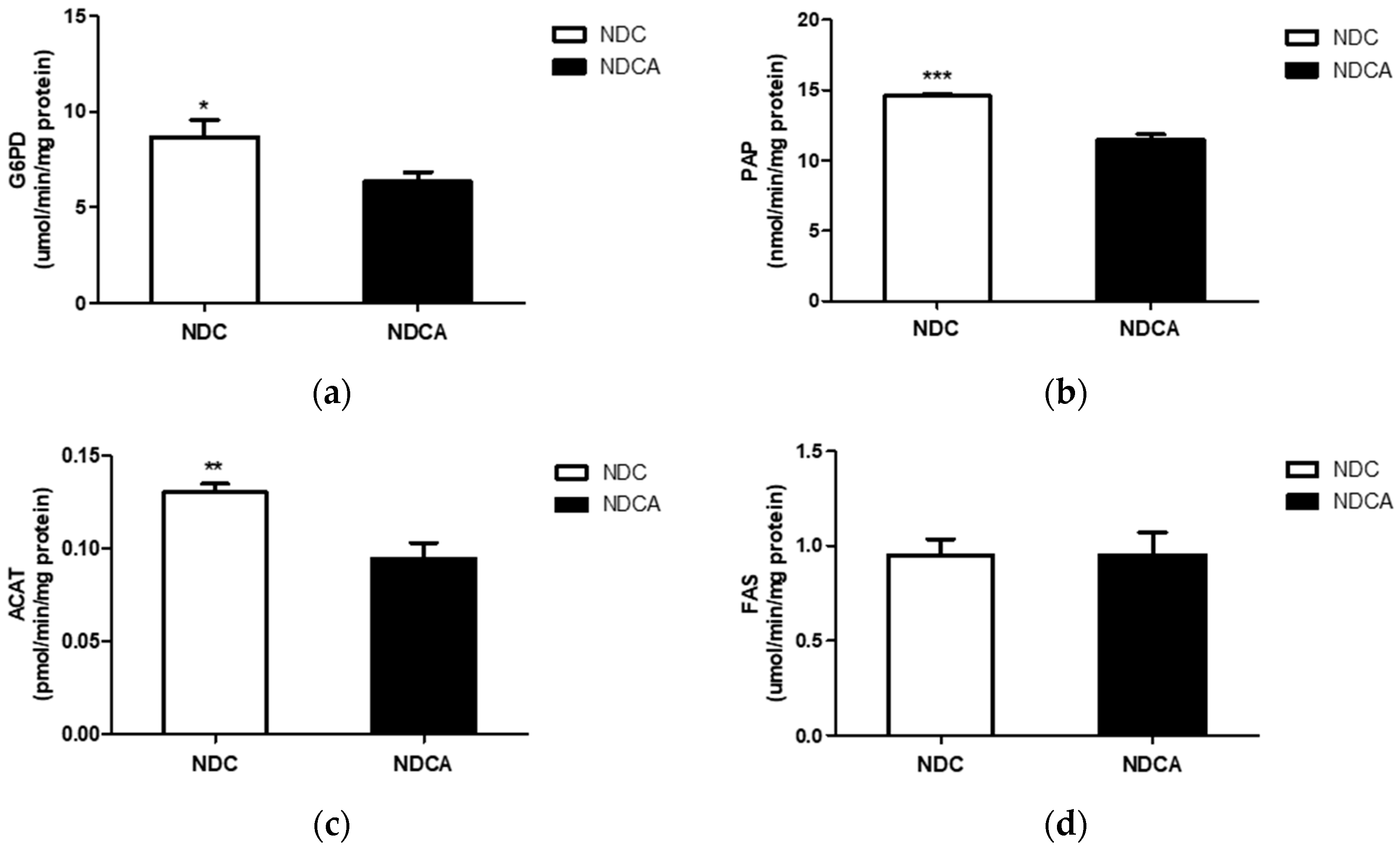
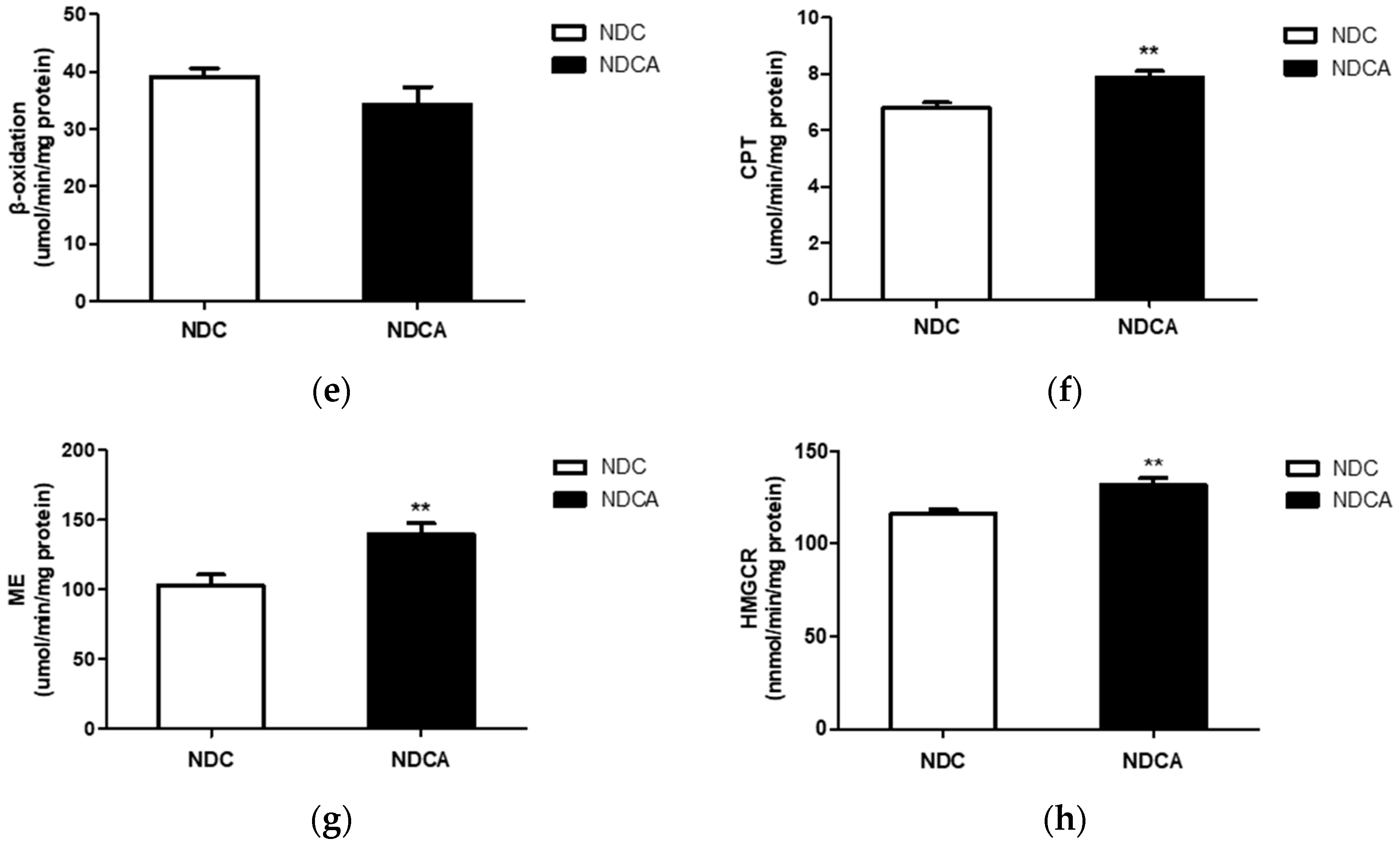
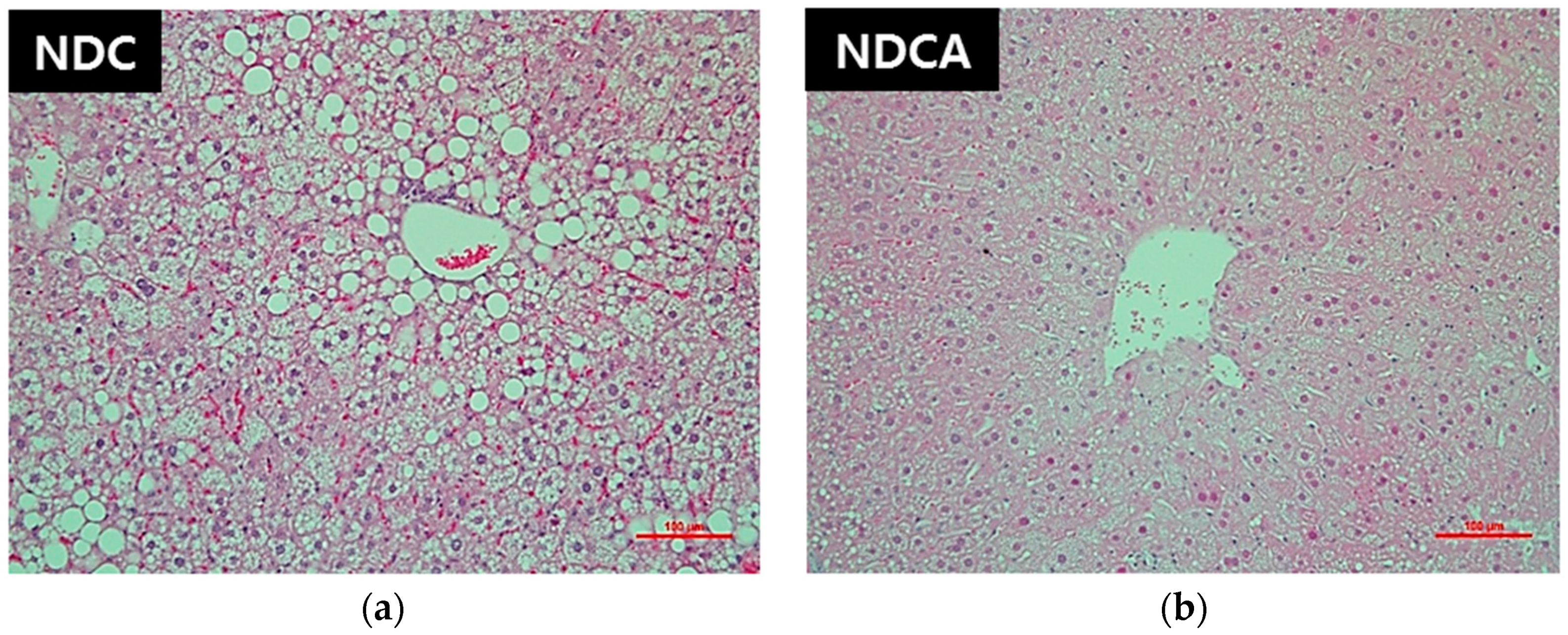
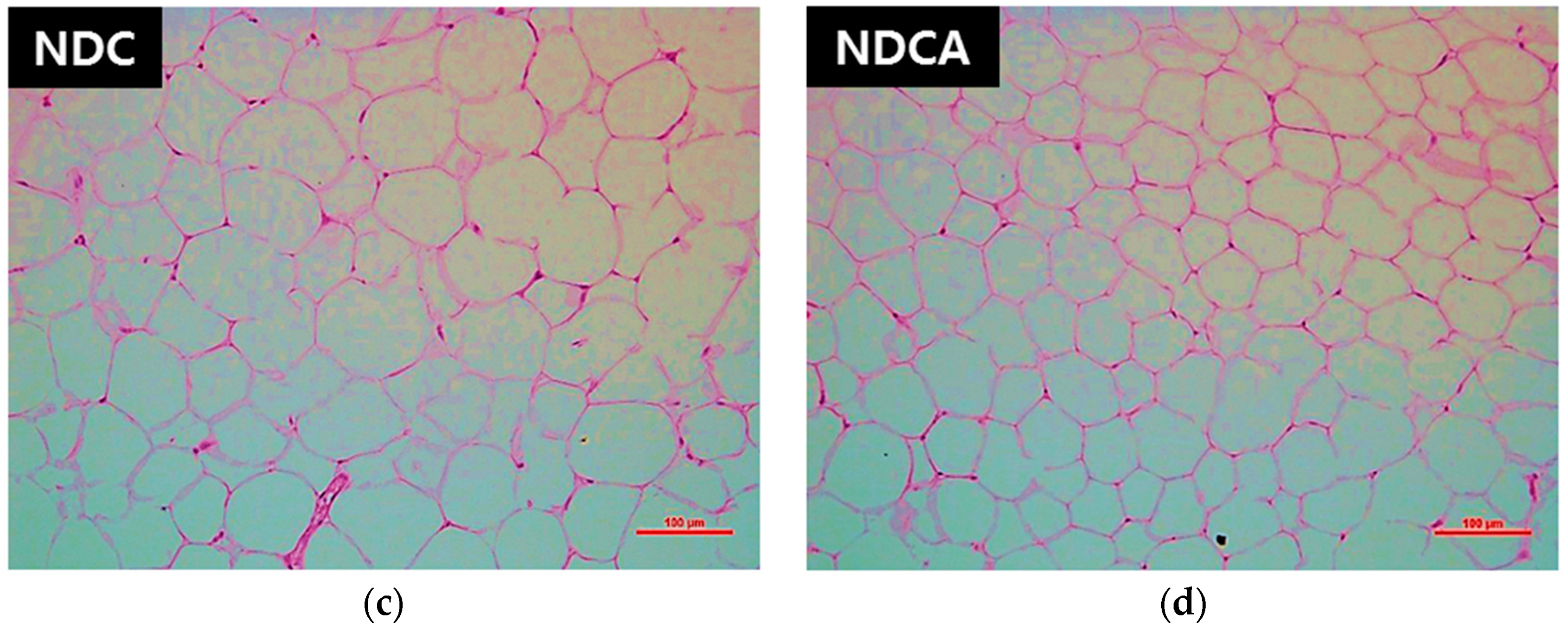
3.4. Hepatic Lipid Profiles, Lipid-Regulating Enzyme Activities, and Tissue Morphology
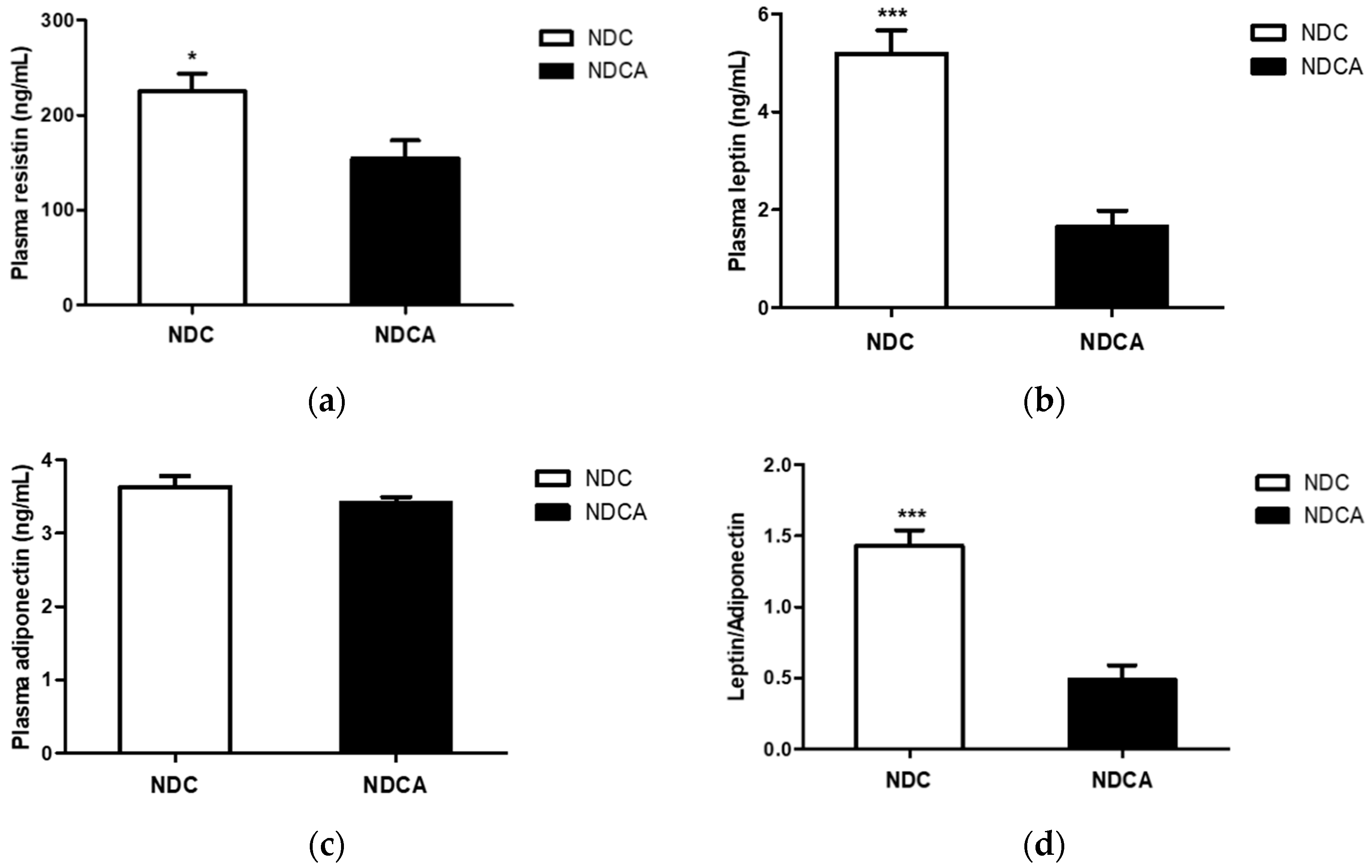
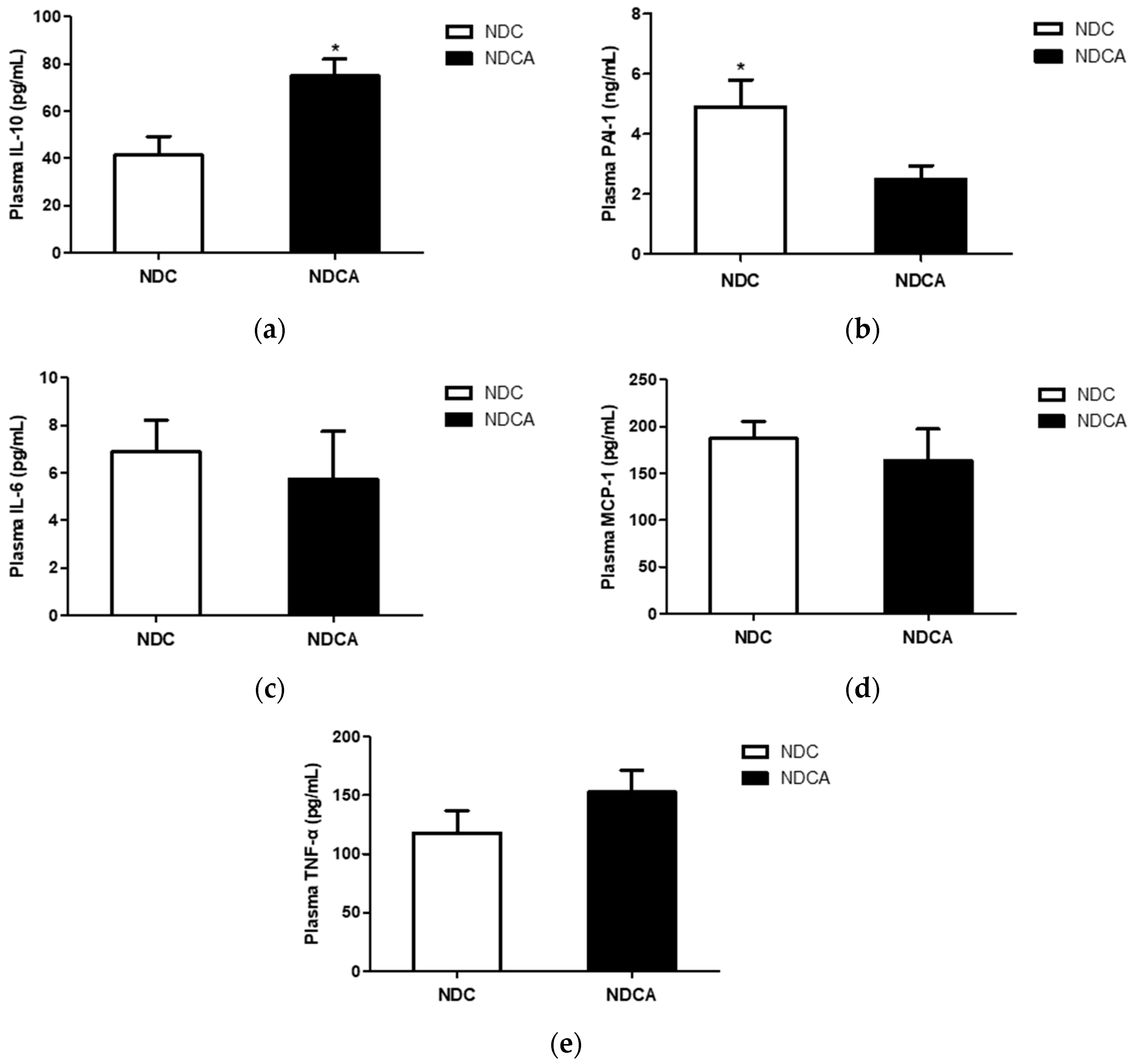
3.5. Plasma Adipokines and Cytokines
4. Discussion
4.1. D-Allulose Increased Fecal Lipid Excretion and Improved Plasma Lipid Profile
4.2. D-Allulose Altered Hepatic Lipid-Regulating Enzyme Activity and Tissue Fat Accumulation
4.3. D-Allulose Reduced Plasma Pro-inflammatory Adipokine Levels
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aguilera, A.A.; Diaz, G.H.; Barcelata, M.L.; Guerrero, O.A.; Ros, R.M. Effects of fish oil on hypertension, plasma lipids, and tumor necrosis factor-α in rats with sucrose-induced metabolic syndrome. J. Nutr. Biochem. 2004, 15, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Smith, G.D.; Ebrahim, S. Socioeconomic position and hormone replacement therapy use: Explaining the discrepancy in evidence from observational and randomized controlled trials. Am. J. Public Health 2004, 94, 2149–2154. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, Y.B.; Drago, S.; Chicco, A.; Fainstein-Day, P.; Gutman, R.; Gagliardino, J.J.; Gomez Dumm, C.L. Long-term administration of a sucrose-rich diet to normal rats: Relationship between metabolic and hormonal profiles and morphological changes in the endocrine pancreas. Metabolism 1996, 45, 1527–1532. [Google Scholar] [CrossRef]
- Ten, S.; Maclaren, N. Insulin resistance syndrome in children. J. Clin. Endocrinol. Metab. 2004, 89, 2526–2539. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.Y.; Oh, D.K.; Lee, K.W. Hypoglycemic health benefits of D-psicose. J. Agric. Food Chem. 2012, 60, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Mizuno, S.; Hama, S.; Oshima, W.; Kawamata, M.; Hossain, A.; Ishihara, Y.; Tokuda, M. Beneficial effects of supplementation of the rare sugar “D-allulose” against hepatic steatosis and severe obesity in Lepob/Lepob mice. J. Food Sci. 2015, 80, H1619–H1626. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Han, H.J.; Kim, A.H.; Choi, J.Y.; Cho, S.J.; Park, Y.B.; Jung, U.J.; Choi, M.S. D-Allulose supplementation normalized the body weight and fat-pad mass in diet-induced obese mice via the regulation of lipid metabolism under isocaloric fed condition. Mol. Nutr. Food Res. 2016, 60, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary fats and cardiovascular disease: A presidential advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Loganathan, R., Jr.; Selvaduray, K.R.; Nesaretnam, K.; Radhakrishnan, A.K. Health promoting effects of phytonutrients found in palm oil. Malays. J. Nutr. 2010, 16, 309–322. [Google Scholar]
- Folch, J.; Ascoli, I.; Lees, M.; Meath, J.A.; Le, B.N. Preparation of lipide extracts from brain tissue. J. Biol. Chem. 1951, 191, 833–841. [Google Scholar]
- Hulcher, F.H.; Oleson, W.H. Simplified spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl CoA reductase by measurement of coenzyme A. J. Lipid Res. 1973, 14, 625–631. [Google Scholar] [PubMed]
- Nepokroeff, C.M.; Lakshmanan, M.R.; Porter, J.W. Fatty acid synthase from rat liver. Methods Enzymol. 1975, 35, 37–44. [Google Scholar] [PubMed]
- Lazarow, P.B. Assay of peroxisomal β-oxidation of fatty acids. Methods Enzymol. 1981, 72, 315–319. [Google Scholar] [PubMed]
- Markwell, M.A.; McGroarty, E.J.; Bieber, L.L.; Tolbert, N.E. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J. Biol. Chem. 1973, 248, 3426–3432. [Google Scholar] [PubMed]
- Pitkanen, E.; Pitkanen, O.; Uotila, L. Enzymatic determination of unbound D-mannose in serum. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Grunberg-Manago, M.; Oritz, P.J.; Ochoa, S. Enzymatic synthesis of nucleic acidlike polynucleotides. Science 1955, 122, 907–910. [Google Scholar] [CrossRef]
- Walton, P.A.; Possmayer, F. Mg2+-dependent phosphatidate phosphohydrolase of rat lung: Development of an assay employing a defined chemical substrate which reflects the phosphohydrolase activity measured using membrane-bound substrate. Anal. Biochem. 1985, 151, 479–486. [Google Scholar] [CrossRef]
- Gillies, P.J.; Rathgeb, K.A.; Perri, M.A.; Robinson, C.S. Regulation of acyl-CoA: Cholesterol acyltransferase activity in normal and atherosclerotic rabbit aortas: Role of a cholesterol substrate pool. Exp. Mol. Pathol. 1986, 44, 329–339. [Google Scholar] [CrossRef]
- Shapiro, D.J.; Nordstrom, J.L.; Mitschelen, J.J.; Rodwell, V.W.; Schimke, R.T. Micro assay for 3-hydroxy-3-methylglutaryl-CoA reductase in rat liver and in L-cell fibroblasts. Biochim. Biophys. Acta 1974, 370, 369–377. [Google Scholar] [CrossRef]
- Shin, S.K.; Ha, T.Y.; McGregor, R.A.; Choi, M.S. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol. Nutr. Food Res. 2011, 55, 1829–1840. [Google Scholar] [CrossRef]
- Drewnowski, A.; Specter, S.E. Poverty and obesity: The role of energy density and energy costs. Am. J. Clin. Nutr. 2004, 79, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.S.; Niskanen, L.K.; Maliranta, H.M.; Savolainen, M.J.; Kesaniemi, Y.A.; Uusitupa, M.I. Lauric and palmitic acid-enriched diets have minimal impact on serum lipid and lipoprotein concentrations and glucose metabolism in healthy young women. J. Nutr. 1995, 125, 466–473. [Google Scholar] [PubMed]
- Zock, P.L.; de Vries, J.H.; Katan, M.B. Impact of myristic acid versus palmitic acid on serum lipid and lipoprotein levels in healthy women and men. Arterioscler. Thromb. 1994, 14, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yu, Y.; Szabo, A.; Wu, Y.; Wang, H.; Camer, D.; Huang, X.F. Palmitic acid induces central leptin resistance and impairs hepatic glucose and lipid metabolism in male mice. J. Nutr. Biochem. 2015, 26, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Diet, Nutrition and the Prevention of Chronic Diseases; WHO Technical Report Series, No. 916; World Health Organization: Geneva, Switzerland, 2003; pp. 1–149.
- Lu, H.; Hao, L.; Li, S.; Lin, S.; Lv, L.; Chen, Y.; Cui, H.; Zi, T.; Chu, X.; Na, L.; et al. Elevated circulating stearic acid leads to a major lipotoxic effect on mouse pancreatic beta cells in hyperlipidaemia via a miR-34a-5p-mediated PERK/p53-dependent pathway. Diabetologia 2016, 59, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Martin, T.L.; Surwit, R.S.; Robidoux, J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: Physiological and molecular characteristics. Physiol. Behav. 2004, 81, 243–248. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef]
- Poirier, H.; Degrace, P.; Niot, I.; Bernard, A.; Besnard, P. Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine. Comparison with fatty acid-binding proteins (FABP). Eur. J. Biochem. 1996, 238, 368–373. [Google Scholar] [CrossRef]
- Greenwalt, D.E.; Scheck, S.H.; Rhinehart-Jones, T. Heart CD36 expression is increased in murine models of diabetes and in mice fed a high fat diet. J. Clin. Investig. 1995, 96, 1382–1388. [Google Scholar] [CrossRef]
- Cai, L.; Eckhardt, E.R.; Shi, W.; Zhao, Z.; Nasser, M.; de Villiers, W.J.; van der Westhuyzen, D.R. Scavenger receptor class B type I reduces cholesterol absorption in cultured enterocyte CaCo-2 cells. J. Lipid Res. 2004, 45, 253–262. [Google Scholar] [CrossRef]
- Bietrix, F.; Yan, D.; Nauze, M.; Rolland, C.; Bertrand-Michel, J.; Comera, C.; Schaak, S.; Barbaras, R.; Groen, A.K.; Perret, B.; et al. Accelerated lipid absorption in mice overexpressing intestinal SR-BI. J. Biol. Chem. 2006, 281, 7214–7219. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.P.; Yao, X.; Crona, J.H.; Hoos, L.M.; Tetzloff, G.; Davis, H.R., Jr.; Graziano, M.P.; Altmann, S.W. Characterization of the putative native and recombinant rat sterol transporter Niemann-Pick C1 Like 1 (NPC1L1) protein. Biochim. Biophys. Acta 2005, 1722, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Heeren, J. The holy grail of metabolic disease: Brown adipose tissue. Curr. Opin. Lipidol. 2012, 23, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.F.; Sinha, M.K.; Kolaczynski, J.W.; Zhang, P.L.; Considine, R.V. Leptin: The tale of an obesity gene. Diabetes 1996, 45, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Guo, J.; Su, Z. Advances in understanding the interrelations between leptin resistance and obesity. Physiol. Behav. 2014, 130, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chu, W.S.; Hemphill, C.; Elbein, S.C. Human resistin gene: Molecular scanning and evaluation of association with insulin sensitivity and type 2 diabetes in Caucasians. J. Clin. Endocrinol. Metab. 2002, 87, 2520–2524. [Google Scholar] [CrossRef] [PubMed]
- Valsamakis, G.; McTernan, P.G.; Chetty, R.; Al Daghri, N.; Field, A.; Hanif, W.; Barnett, A.H.; Kumar, S. Modest weight loss and reduction in waist circumference after medical treatment are associated with favorable changes in serum adipocytokines. Metabolism 2004, 53, 430–434. [Google Scholar] [CrossRef]
- Diez, J.J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef]
- Renaldi, O.; Pramono, B.; Sinorita, H.; Purnomo, L.B.; Asdie, R.H.; Asdie, A.H. Hypoadiponectinemia: A risk factor for metabolic syndrome. Acta Med. Indones. 2009, 41, 20–24. [Google Scholar] [PubMed]
- Gustafson, B.; Hammarstedt, A.; Andersson, C.X.; Smith, U. Inflamed adipose tissue: A culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2276–2283. [Google Scholar] [CrossRef] [PubMed]

| (a) Composition of the Experimental Diets | ||
| Negative Control Group | Test Group | |
| Diet component 1) | HFD with non-dairy creamer* (NDC) | HFD with non-dairy creamer* +5% D-allulose (NDCA) |
| Casein | 20 | 20 |
| DL-methionine | 0.3 | 0.3 |
| Corn starch | 11.1 | 11.1 |
| Sucrose | 37 | 32 |
| Cellulose | 5 | 5 |
| Non-dairy creamer 2) | 14.6 | 14.6 |
| Lard | 5.4 | 5.4 |
| Mineral mix 3) | 4.2 | 4.2 |
| Vitamin mix 4) | 1.2 | 1.2 |
| Choline bitartrate | 0.2 | 0.2 |
| Cholesterol | 1 | 1 |
| tert-Butylhydroquinone | 0.004 | 0.004 |
| D-allulose | - | 5 |
| Total (%) | 100 | 100 |
| kcal/g | 4.047 | 3.847 |
| (b) Composition of Fatty Acid Components in Non-Dairy Creamer | ||
| Fatty Acid | Percentage of Fatty Acid in Non-Dairy Creamer (%) | |
| Lauric acid | 15.06 | |
| Myristic acid | 5.78 | |
| Stearic acid | 4.03 | |
| Palmitic acid | 3.40 | |
| Caprylic acid | 2.67 | |
| Capric acid | 1.95 | |
| Caproic acid | 0.22 | |
| Arachidic acid | 0.05 | |
| Oleic acid | 0.04 | |
| Heptadecanoic acid | 0.02 | |
| Pentadecanoic acid | 0.01 | |
| Lignoceric acid | 0.01 | |
| Behenic acid | 0.01 | |
| Tridecanoic acid | 0.01 | |
| Phospholipid | 0.015 | |
| Total fat | 33.275 | |
| NDC | NDCA | |
|---|---|---|
| Glucose (mM) | 16.31 ± 1.04 * | 11.93 ± 1.23 |
| Free fatty acids (mM) | 1.03 ± 0.19 | 0.98 ± 0.25 |
| Triglycerides (mM) | 1.21 ± 0.035 *** | 0.66 ± 0.089 |
| Total C (mM) | 5.09 ± 0.13 | 4.88 ± 0.28 |
| HDL C (mM) | 2.84 ± 0.60 | 2.69 ± 0.18 |
| Non-HDL C (mM) | 2.27 ± 0.14 | 2.19 ± 0.12 |
| HTR (%) | 55.44 ± 1.78 | 54.99 ± 1.44 |
| AI | 0.81 ± 0.058 | 0.82 ± 0.048 |
| Apo A-I (mg/dL) | 35.93 ± 1.36 | 38.42 ± 0.39 |
| Apo B (mg/dL) | 1.80 ± 0.20 | 1.43 ± 0.23 |
| Apo B/Apo A-I | 0.050 ± 0.0055 | 0.037 ± 0.0060 |
| GOT (IU/L) | 37.18 ± 3.16 | 35.78 ± 3.58 |
| GPT (IU/L) | 23.85 ± 2.83 * | 16.43 ± 1.48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, G.Y.; Kwon, E.-Y.; Kim, Y.J.; Han, Y.; Kim, S.-B.; Kim, Y.H.; Choi, M.-S. Supplementation of Non-Dairy Creamer-Enriched High-Fat Diet with D-Allulose Ameliorated Blood Glucose and Body Fat Accumulation in C57BL/6J Mice. Appl. Sci. 2019, 9, 2750. https://doi.org/10.3390/app9132750
Do GY, Kwon E-Y, Kim YJ, Han Y, Kim S-B, Kim YH, Choi M-S. Supplementation of Non-Dairy Creamer-Enriched High-Fat Diet with D-Allulose Ameliorated Blood Glucose and Body Fat Accumulation in C57BL/6J Mice. Applied Sciences. 2019; 9(13):2750. https://doi.org/10.3390/app9132750
Chicago/Turabian StyleDo, Ga Young, Eun-Young Kwon, Yun Jin Kim, Youngji Han, Seong-Bo Kim, Yang Hee Kim, and Myung-Sook Choi. 2019. "Supplementation of Non-Dairy Creamer-Enriched High-Fat Diet with D-Allulose Ameliorated Blood Glucose and Body Fat Accumulation in C57BL/6J Mice" Applied Sciences 9, no. 13: 2750. https://doi.org/10.3390/app9132750
APA StyleDo, G. Y., Kwon, E.-Y., Kim, Y. J., Han, Y., Kim, S.-B., Kim, Y. H., & Choi, M.-S. (2019). Supplementation of Non-Dairy Creamer-Enriched High-Fat Diet with D-Allulose Ameliorated Blood Glucose and Body Fat Accumulation in C57BL/6J Mice. Applied Sciences, 9(13), 2750. https://doi.org/10.3390/app9132750





