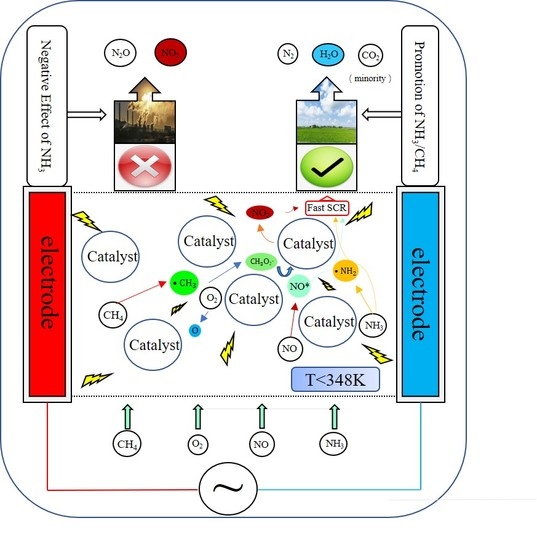
NO Removal by Plasma-Enhanced NH3-SCR Using Methane as an Assistant Reduction Agent at Low Temperature
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
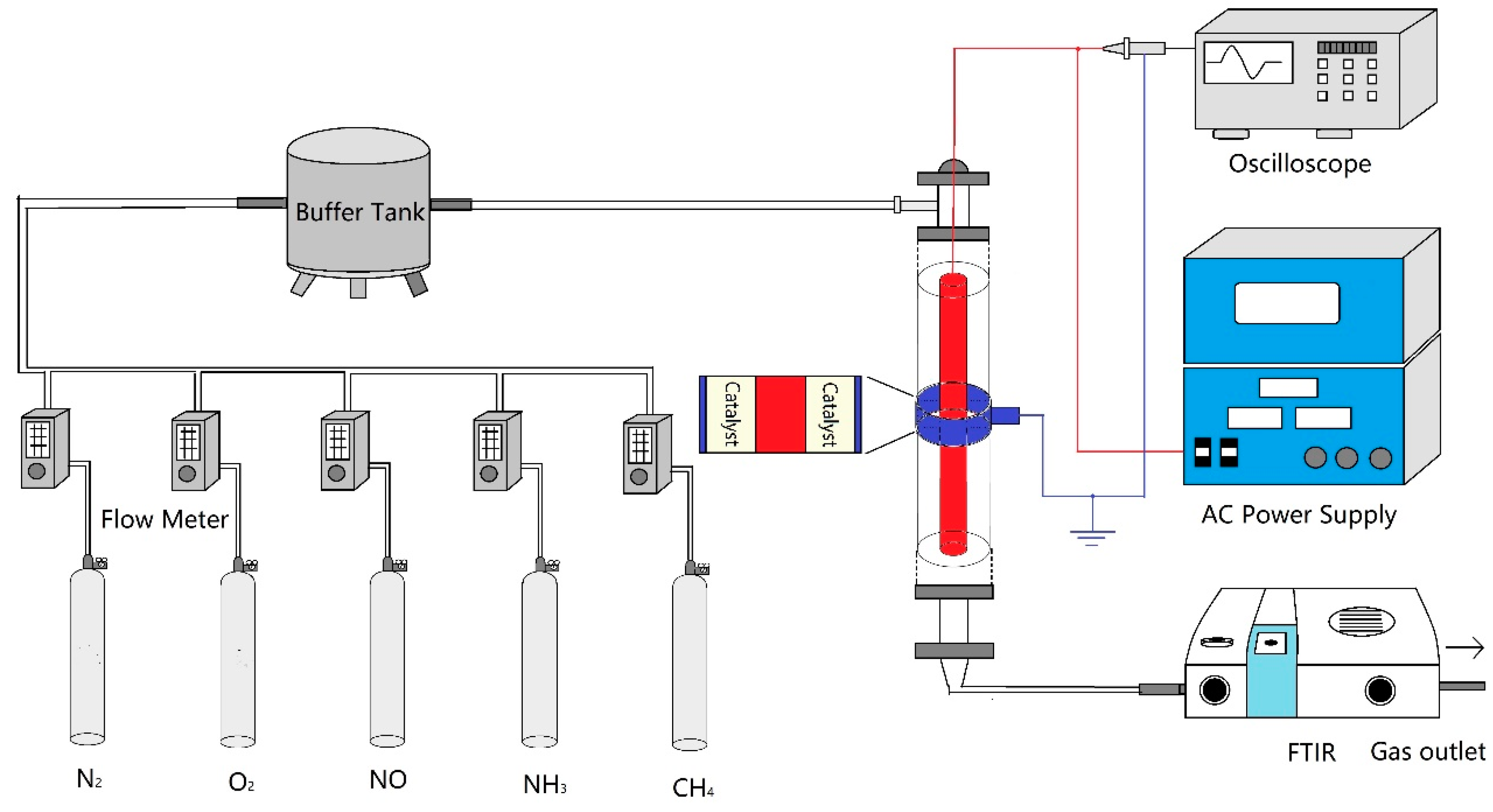
2.2. Experimental Setup and Procedure
2.3. Analytical Measurements
3. Results and Discussion
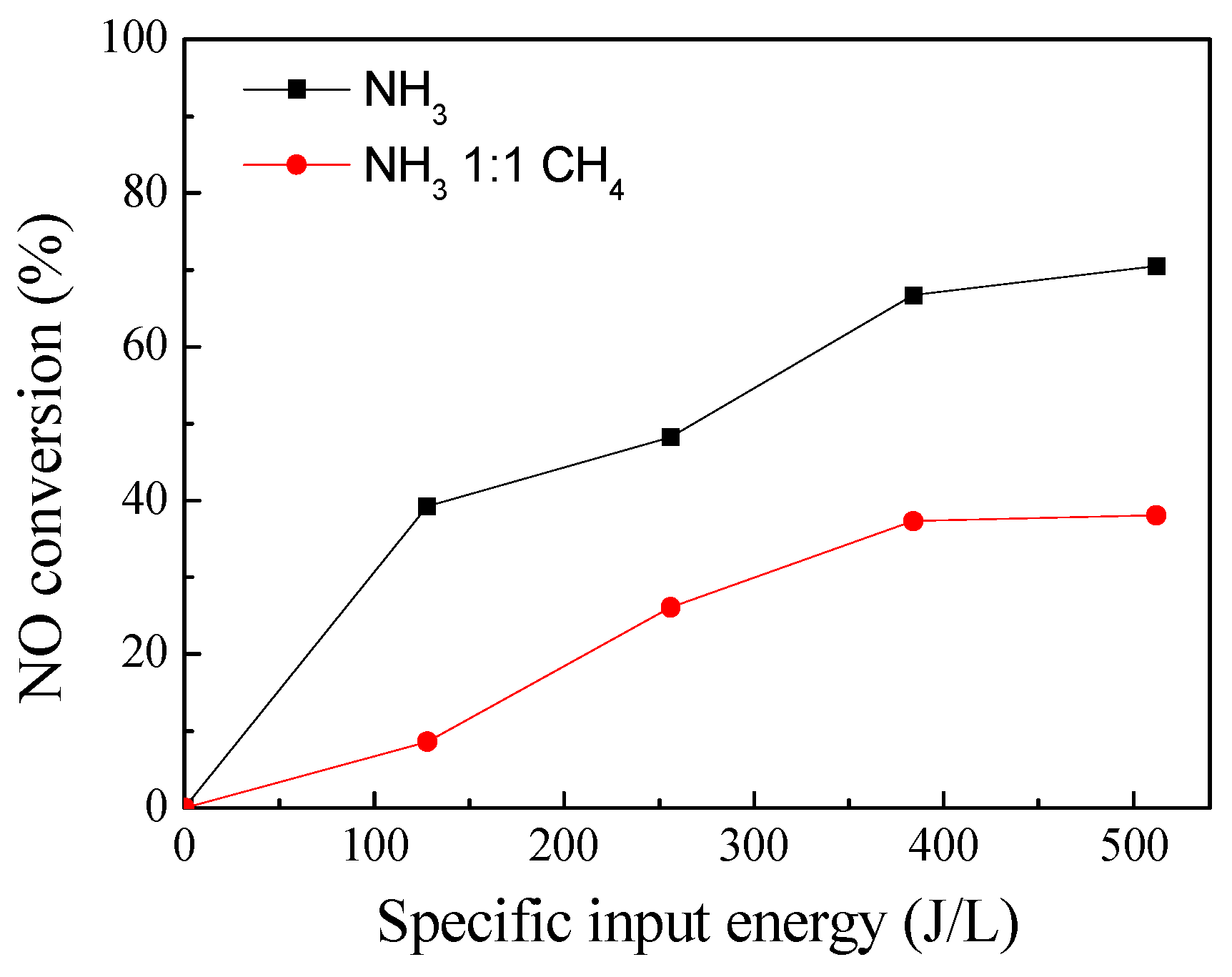
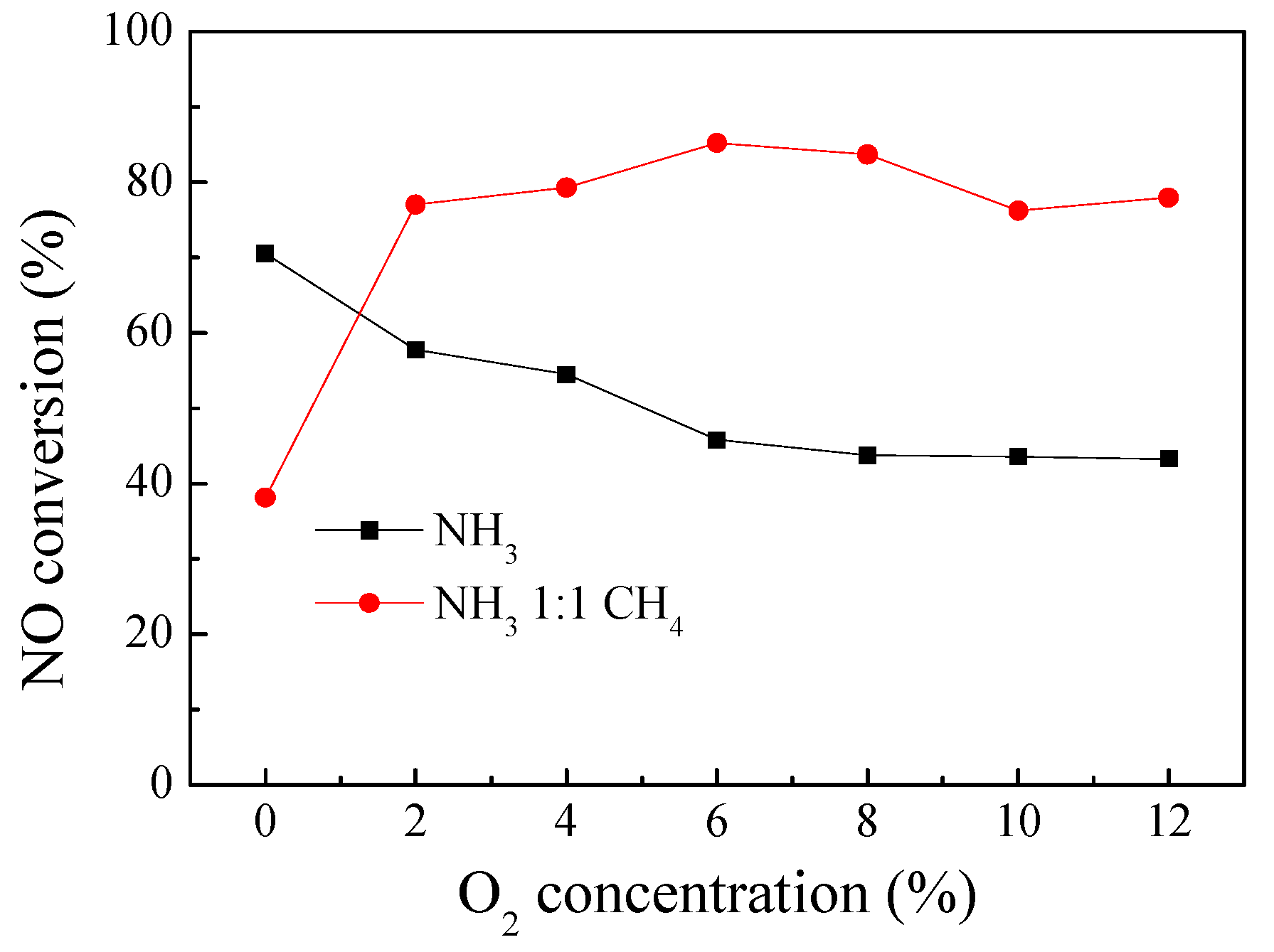
3.1. Performances of the DBD–NH3–CH4–SCR and DBD–NH3–SCR Hybrid System
3.2. Effect of CH4 and NH3 on the Product Selectivity of the DBD–SCR Hybrid System
3.3. Effect of Different Ratios of CH4 as An Assistant Agent on Final Products
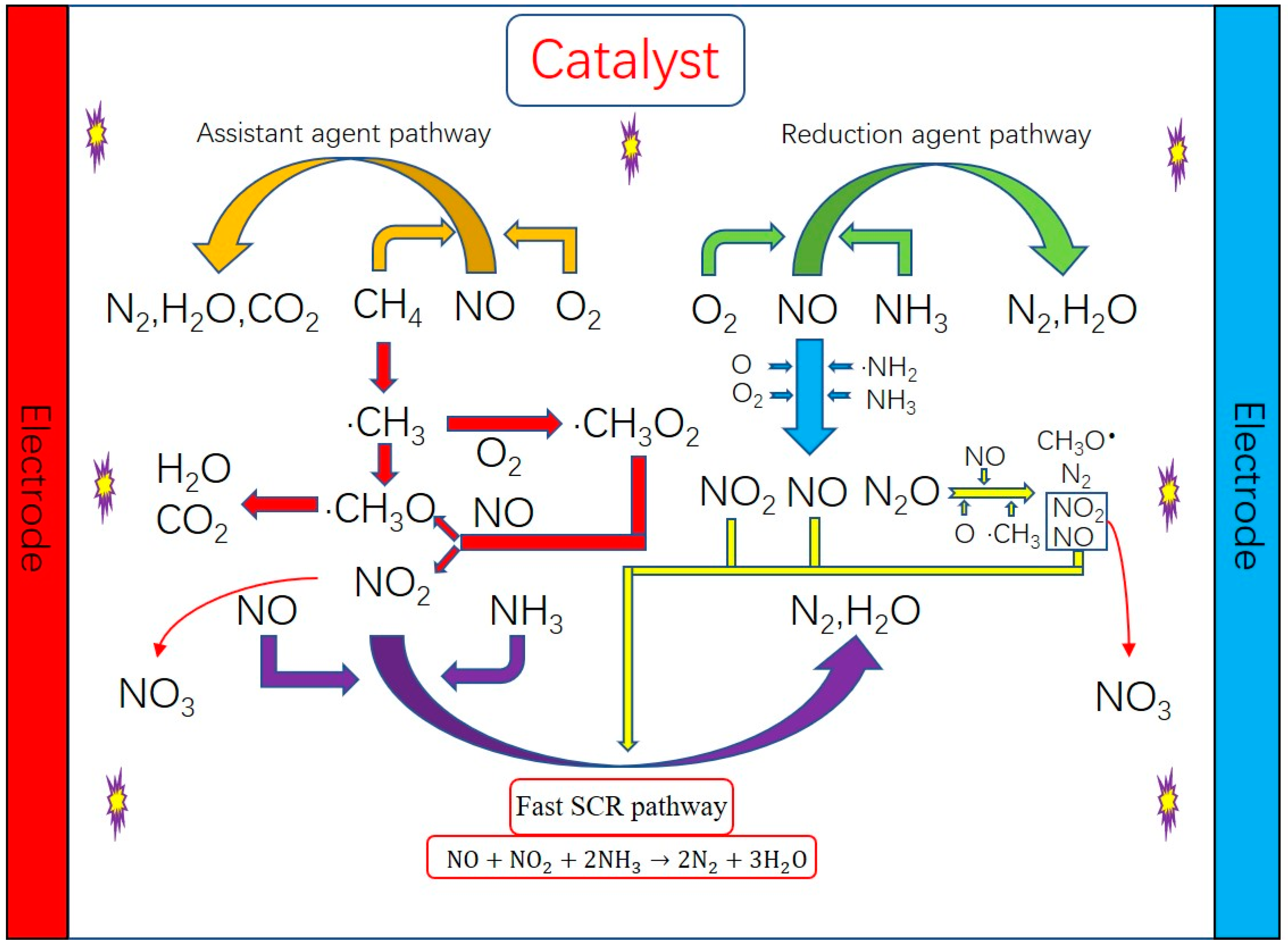
4. Reaction Mechanism
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Devahasdin, S.; Fan, C., Jr.; Li, K.; Chen, D.H. TiO2, photocatalytic oxidation of nitric oxide: Transient behavior and reaction kinetics. J. Photochem. Photobiol. A Chem. 2003, 156, 161–170. [Google Scholar] [CrossRef]
- Vestreng, V.; Ntziachristos, L.; Semb, A.; Reis, S.; Isaksen, I.S.A.; Tarrasón, L. Evolution of NOx emissions in Europe with focus on road transport control measures. Atmos. Chem. Phys. 2008, 8, 10697–10747. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A Gen. 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Zhao, L.; Li, C.; Wang, Y.; Gao, L.; Zhang, J.; Zeng, G. Simultaneous removal of elemental mercury and NO from simulated flue gas using a CeO2 modified V2O5–WO3/TiO2 catalyst. Catal. Sci. Technol. 2016, 6, 6076–6086. [Google Scholar] [CrossRef]
- Burch, R.; Breen, J.; Meunier, F.; Meunier, F. A review of the selective reduction of NOx with hydrocarbons under lean-burn conditions with non-zeolitic oxide and platinum group metal catalysts. Appl. Catal. B Environ. 2002, 39, 283–303. [Google Scholar] [CrossRef]
- He, H.; Yu, Y. Selective catalytic reduction of NOx over Ag/Al2O3 catalyst: From reaction mechanism to diesel engine test. Catal. Today 2005, 100, 37–47. [Google Scholar] [CrossRef]
- Granger, P.; Pârvulescu, V.I. Catalytic NOxAbatement Systems for Mobile Sources: From Three-Way to Lean Burn after-Treatment Technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Satsuma, A. Selective catalytic reduction of NO over supported silver catalysts—Practical and mechanistic aspects. Phys. Chem. Chem. Phys. 2006, 8, 2677–2695. [Google Scholar] [CrossRef]
- Liu, Z.; Woo, S.I. Recent Advances in Catalytic DeNOX Science and Technology. Catal. Rev. 2006, 48, 43–89. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Junaid, A.S. Knowledge and know-how in improving the sulfur tolerance of deNOx catalysts. Catal. Today 2010, 153, 95–102. [Google Scholar] [CrossRef]
- Burch, R. Knowledge and Know-How in Emission Control for Mobile Applications. Catal. Rev. 2004, 46, 271–334. [Google Scholar] [CrossRef]
- Li, J.; Ke, R.; Li, W.; Hao, J. A comparison study on non-thermal plasma-assisted catalytic reduction of NO by C3H6 at low temperatures between Ag/USY and Ag/Al2O3 catalysts. Catal. Today 2007, 126, 272–278. [Google Scholar] [CrossRef]
- Gunnarsson, F.; Pihl, J.A.; Toops, T.J.; Skoglundh, M.; Härelind, H. Lean NOx reduction over Ag/alumina catalysts via ethanol-SCR using ethanol/gasoline blends. Appl. Catal. B Environ. 2017, 202, 42–50. [Google Scholar] [CrossRef]
- Salazar, M.; Hoffmann, S.; Singer, V. Hybrid catalysts for the selective catalytic reduction (SCR) of NO by NH3. On the role of fast SCR in the reaction network. Appl. Catal. B Environ. 2016, 199, 433–438. [Google Scholar] [CrossRef]
- Salazar, M.; Hoffmann, S.; Tkachenko, O.P.; Becker, R.; Grünert, W. Hybrid catalysts for the selective catalytic reduction of NO by NH3: The influence of component separation on the performance of hybrid systems. Appl. Catal. B Environ. 2015, 182, 213–219. [Google Scholar] [CrossRef]
- Salazar, M.; Becker, R.; Grünert, W. Hybrid catalysts—An innovative route to improve catalyst performance in the selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2015, 165, 316–327. [Google Scholar] [CrossRef]
- Liese, T.; Löffler, E.; Grunert, W. Selective Catalytic Reduction of NO by Methane over CeO2–Zeolite Catalysts—Active Sites and Reaction Steps. J. Catal. 2001, 197, 123–130. [Google Scholar] [CrossRef]
- Salazar, M.; Hoffmann, S.; Tillmann, L.; Singer, V.; Becker, R.; Grünert, W. Hybrid catalysts for the selective catalytic reduction (SCR) of NO by NH3: Precipitates and physical mixtures. Appl. Catal. B Environ. 2017, 218, 793–802. [Google Scholar] [CrossRef]
- Lietti, L.; Forzatti, P.; Bregani, F. Steady-State and Transient Reactivity Study of TiO2-Supported V2O5−WO3De-NOxCatalysts: Relevance of the Vanadium−Tungsten Interaction on the Catalytic Activity. Ind. Eng. Chem. Res. 1996, 35, 3884–3892. [Google Scholar] [CrossRef]
- Madia, G.; Elsener, M.; Koebel, M.; Raimondi, F.; Wokaun, A. Thermal stability of vanadia-tungsta-titania catalysts in the SCR process. Appl. Catal. B Environ. 2002, 39, 181–190. [Google Scholar] [CrossRef]
- Weisweiler, W. Removal of NOx from automotive exhaust under lean conditions. Symp. Diesel Mot. Technol. 2000, 72, 441–449. [Google Scholar]
- Alemany, L.J.; Ferlazzo, N.; Forzatti, P.; Lietti, L.; Giamello, E.; Bregani, F. Physico-chemical characterisation and catalytic behavior of V2O5-WO3-TiO2 catalysts. J. Account. Audit. Financ. 1995, 31, 593–614. [Google Scholar]
- Chen, J.; Yang, R. Role of WO3 in mixed V2O5-WO3/TiO2catalysts for selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. A Gen. 1992, 80, 135–148. [Google Scholar] [CrossRef]
- Ramis, G.; Busca, G.; Cristiani, C.; Lietti, L.; Forzatti, P.; Bregani, F. Characterization of tungsta-titania catalysts. Langmuir 1992, 8, 1744–1749. [Google Scholar] [CrossRef]
- Kompio, P.G.; Brückner, A.; Hipler, F.; Manoylova, O.; Auer, G.; Mestl, G.; Grünert, W. V2O5-WO3/TiO2, catalysts under thermal stress: Responses of structure and catalytic behavior in the selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2017, 217, 365–377. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. Poisoning effect of alkali metals doping over nano V2O5–WO3/TiO2 catalysts on selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2011, 170, 531–537. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, Q. F-Doped V2O5-WO3/TiO2 as a Catalyst for NO Reduction with NH3 at Low-Temperature. IJESD Org. 2012, 3, 441–445. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, X.; Xie, A.; Luo, S.; Yao, C.; Li, X.; Zuo, S. V2O5-decorated Mn-Fe/attapulgite catalyst with high SO2 tolerance for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 326, 1074–1085. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, D.; Liu, X.; Shi, L.; Maitarad, P.; Li, H.; Zhang, J.; Cao, W. Enhanced catalytic performance of V2O5-WO3/Fe2O3/TiO2 microspheres for selective catalytic reduction of NO by NH3. Catal Sci. Technol. 2013, 3, 191–199. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Zhong, Q. Promotional effect of F-doped V2O5–WO3/TiO2 catalyst for NH3-SCR of NO at low-temperature. Appl. Catal. A Gen. 2012, 435, 156–162. [Google Scholar] [CrossRef]
- Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A Review of Low Temperature NH3-SCR for Removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef]
- Damma, D.; Boningari, T.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. Direct Decomposition of NOx over TiO2 Supported Transition Metal Oxides at Low Temperatures. Ind. Eng. Chem. Res. 2018, 57, 16615–16621. [Google Scholar] [CrossRef]
- Martín, N.; Paris, C.; Vennestrøm, P.N.; Thøgersen, J.R.; Moliner, M.; Corma, A. Cage-based small-pore catalysts for NH3-SCR prepared by combining bulky organic structure directing agents with modified zeolites as reagents. Appl. Catal. B Environ. 2017, 217, 125–136. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, R.; Zhao, R.; Shi, C.; Gies, H.; Xiao, F.S.; De Vos, D.; Yokoi, T.; Bao, X.; Kolb, U.; et al. Cu-Exchanged Al-rich SSZ-13 Zeolite from Organotemplate-Free Synthesis as NH3-SCR Catalyst: Effects of Na+, Ions on the Activity and Hydrothermal Stability. Appl. Catal. B Environ. 2017, 217, 421–428. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Bai, M.; Leng, B.; Mao, S. Flue Gas Desulfurization by Dielectric Barrier Discharge. Plasma Chem. Plasma Process. 2016, 36, 511–521. [Google Scholar] [CrossRef]
- Liu, H.; Wang, T.; Zhang, X.; Guo, Y.; Sun, B. Influence of the TiO2/multi-walled carbon nanotubes (MWCNTs) mass ratio on NO removal over the Mn/TiO2, (x)-MWCNTs (1 − x) catalyst assisted by plasma. React. Kinet. Mech. Catal. 2017, 121, 735–749. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.; Zhang, X.; Guo, Y.; Zhang, Y.; Wang, Y.; Sun, B. A plasma-assisted catalytic system for NO removal over CuCe/ZSM-5 catalysts at ambient temperature. Fuel Process. Technol. 2017, 158, 199–205. [Google Scholar] [CrossRef]
- Chen, J.X.; Pan, K.L.; Yu, S.J. Combined fast selective reduction using Mn-based catalysts and nonthermal plasma for NOx removal. Environ. Sci. Pollut. Res. 2017, 24, 1–13. [Google Scholar] [CrossRef]
- Wang, J.; Yi, H.; Tang, X.; Zhao, S.; Gao, F.; Yang, Z. Oxygen plasma-catalytic conversion of NO over MnOx: Formation and reactivity of adsorbed oxygen. Catal. Commun. 2017, 100, 227–231. [Google Scholar] [CrossRef]
- Guo, Y.; Liao, X.; Fu, M.; Huang, H.; Ye, D. Toluene decomposition performance and NOx by-product formation during a DBD-catalyst process. J. Environ. Sci. 2015, 28, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, Y.; Wang, J.; Zhang, L.; Li, X. Research on the Effect of C3H6 on NO Conversion Rate in a NTP Reactor. In Proceedings of the IEEE International Conference on Optoelectronics and Image Processing, Haiko, China, 11–12 November 2010; pp. 297–300. [Google Scholar]
- Anaghizi, S.J.; Talebizadeh, P.; Rahimzadeh, H.; Ghomi, H. The Configuration Effects of Electrode on the Performance of Dielectric Barrier Discharge Reactor for NOx, Removal. IEEE Trans. Plasma Sci. 2015, 43, 1944–1953. [Google Scholar] [CrossRef]
- Cho, B.K.; Lee, J.H.; Crellin, C.C.; Olson, K.L.; Hilden, D.L.; Kim, M.K.; Kim, P.S.; Heo, I.; Oh, S.H.; Nam, I.S.; et al. Selective catalytic reduction of NOx, by diesel fuel: Plasma-assisted HC/SCR system. Catal. Today 2012, 191, 20–24. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.O.; Kim, K.T. Characteristics of plasma-assisted hydrocarbon SCR system. Int. J. Hydrogen Energy 2011, 36, 11718–11726. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, A.-M.; Yang, X.; Niu, J.; Xu, Y. Formation of NOx from N2 and O2 in catalyst-pellet filled dielectric barrier discharges at atmospheric pressure. Chem. Commun. 2003, 12, 1418. [Google Scholar] [CrossRef]
- Kim, H.-H.; Ogata, A.; Futamura, S. Effect of different catalysts on the decomposition of VOCs using flow-type plasma-driven catalysis. IEEE Trans. Plasma Sci. 2006, 34, 984–995. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Hou, H.; Zhang, R. NO reduction using low-temperature SCR assisted by a DBD method. Plasma Sci. Technol. 2018, 20, 014002. [Google Scholar] [CrossRef]
- Miessner, H.; Francke, K.P.; Rudolph, R.; Hammer, T. NOX, removal in excess oxygen by plasma-enhanced selective catalytic reduction. Catal. Today 2002, 75, 325–330. [Google Scholar] [CrossRef]
- Guan, B.; Lin, H.; Cheng, Q.; Huang, Z. Removal of NOx with selective catalytic reduction based on nonthermal plasma peroxidation. Ind. Eng. Chem. Res. 2011, 50, 5401–5413. [Google Scholar] [CrossRef]
- Fan, H.Y.; Shi, C.; Li, X.S.; Yang, X.F.; Xu, Y.; Zhu, A.M. Low-temperature NOx selective reduction by hydrocarbons on H-Mordenite catalysts in dielectric barrier discharge plasma. Plasma Chem. Plasma Process. 2009, 29, 43–53. [Google Scholar] [CrossRef]
- Pan, H.; Guo, Y.; Jian, Y.; He, C. Synergistic Effect of Non-thermal Plasma on NOx Reduction by CH4 over an In/H-BEA Catalyst at Low Temperatures. Energy Fuels 2015, 29, 5282–5289. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, F.; Liu, Y.; Zhang, R.; Hou, H. Effects of Electrode Structure and Electron Energy on Abatement of NO in Dielectric Barrier Discharge Reactor. Appl. Sci. 2018, 8, 618. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, J.Y.; Zhong, K.; Wang, L.M.; Guan, Z.C. Synergy study of plasma-facilitated C2H4 selective catalytic reduction of NOx over Ag/γ-Al2O3 catalyst. IEEE Trans. Plasma Sci. 2007, 35, 663–669. [Google Scholar] [CrossRef]
- Wang, X.Q.; Li, Y.; Chen, W.; Lv, G.H.; Huang, J.; Zhu, G.X.; Wang, X.Q.; Zhang, X.H.; Wang, D.C.; Feng, K.C.; et al. Characteristics of NOx removal combing dielectric barrier discharge plasma with selective catalytic reduction by C2H5OH. J. Appl. Phys. 2009, 106, 013309. [Google Scholar] [CrossRef]
- Marberger, A.; Ferri, D.; Elsener, M.; Sagar, A.; Artner, C.; Schermanz, K.; Kröcher, O. Relationship between structures and activities of supported metal vanadates for the selective catalytic reduction of NO by NH3. Appl. Catal. B 2017, 218, 731–742. [Google Scholar] [CrossRef]
- Cobos, C.J.; Hippler, H.; Troe, J. The Reaction of NH2 + O2 at High Temperatures. J. Phys. Chem. 1995, 99, 342–349. [Google Scholar]
- Cohen, N.; Westberg, K.R. Chemical Kinetic Data Sheets for High-Temperature Reactions. Part II. J. Phys. Chem. Ref. Data 1991, 20, 1211–1311. [Google Scholar] [CrossRef]
- Nishida, Y.; Chen, T.-C.; Cheng, C.-Z. Effects of Multiple Pulses on Decomposition of Hydrocarbons for Hydrogen Production. IEEE Trans. Plasma Sci. 2018, 46, 962–966. [Google Scholar] [CrossRef]
- Lin, H.; Guan, B.; Cheng, Q.; Huang, Z. An Investigation on the Principal Paths to Plasma Oxidation of Propylene and NO. Energy Fuels 2010, 24, 5418–5425. [Google Scholar] [CrossRef]
- Jabłońska, M.; Delahay, G.; Kruczała, K.; Błachowski, A.; Tarach, K.A.; Brylewska, K.M.; Petitto, C.; Góra-Marek, K. Standard and Fast Selective Catalytic Reduction of NO with NH3 on Zeolites Fe-BEA. J. Phys. Chem. C 2016, 120, 16831–16842. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Raimondi, F.; Wokaun, A. Enhanced Reoxidation of Vanadia by NO2 in the Fast SCR Reaction. J. Catal. 2002, 209, 159–165. [Google Scholar] [CrossRef]
- Cao, L.; Chen, L.; Wu, X.; Ran, R.; Xu, T.; Chen, Z.; Weng, D. TRA and DRIFTS studies of the fast SCR reaction over CeO2/TiO2 catalyst at low temperatures. Appl. Catal. A Gen. 2018, 557, 46–54. [Google Scholar] [CrossRef]
- Tomeczek, J.; Gradoń, B. The role of N2O and NNH in the formation of NO via HCN in hydrocarbon flames. Combust. Flame 2003, 133, 311–322. [Google Scholar] [CrossRef]
- Nancy, E.; Meagher, W.R. Anderson. Kinetics of the O(3P) + N2O Reaction. 2. Interpretation and Recommended Rate Coefficients. J. Phys. Chem. A 2000, 104, 6013–6031. [Google Scholar]
- Mebel, A.M.; Lin, M.C.; Morokuma, K.; Melius, C. Theoretical study of reactions of N2O with NO and OH radicals. Int. J. Chem. Kinet. 1996, 28, 693–703. [Google Scholar] [CrossRef]
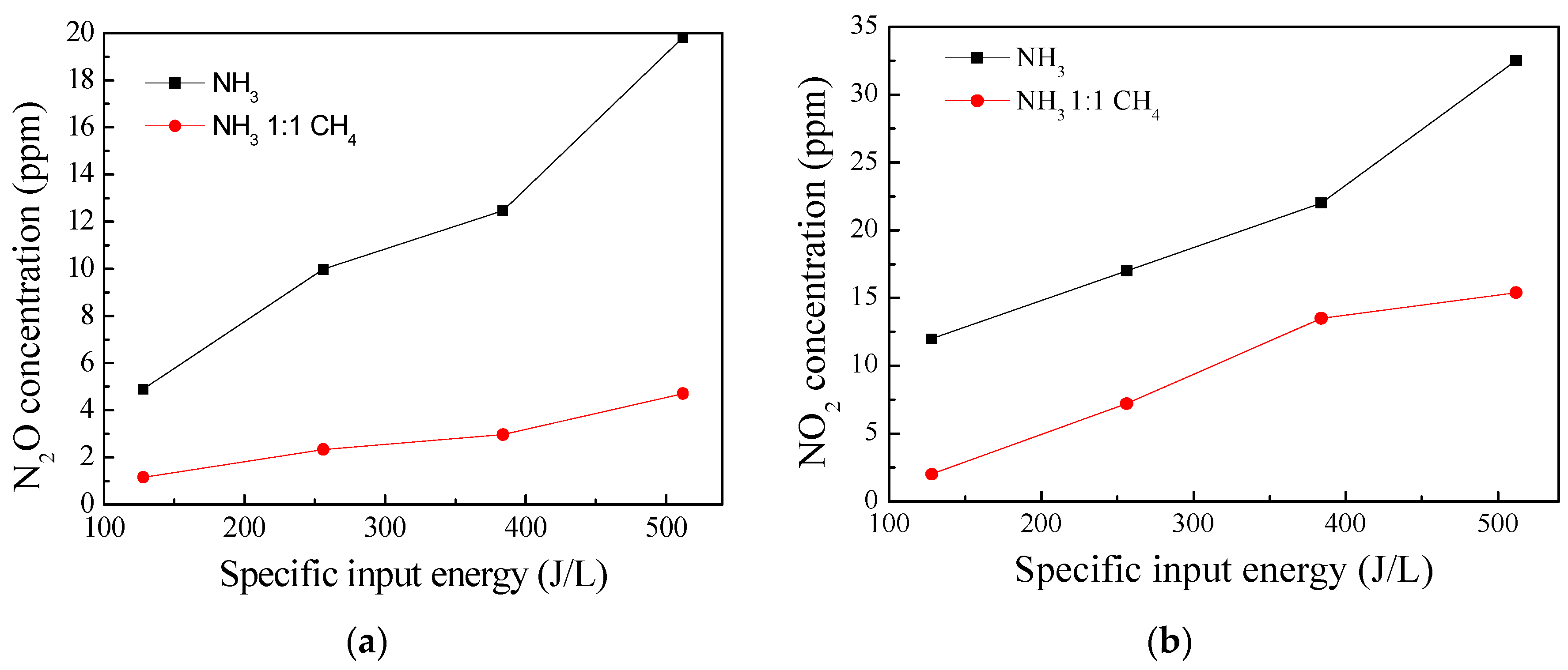

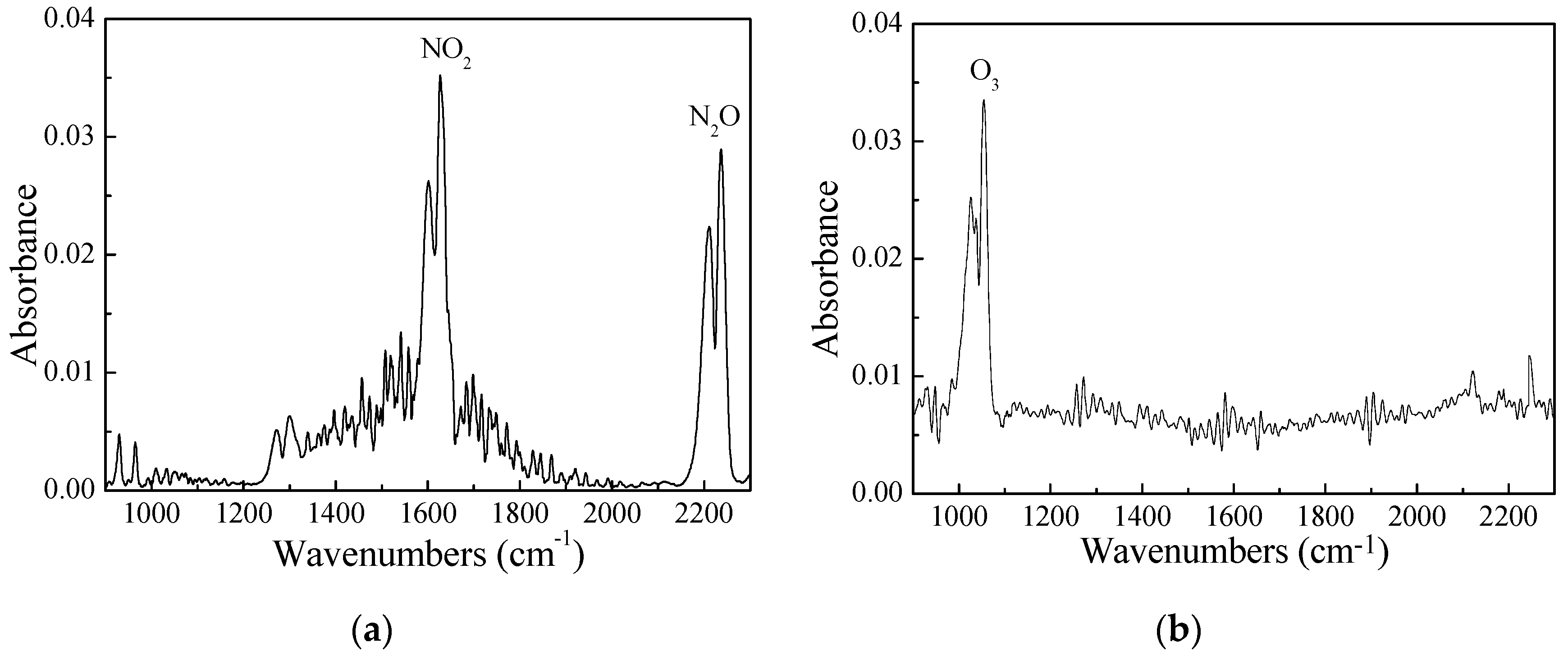
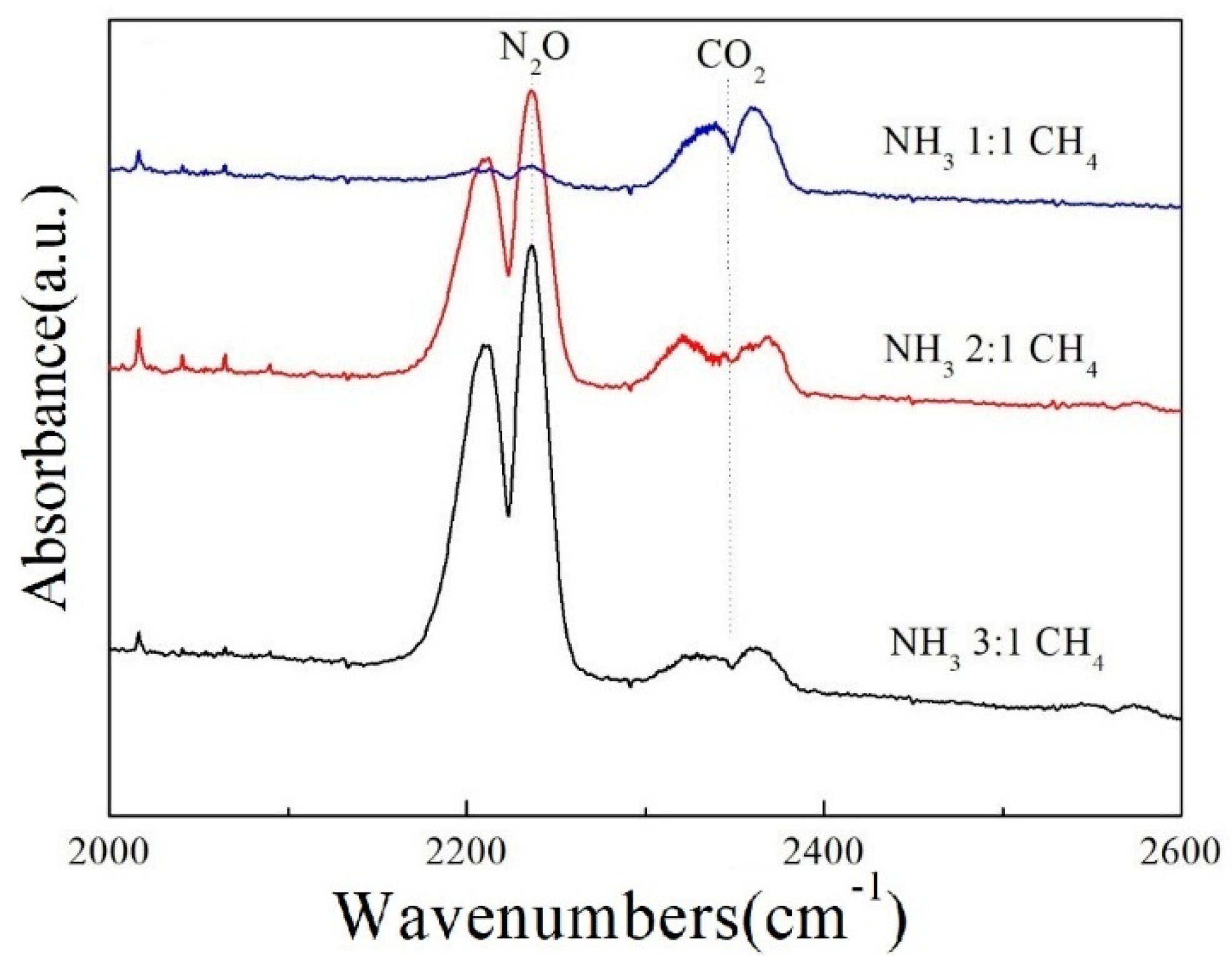
| Feed Gas Concentration of Inlet (ppm) | Product Concentration of Outlet (ppm) | N2 Selectivity (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NO | CH4 | NH3 | NO | NO2 | N2O | NO3 | NH3 | ||
| DBD–NH3–SCR | 400 | 0 | 90 | 220 | 32.5 | 19.8 | 37.2 | 7.7 | 31.2 |
| DBD–NH3–CH4–SCR | 400 | 45 | 45 | 80 | 15.4 | 4.71 | 25.5 | 3.8 | 69.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Liu, Y.; Wei, H.; Zhang, R.; Luo, G.; Hou, H.; Chen, S.; Zhang, R. NO Removal by Plasma-Enhanced NH3-SCR Using Methane as an Assistant Reduction Agent at Low Temperature. Appl. Sci. 2019, 9, 2751. https://doi.org/10.3390/app9132751
Zhao W, Liu Y, Wei H, Zhang R, Luo G, Hou H, Chen S, Zhang R. NO Removal by Plasma-Enhanced NH3-SCR Using Methane as an Assistant Reduction Agent at Low Temperature. Applied Sciences. 2019; 9(13):2751. https://doi.org/10.3390/app9132751
Chicago/Turabian StyleZhao, Weixuan, Yanghaichao Liu, Heng Wei, Renxi Zhang, Gang Luo, Huiqi Hou, Shanping Chen, and Ruina Zhang. 2019. "NO Removal by Plasma-Enhanced NH3-SCR Using Methane as an Assistant Reduction Agent at Low Temperature" Applied Sciences 9, no. 13: 2751. https://doi.org/10.3390/app9132751
APA StyleZhao, W., Liu, Y., Wei, H., Zhang, R., Luo, G., Hou, H., Chen, S., & Zhang, R. (2019). NO Removal by Plasma-Enhanced NH3-SCR Using Methane as an Assistant Reduction Agent at Low Temperature. Applied Sciences, 9(13), 2751. https://doi.org/10.3390/app9132751