Channelrhodopsin-2 Function is Modulated by Residual Hydrophobic Mismatch with the Surrounding Lipid Environment
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Membrane Deformation
2.2. Molecular Biology, mRNA Synthesis and Oocyte Injection
2.3. Electrophysiology
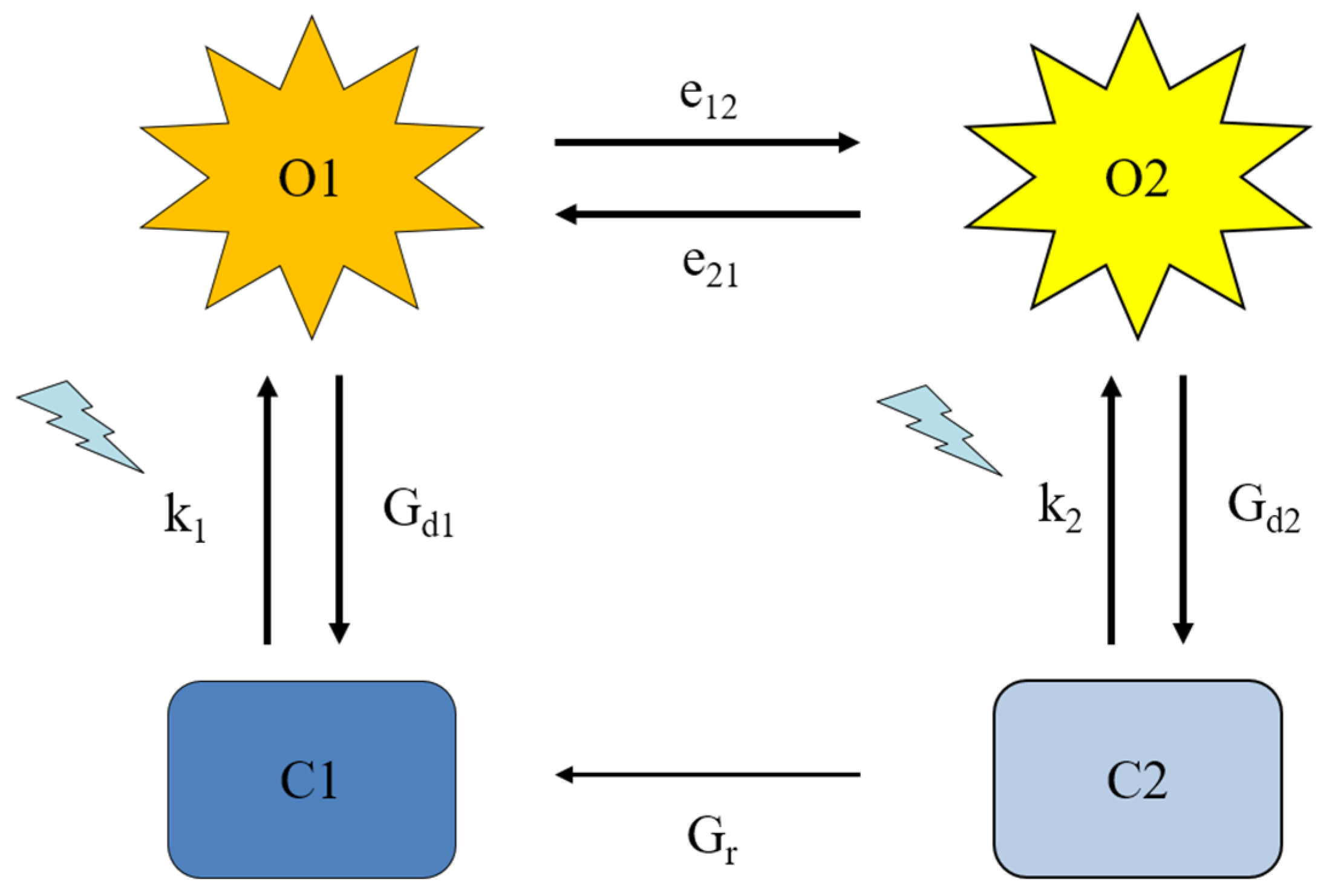
2.4. Four-state modeling of photocycle kinetics
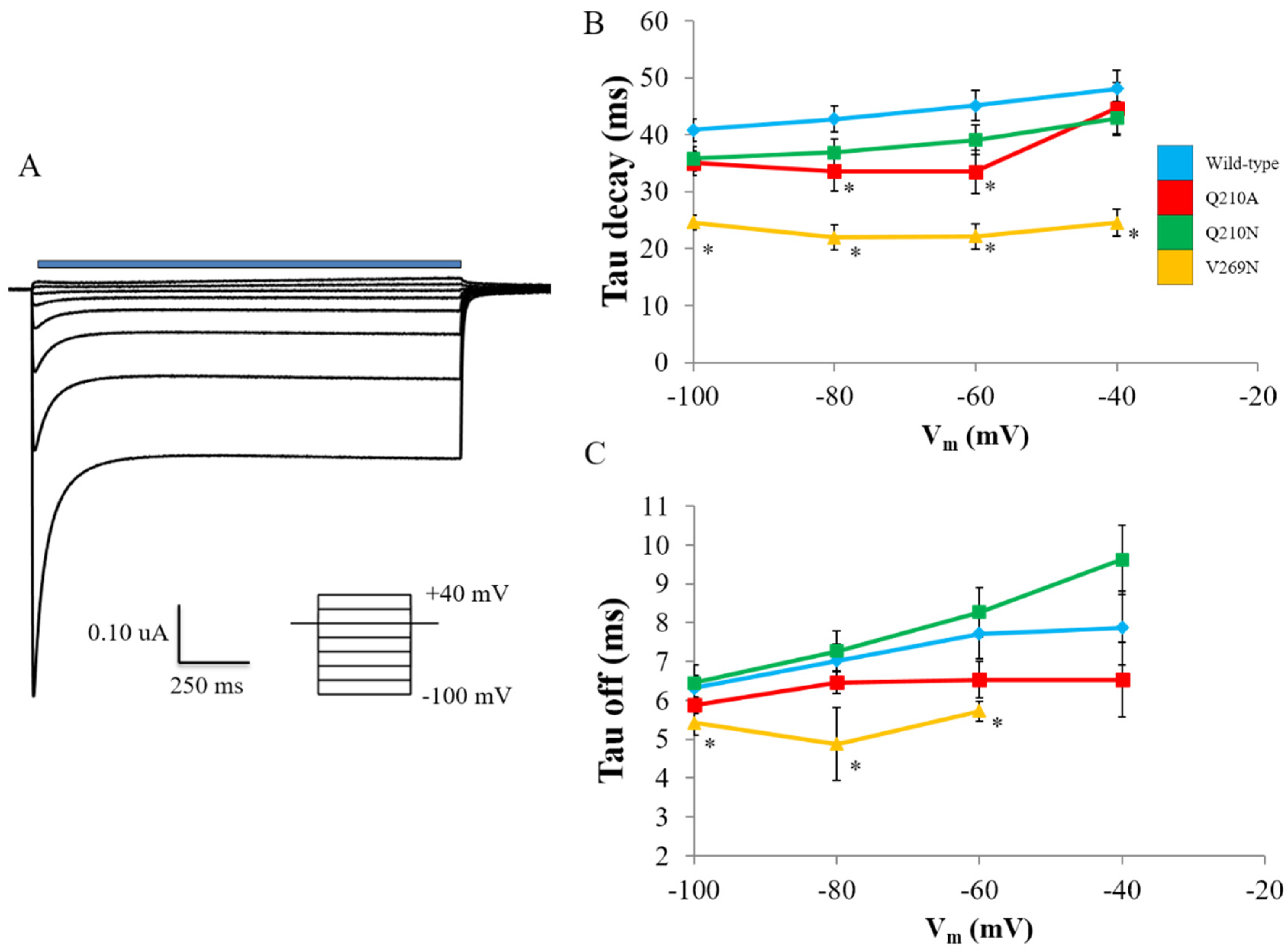
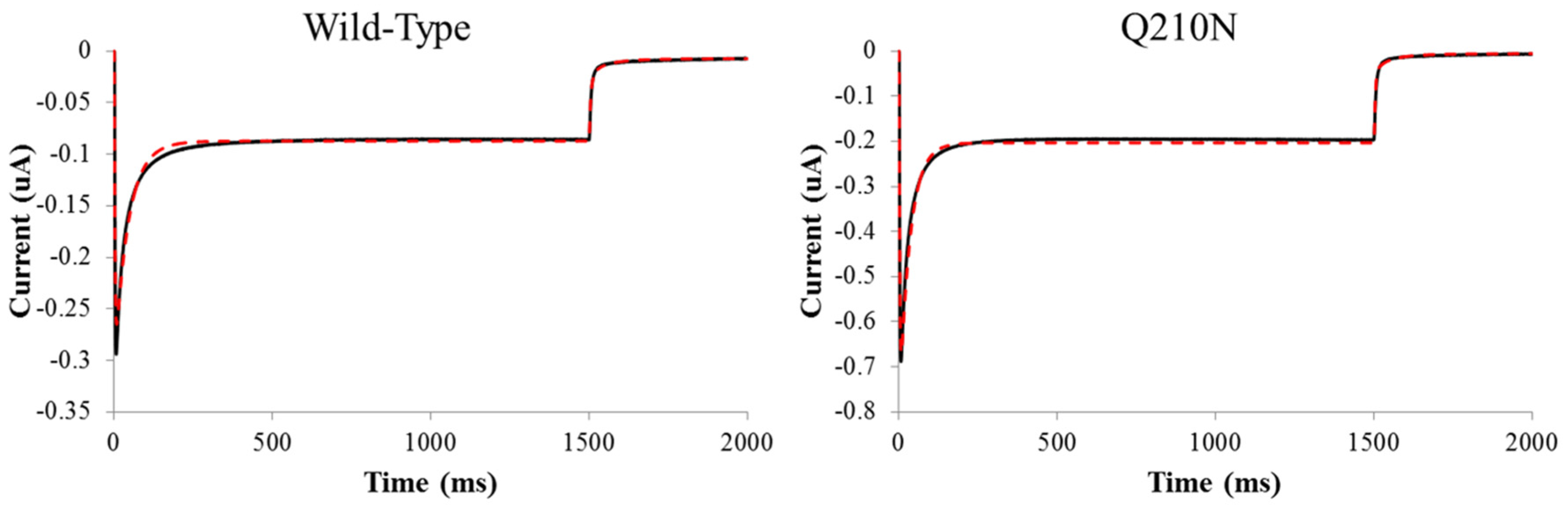
3. Results
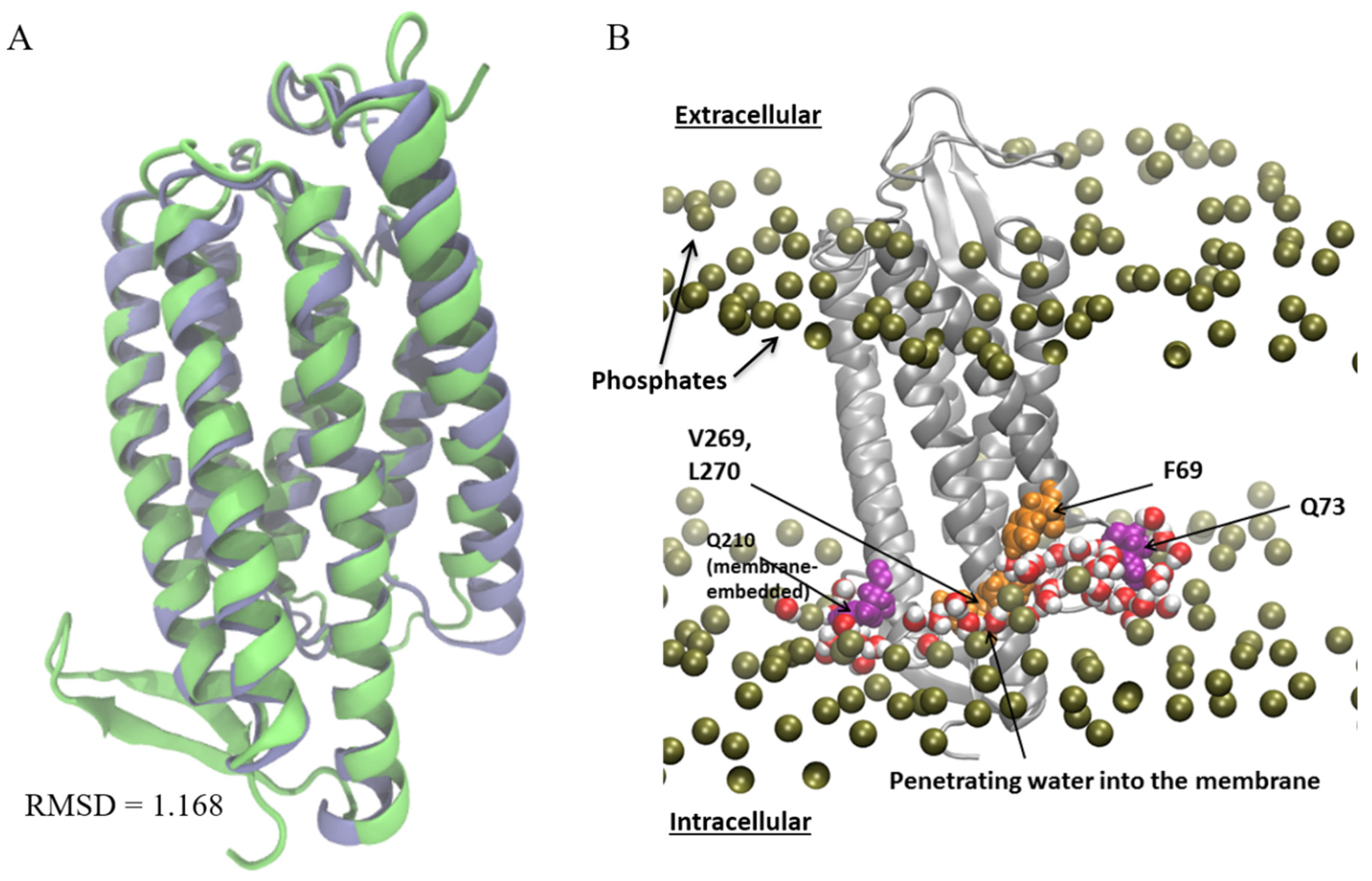
3.1. Identification and Quantification of Residual Hydrophobic Mismatch
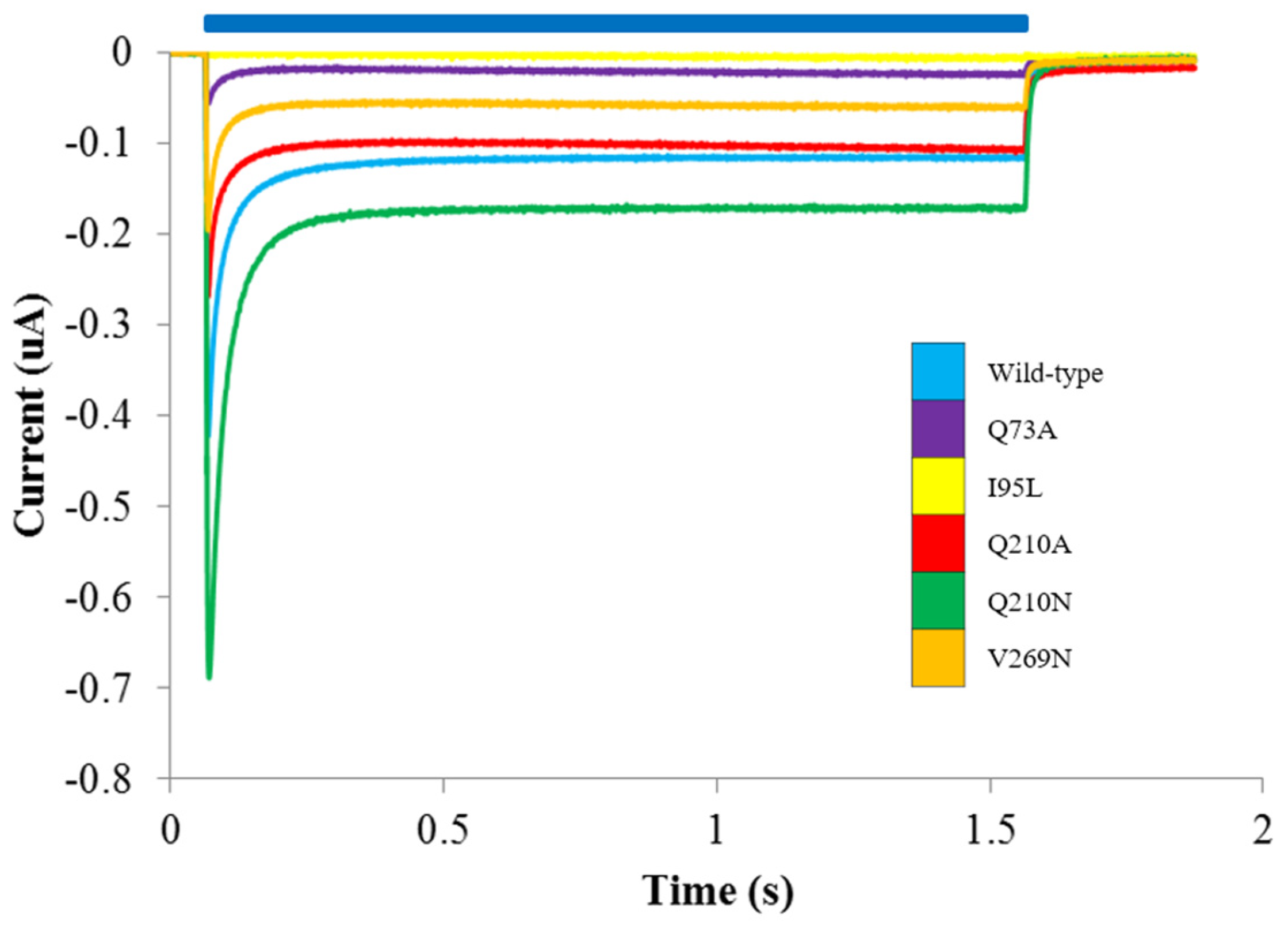
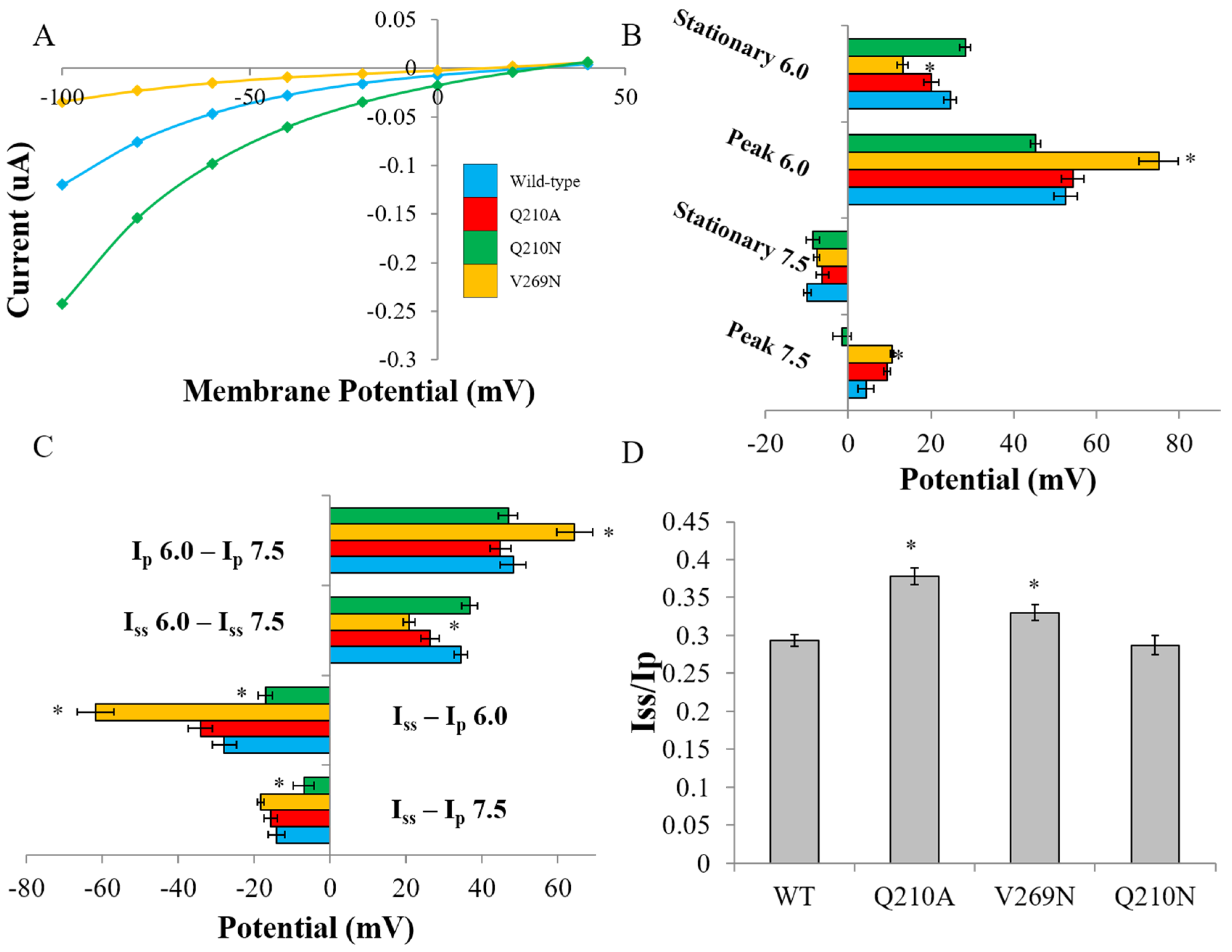
3.2. Probing the Effects of Predicted RHM Changes with Experimental Measurements of Function for ChR2 Constructs
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagel, G.; Ollig, D.; Fuhrmann, M.; Kateriya, S.; Mustl, A.M.; Bamberg, E.; Hegemann, P. Channelrhodopsin-1: A light-gated proton channel in green algae. Science 2002, 296, 2395–2398. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.; Dempski, R.E. From phototaxis to biomedical applications: Investigating the molecular mechanism of channelrhodopsins. In Electrophysiology of Unconventional Channels and Pores; Delcour, A.H., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 361–381. [Google Scholar] [CrossRef]
- Spudich, J.L.; Sineshchekov, O.A.; Govorunova, E.G. Mechanism divergence in microbial rhodopsins. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Lin, M.Z.; Steinbach, P.; Tsien, R.Y. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 2009, 96, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Yizhar, O.; Gunaydin, L.A.; Hegemann, P.; Deisseroth, K. Bi-stable neural state switches. Nat. Neurosci. 2009, 12, 229–234. [Google Scholar] [CrossRef]
- Bamann, C.; Kirsch, T.; Nagel, G.; Bamberg, E. Spectral characteristics of the photocycle of channelrhodopsin-2 and its implication for channel function. J. Mol. Biol. 2008, 375, 686–694. [Google Scholar] [CrossRef]
- Berndt, A.; Prigge, M.; Gradmann, D.; Hegemann, P. Two open states with progressive proton selectivities in the branched channelrhodopsin-2 photocycle. Biophys. J. 2010, 98, 753–761. [Google Scholar] [CrossRef]
- Nikolic, K.; Degenaar, P.; Toumazou, C. Modeling and engineering aspects of channelrhodopsin2 system for neural photostimulation. In Proceedings of the Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; Volume 1, pp. 1626–1629. [Google Scholar] [CrossRef]
- Hegemann, P.; Ehlenbeck, S.; Gradmann, D. Multiple photocycles of channelrhodopsin. Biophys. J. 2005, 89, 3911–3918. [Google Scholar] [CrossRef]
- Nikolic, K.; Grossman, N.; Grubb, M.S.; Burrone, J.; Toumazou, C.; Degenaar, P. Photocycles of channelrhodopsin-2. Photochem. Photobiol. 2009, 85, 400–411. [Google Scholar] [CrossRef]
- Richards, R.; Dempski, R.E. Adjacent channelrhodopsin-2 residues within transmembranes 2 and 7 regulate cation selectivity and distribution of the two open states. J. Biol. Chem. 2017, 292, 7314–7326. [Google Scholar] [CrossRef] [PubMed]
- Radu, I.; Bamann, C.; Nack, M.; Nagel, G.; Bamberg, E.; Heberle, J. Conformational changes of channelrhodopsin-2. J. Am. Chem. Soc. 2009, 131, 7313–7319. [Google Scholar] [CrossRef] [PubMed]
- Lorenz-Fonfria, V.A.; Bamann, C.; Resler, T.; Schlesinger, R.; Bamberg, E.; Heberle, J. Temporal evolution of helix hydration in a light-gated ion channel correlates with ion conductance. Proc. Natl. Acad. Sci. USA 2015, 112, E5796–E5804. [Google Scholar] [CrossRef] [PubMed]
- Sattig, T.; Rickert, C.; Bamberg, E.; Steinhoff, H.J.; Bamann, C. Light-induced movement of the transmembrane HelixB in channelrhodopsin-2. Angew. Chem. (Int. Ed.) 2013, 52, 9705–9708. [Google Scholar] [CrossRef] [PubMed]
- Kuhne, J.; Eisenhauer, K.; Ritter, E.; Hegemann, P.; Gerwert, K.; Bartl, F. Early formation of the ion-conducting pore in channelrhodopsin-2. Angew. Chem. (Int. Ed.) 2015, 54, 4953–4957. [Google Scholar] [CrossRef]
- Mondal, S.; Khelashvili, G.; Weinstein, H. Not just an oil slick: How the energetics of protein-membrane interactions impacts the function and organization of transmembrane proteins. Biophys. J. 2014, 106, 2305–2316. [Google Scholar] [CrossRef]
- Mondal, S.; Johnston, J.M.; Wang, H.; Khelashvili, G.; Filizola, M.; Weinstein, H. Membrane driven spatial organization of GPCRs. Sci. Rep. 2013, 3, 2909. [Google Scholar] [CrossRef]
- Chawla, U.; Jiang, Y.; Zheng, W.; Kuang, L.; Perera, S.M.; Pitman, M.C.; Brown, M.F.; Liang, H. A usual g-protein-coupled receptor in unusual membranes. Angew. Chem. Int. Ed. Engl. 2016, 55, 588–592. [Google Scholar] [CrossRef]
- Periole, X. Interplay of G protein-coupled receptors with the membrane: Insights from supra-atomic coarse grain molecular dynamics simulations. Chem. Rev. 2017, 117, 156–185. [Google Scholar] [CrossRef]
- Mondal, S.; Khelashvili, G.; Shi, L.; Weinstein, H. The cost of living in the membrane: A case study of hydrophobic mismatch for the multi-segment protein LeuT. Chem. Phys. Lipids 2013, 169, 27–38. [Google Scholar] [CrossRef][Green Version]
- Botelho, A.V.; Huber, T.; Sakmar, T.P.; Brown, M.F. Curvature and hydrophobic forces drive oligomerization and modulate activity of rhodopsin in membranes. Biophys. J. 2006, 91, 4464–4477. [Google Scholar] [CrossRef] [PubMed]
- Khelashvili, G.; Weinstein, H. Functional mechanisms of neurotransmitter transporters regulated by lipid-protein interactions of their terminal loops. Biochim. Biophys. Acta 2015, 1848, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- De Jesus-Perez, J.J.; Cruz-Rangel, S.; Espino-Saldana, A.E.; Martinez-Torres, A.; Qu, Z.; Hartzell, H.C.; Corral-Fernandez, N.E.; Perez-Cornejo, P.; Arreola, J. Phosphatidylinositol 4,5-bisphosphate, cholesterol and fatty acids modulate the calcium-activated chloride channel TMEM16A (ANO1). Biochim. Biophys. Acta 2018, 1863, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.J.; Gilchrist, J.M.; Tien, J.; Bethel, N.P.; Qi, L.; Chen, T.; Wang, L.; Jan, Y.N.; Grabe, M.; Jan, L.Y. the sixth transmembrane segment is a major gating component of the tmem16a calcium-activated chloride channel. Neuron 2018, 97, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Khelashvili, G.; Shan, J.; Andersen, O.S.; Weinstein, H. Quantitative modeling of membrane deformations by multihelical membrane proteins: Application to G-protein coupled receptors. Biophys. J. 2011, 101, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys. J. 1986, 50, 1061–1070. [Google Scholar] [CrossRef]
- Arenkiel, B.R.; Peca, J.; Davison, I.G.; Feliciano, C.; Deisseroth, K.; Augustine, G.J.; Ehlers, M.D.; Feng, G.P. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 2007, 54, 205–218. [Google Scholar] [CrossRef]
- Douglass, A.D.; Kraves, S.; Deisseroth, K.; Schier, A.F.; Engert, F. Escape behavior elicited by single, Channelrhodopsin-2-evoked spikes in zebrafish somatosensory neurons. Curr. Biol. 2008, 18, 1133–1137. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.-P.; Boyden, E.S.; Deisseroth, K. Channelrhodopsin-2 and optical control of excitable cells. Nat. Methods 2006, 3, 785–792. [Google Scholar] [CrossRef]
- Richards, R.; Dempski, R.E. Re-introduction of transmembrane serine residues reduce the minimum pore diameter of channelrhodopsin-2. PLoS ONE 2012, 7, e50018. [Google Scholar] [CrossRef]
- Shan, J.; Khelashvili, G.; Mondal, S.; Mehler, E.L.; Weinstein, H. Ligand-dependent conformations and dynamics of the serotonin 5-HT(2A) receptor determine its activation and membrane-driven oligomerization properties. PLoS Comput. Biol. 2012, 8, e1002473. [Google Scholar] [CrossRef]
- Khelashvili, G.; Doktorova, M.; Sahai, M.A.; Johner, N.; Shi, L.; Weinstein, H. Computational modeling of the N-terminus of the human dopamine transporter and its interaction with PIP2-containing membranes. Proteins 2015, 83, 952–969. [Google Scholar] [CrossRef] [PubMed]
- Khelashvili, G.; Stanley, N.; Sahai, M.A.; Medina, J.; LeVine, M.V.; Shi, L.; De Fabritiis, G.; Weinstein, H. Spontaneous inward opening of the dopamine transporter is triggered by PIP2-regulated dynamics of the N-terminus. ACS Chem. Neurosci. 2015, 6, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.E.; Zhang, F.; Yizhar, O.; Ramakrishnan, C.; Nishizawa, T.; Hirata, K.; Ito, J.; Aita, Y.; Tsukazaki, T.; Hayashi, S.; et al. Crystal structure of the channelrhodopsin light-gated cation channel. Nature 2012, 482, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.; Dempski, R.E. Cysteine substitution and labeling provide insight into channelrhodopsin-2 ion conductance. Biochemistry 2015, 54, 5665–5668. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Eargle, J.; Wright, D.; Luthey-Schulten, Z. MultiSeq: Unifying sequence and structure data for evolutionary analysis. BMC Bioinform. 2006, 7, 382. [Google Scholar] [CrossRef]
- Volkov, O.; Kovalev, K.; Polovinkin, V.; Borshchevskiy, V.; Bamann, C.; Astashkin, R.; Marin, E.; Popov, A.; Balandin, T.; Willbold, D.; et al. Structural insights into ion conduction by channelrhodopsin 2. Science 2017, 358, eaan8862. [Google Scholar] [CrossRef]
- Yau, W.M.; Wimley, W.C.; Gawrisch, K.; White, S.H. The preference of tryptophan for membrane interfaces. Biochemistry 1998, 37, 14713–14718. [Google Scholar] [CrossRef]
- Ben-Tal, N.; Ben-Shaul, A.; Nicholls, A.; Honig, B. Free-energy determinants of alpha-helix insertion into lipid bilayers. Biophys. J. 1996, 70, 1803–1812. [Google Scholar] [CrossRef]
- Sankararamakrishnan, R.; Weinstein, H. Positioning and stabilization of dynorphin peptides in membrane bilayers: The mechanistic role of aromatic and basic residues revealed from comparative MD simulations. J. Phys. Chem. B 2002, 106, 209–218. [Google Scholar] [CrossRef]
- Gray, T.M.; Matthews, B.W. Intrahelical hydrogen bonding of serine, threonine and cysteine residues within alpha-helices and its relevance to membrane-bound proteins. J. Mol. Biol. 1984, 175, 75–81. [Google Scholar] [CrossRef]
- Eisenhaber, F.; Lijnzaad, P.; Argos, P.; Sander, C.; Scharf, M. The double cubic lattice method—Efficient approaches to numerical-integration of surface-area and volume and to dot surface contouring of molecular assemblies. J. Comput. Chem. 1995, 16, 273–284. [Google Scholar] [CrossRef]
- Nielsen, C.; Goulian, M.; Andersen, O.S. Energetics of inclusion-induced bilayer deformations. Biophys. J. 1998, 74, 1966–1983. [Google Scholar] [CrossRef]
- Gaiko, O.; Dempski, R.E. Transmembrane domain three contributes to the ion conductance pathway of channelrhodopsin-2. Biophys. J. 2013, 104, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Talathi, S.S.; Carney, P.R.; Khargonekar, P.P. Control of neural synchrony using channelrhodopsin-2: A computational study. J. Comput. Biol. 2011, 31, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; Xu, J.; Lu, Z.; Klimas, A.; Chen, X.; Ambrosi, C.M.; Cohen, I.S.; Entcheva, E. Computational optogenetics: Empirically-derived voltage- and light-sensitive channelrhodopsin-2 model. PLoS Comput. Biol. 2013, 9, e1003220. [Google Scholar] [CrossRef]
- Wang, J.; Hasan, M.T.; Seung, H.S. Laser-evoked synaptic transmission in cultured hippocampal neurons expressing channelrhodopsin-2 delivered by adeno-associated virus. J. Neurosci. Methods 2009, 183, 165–175. [Google Scholar] [CrossRef]
- Bruegmann, T.; Malan, D.; Hesse, M.; Beiert, T.; Fuegemann, C.J.; Fleischmann, B.K.; Sasse, P. Optogenetic control of heart muscle in vitro and in vivo. Nat. Methods 2010, 7, 897–900. [Google Scholar] [CrossRef]
- Khelashvili, G.; Mondal, S.; Andersen, O.S.; Weinstein, H. Cholesterol modulates the membrane effects and spatial organization of membrane-penetrating ligands for G-protein coupled receptors. J. Phys. Chem. B 2010, 114, 12046–12057. [Google Scholar] [CrossRef]
- Krause, N.; Engelhard, C.; Heberle, J.; Schlesinger, R.; Bittl, R. Structural differences between the closed and open states of channelrhodopsin-2 as observed by EPR spectroscopy. FEBS Lett. 2013, 587, 3309–3313. [Google Scholar] [CrossRef]
- Arango-Lievano, M.; Sensoy, O.; Borie, A.; Corbani, M.; Guillon, G.; Sokoloff, P.; Weinstein, H.; Jeanneteau, F. A GIPC1-palmitate switch modulates dopamine drd3 receptor trafficking and signaling. Mol. Cell. Biol. 2016, 36, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Sensoy, O.; Weinstein, H. A mechanistic role of Helix 8 in GPCRs: Computational modeling of the dopamine D2 receptor interaction with the GIPC1-PDZ-domain. Biochim. Biophys. Acta 2015, 1848, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Perez-Aguilar, J.M.; Shan, J.; LeVine, M.V.; Khelashvili, G.; Weinstein, H. A functional selectivity mechanism at the serotonin-2A GPCR involves ligand-dependent conformations of intracellular loop 2. J. Am. Chem. Soc. 2014, 136, 16044–16054. [Google Scholar] [CrossRef] [PubMed]
- Kaczor, A.A.; Rutkowska, E.; Bartuzi, D.; Targowska-Duda, K.M.; Matosiuk, D.; Selent, J. Computational methods for studying G protein-coupled receptors (GPCRs). Methods Cell Biol. 2016, 132, 359–399. [Google Scholar] [CrossRef] [PubMed]
- LeVine, M.V.; Khelashvili, G.; Shi, L.; Quick, M.; Javitch, J.A.; Weinstein, H. Role of annular lipids in the functional properties of leucine transporter LeuT proteomicelles. Biochemistry 2016, 55, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Razavi, A.M.; Khelashvili, G.; Weinstein, H. How structural elements evolving from bacterial to human SLC6 transporters enabled new functional properties. BMC Biol. 2018, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Kato, H.E.; Koyama, M.; Ito, J.; Kamiya, M.; Hayashi, S.; Maturana, A.D.; Deisseroth, K.; Ishitani, R.; Nureki, O. Molecular dynamics of channelrhodopsin at the early stages of channel opening. PLoS ONE 2015, 10, e0131094. [Google Scholar] [CrossRef]
- Nakanishi, T.; Kanada, S.; Murakami, M.; Ihara, K.; Kouyama, T. Large deformation of helix F during the photoreaction cycle of pharaonis halorhodopsin in complex with azide. Biophys. J. 2013, 104, 377–385. [Google Scholar] [CrossRef]
- Radzwill, N.; Gerwert, K.; Steinhoff, H.J. Time-resolved detection of transient movement of helices F and G in doubly spin-labeled bacteriorhodopsin. Biophys. J. 2001, 80, 2856–2866. [Google Scholar] [CrossRef]
- Muller, M.; Bamann, C.; Bamberg, E.; Kuhlbrandt, W. Light-induced helix movements in channelrhodopsin-2. J. Mol. Biol. 2015, 427, 341–349. [Google Scholar] [CrossRef]
- Piscitelli, C.L.; Krishnamurthy, H.; Gouaux, E. Neurotransmitter/sodium symporter orthologue LeuT has a single high–affinity substrate site. Nature 2010, 468, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, K.; Kim, H.-Y. Differential modification of the phospholipid profile by transient ischemia in rat hippocampal CA1 and CA3 regions. ProstaglandinsLeukot. Essent. Fat. Acids 2013, 88, 299–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, H.E.; Harwood, J.L.; Bowen, I.D.; Griffiths, G. Lipid composition of subcellular membranes from larvae and prepupae of Drosophila melanogaster. Lipids 1992, 27, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Norton, W.T.; Abe, T.; Poduslo, S.E.; DeVries, G.H. The lipid composition of isolated brain cells and axons. J. Neurosci. Res. 1975, 1, 57–75. [Google Scholar] [CrossRef] [PubMed]
| WT (kT) | V259S (kT) (% RHM Decrease) | V269N (kT) (% RHM Decrease) | Q210A (kT) (% RHM Decrease) |
|---|---|---|---|
| 12.1 | 7.7 (36%) | 5.8 (52%) | 3.1 (74%) |
| 7 | 3.7 (47%) | 3 (56%) | 2.3 (67%) |
| Construct | Ip7.5 | Iss7.5 | Ip6.0 | Iss6.0 | Iss-Ip 7.5 | Iss-Ip 6.0 | Iss6.0 – Iss7.5 | Ip6.0 – Ip7.5 |
|---|---|---|---|---|---|---|---|---|
| WT | 4.30 ± 1.9 | −9.84 ± 0.8 | 52.5 ± 2.7 | 24.6 ± 1.5 | −14.1 ± 2.1 | −27.8 ± 3.1 | 34.5 ± 1.7 | 48.2 ± 3.3 |
| Q210A | 9.39 ± 0.8 | −6.20 ± 1.5 | 54.2 ± 2.6 | 20.1 ± 1.8 | −15.6 ± 1.8 | −34.1 ± 3.2 | 26.3 ± 2.4 | 44.8 ± 2.7 |
| V269N | 10.6 ± 0.4 | −7.58 ± 0.7 | 75.0 ± 4.6 * | 13.2 ± 1.2 * | −18.2 ± 0.8 | −61.8 ± 4.8 * | 20.7 ± 1.4 * | 64.4 4.7 * |
| Q210N | −1.55 ± 2.2 | −8.5 ± 1.6 | 45.3 ± 1.2 | 28.2 ± 1.3 | −6.9 ± 2.7 * | −17.0 ± 1.8 * | 36.7 ± 2.1 | 46.9 ± 2.5 |
| Construct | k1 | k2 | Gd1 (ms−1) | Gd2 (ms−1) | e12 (ms−1) | e21 (ms−1) | Imax | γ | Gr (ms−1) | TChR2 (ms) |
|---|---|---|---|---|---|---|---|---|---|---|
| WT | 0.26 ± 0.01 | 0.081 ± 0.01 | 0.24 ± 0.03 | 0.015 ± 0.005 | 0.038 ± 0.004 | 0.0057 ± 0.0004 | 0.57 ± 0.06 | 0.036 ± 0.007 | 0.0071 ± 0.001 | 0.73 ± 0.08 |
| Q210A | 0.30 ± 0.001 | 0.028 ± 0.007 | 0.16 ± 0.04 | 0.0055 ± 0.001 * | 0.036 ± 0.003 | 0.010 ± 0.001 * | 0.45 ± 0.08 | 0.050 ± 0.01 | 0.0093 ± 0.002 * | 0.61 ± 0.03 |
| V269N | 0.30 ± 0.002 | 0.037 ± 0.02 | 0.13 ± 0.05 | 0.006 ± 0.003 | 0.061 ± 0.009 * | 0.012 ± 0.001 * | 0.25 ± 0.05 | 0.035 ± 0.005 | 0.030 ± 0.01 * | 0.63 ± 0.2 |
| Q210N | 0.30 ± 0.03 | 0.058 ± 0.01 | 0.34 ± 0.03 | 0.013 ± 0.005 | 0.052 ± 0.002 * | 0.0067 ± 0.0006 | 1.7 ± 0.2 | 0.020 ± 0.004 | 0.0074 ± 0.001 | 1.45 ± 0.05 |
| State Population | WT | Q210A | V269N | Q210N |
|---|---|---|---|---|
| Time to Peak (ms) | 8.0 | 7.7 | 5.3 | 7.3 |
| O1 Peak | 0.46 | 0.55 | 0.52 | 0.38 |
| O2 Peak | 0.09 | 0.10 | 0.11 | 0.09 |
| O1 Stat | 0.13 | 0.19 | 0.16 | 0.10 |
| O2 Stat | 0.65 | 0.61 | 0.67 | 0.65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richards, R.; Mondal, S.; Weinstein, H.; Dempski, R.E. Channelrhodopsin-2 Function is Modulated by Residual Hydrophobic Mismatch with the Surrounding Lipid Environment. Appl. Sci. 2019, 9, 2674. https://doi.org/10.3390/app9132674
Richards R, Mondal S, Weinstein H, Dempski RE. Channelrhodopsin-2 Function is Modulated by Residual Hydrophobic Mismatch with the Surrounding Lipid Environment. Applied Sciences. 2019; 9(13):2674. https://doi.org/10.3390/app9132674
Chicago/Turabian StyleRichards, Ryan, Sayan Mondal, Harel Weinstein, and Robert E. Dempski. 2019. "Channelrhodopsin-2 Function is Modulated by Residual Hydrophobic Mismatch with the Surrounding Lipid Environment" Applied Sciences 9, no. 13: 2674. https://doi.org/10.3390/app9132674
APA StyleRichards, R., Mondal, S., Weinstein, H., & Dempski, R. E. (2019). Channelrhodopsin-2 Function is Modulated by Residual Hydrophobic Mismatch with the Surrounding Lipid Environment. Applied Sciences, 9(13), 2674. https://doi.org/10.3390/app9132674






