The Learning Curve of Robotic Thyroid Surgery and the Avoidance of Temporary Hypoparathyroidism after Total Thyroidectomy and Concomitant Central Compartment Node Dissection: A Single Surgeon’s Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Robotic Procedures and Instruments
2.3. Surgical Complications
2.4. Learning Curve of Robotic Thyroid Surgery
2.5. Inclusion and Exclusion Criteria of Robotic Thyroid Surgery for PTC
2.6. Surgical Strategy in Patients with Thyroid Cancer
2.7. Postoperative Follow-up Protocol
2.8. Statistics
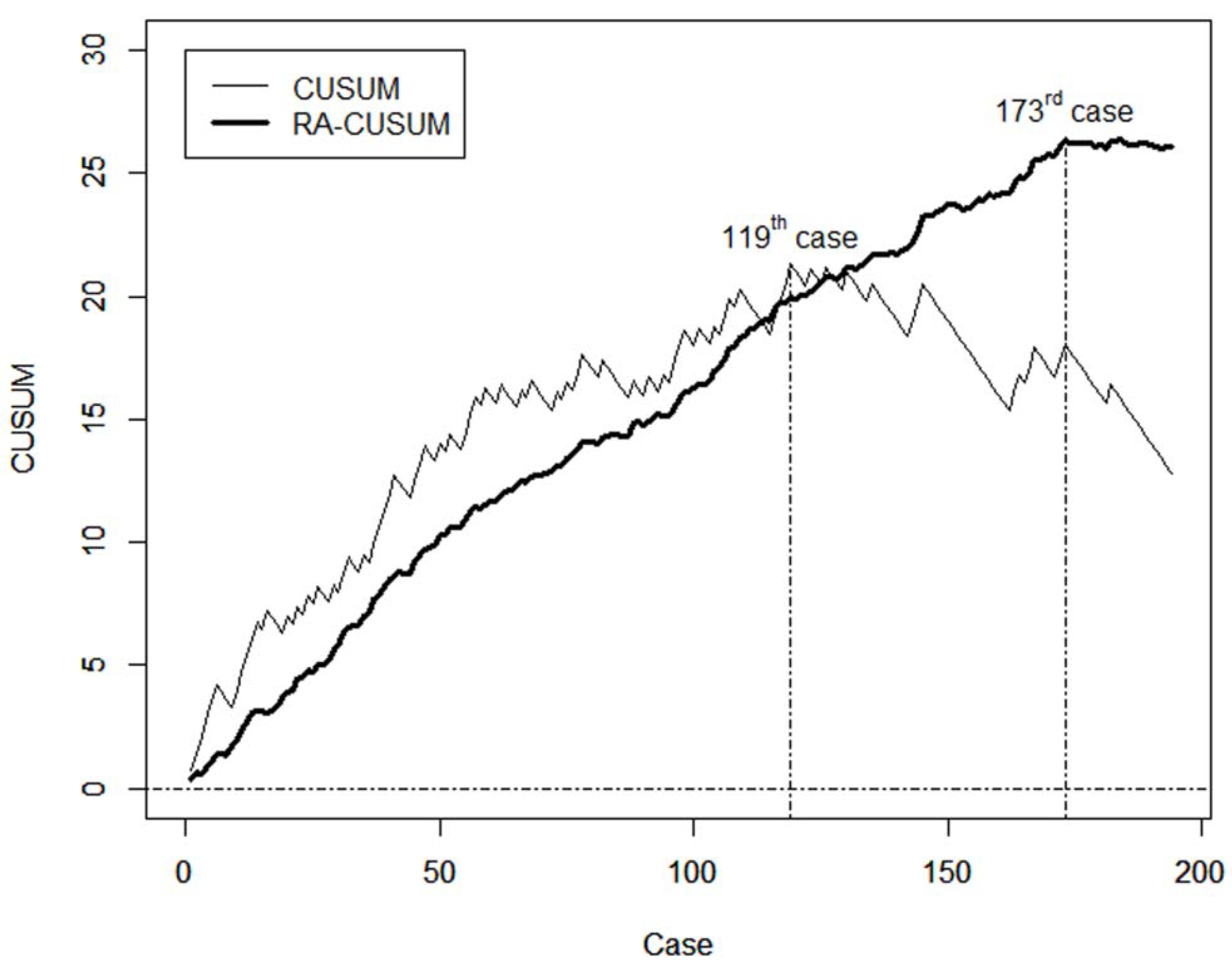
2.8.1. Cumulative Sum (CUSUM)
2.8.2. Risk-Adjusted CUSUM (RA-CUSUM)
3. Results
3.1. Baseline Clinicopathologic Features
3.2. Technical Outcomes of Robotic Thyroid Surgery
3.3. Surgical Completeness of Robotic Rhyroid Surgery
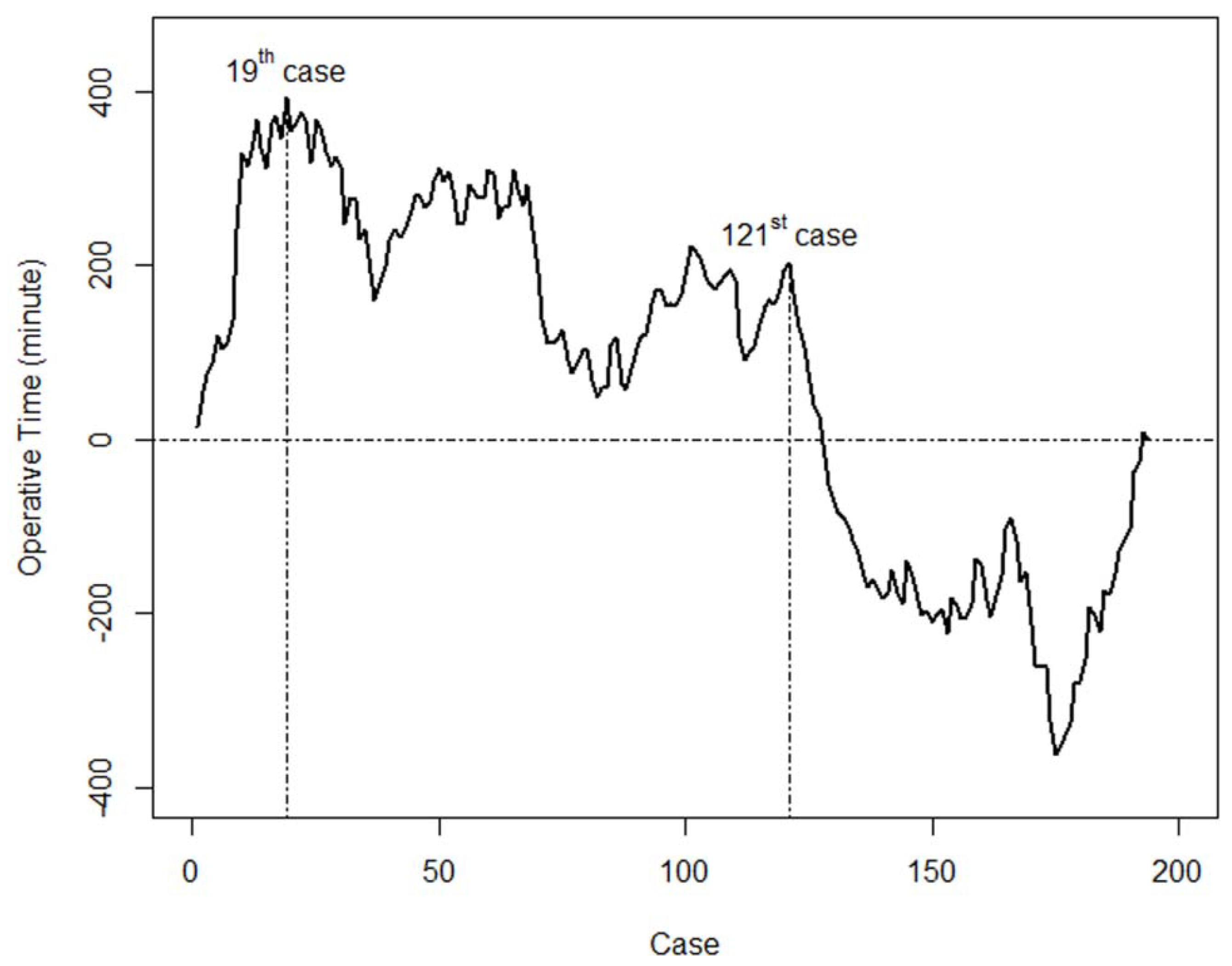
3.4. Learning Curve for Operation Time and the Incidence of Temporary Hypoparathyroidism
3.5. Comparison of Basic Clinicopathologic Features and Surgical Completeness before and after the 120th Case
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Napoli, N.; Kauffmann, E.F.; Palmeri, M.; Miccoli, M.; Costa, F.; Vistoli, F.; Amorese, G.; Boggi, U. The Learning Curve in Robotic Pancreacticoduodenectomy. Dig. Surg. 2016, 33, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Saito, F.J.; Dall’Oglio, M.F.; Ebaid, G.X.; Bruschini, H.; Chade, D.C.; Srougi, M. Learning curve for radical retropubic prostatectomy. Int. Braz. J. Urol. 2011, 37, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.C.; Law, W.L. The Learning Curve of Robotic-Assisted Low Rectal Resection of a Novice Rectal Surgeon. World J. Surg. 2016, 40, 456–462. [Google Scholar] [CrossRef] [PubMed]
- de Meritens, A.B.; Kim, J.; Dinkelspiel, H.; Chapman-Davis, E.; Caputo, T.; Holcomb, K.M. Feasibility and Learning Curve of Robotic. Laparoendoscopic Single Site Surgery in Gynecology. J. Minim. Invasive. Gynecol. 2017, 24, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Lee, S.C.; Lee, S.H.; Lee, K.Y.; Jeong, J.J.; Lee, Y.S.; Nam, K.H.; Chang, H.S.; Chung, W.Y.; Park, C.S. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery 2009, 146, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yun, J.H.; Nam, K.H.; Soh, E.Y.; Chung, W.Y. The learning curve for robotic thyroidectomy: A multicenter study. Ann. Surg. Oncol. 2011, 18, 226–232. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.H.; Nah, K.Y.; Soh, E.Y.; Chung, W.Y. Comparison of endoscopic and robotic thyroidectomy. Ann. Surg. Oncol. 2011, 18, 1439–1446. [Google Scholar] [CrossRef]
- Kandil, E.H.; Noureldine, S.I.; Yao, L.; Slakey, D.P. Robotic transaxillary thyroidectomy: an examination of the first one hundred cases. J. Am. Coll. Surg. 2012, 214, 558–564. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.; Hakim, N.A.; Kim, H.Y.; Kang, S.W.; Jeong, J.J.; Nam, K.H.; Bae, K.S.; Kang, S.J.; Chung, W.Y. Robotic thyroidectomy learning curve for beginning surgeons with little or no experience of endoscopic surgery. Head Neck. 2015, 37, 1705–1711. [Google Scholar] [CrossRef]
- Kim, W.W.; Jung, J.H.; Park, H.Y. A single surgeon’s experience and surgical outcomes of 300 robotic thyroid surgeries using a bilateral axillo-breast approach. J. Surg. Oncol. 2015, 111, 135–140. [Google Scholar] [CrossRef]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; Schlumberger, M.; et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2019, 19, 1167–1214. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, C.W.; Cho, M.S.; Baik, S.H.; Kim, D.W.; Min, B.S.; Lee, K.Y.; Kim, N.K. Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3-phase learning process comparison. Surg. Endosc. 2014, 28, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, C.W.; Cho, M.S.; Kim, D.W.; Min, B.S.; Baik, S.H.; Lee, K.Y.; Kim, N.K. Is the learning curve of robotic low anterior resection shorter than laparoscopic low anterior resection for rectal cancer? A comparative analysis of clinicopathologic outcomes between robotic and laparoscopic surgeries. Medicine 2014, 93, e109. [Google Scholar] [CrossRef] [PubMed]
- Tekkis, P.P.; Senagore, A.J.; Delaney, C.P.; Fazio, V.W. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann. Surg. 2005, 242, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.H.; Cook, R.J.; Farewell, V.T.; Treasure, T. Monitoring surgical performance using risk-adjusted cumulative sum charts. Biostatistics 2000, 1, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Nah, K.Y.; Kim, R.M.; Ahn, Y.H.; Soh, E.Y.; Chung, W.Y. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg. Endosc. 2010, 24, 3186–3194. [Google Scholar] [CrossRef] [PubMed]
- Landry, C.S.; Grubbs, E.G.; Morris, G.S.; Turner, N.S.; Holsinger, F.C.; Lee, J.E.; Perrier, N.D. Robot assisted transaxillary surgery (RATS) for the removal of thyroid and parathyroid glands. Surgery 2011, 149, 549–555. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, H.R.; Park, J.H.; Kim, K.H.; Kang, S.W.; Jeong, J.J.; Nam, K.H.; Chung, W.Y.; Park, C.S. Early surgical outcomes comparison between robotic and conventional open thyroid surgery for papillary thyroid microcarcinoma. Surgery 2012, 151, 724–730. [Google Scholar] [CrossRef]
- Tae, K.; Ji, Y.B.; Cho, S.H.; Lee, S.H.; Kim, D.S.; Kim, T.W. Early surgical outcomes of robotic thyroidectomy by a gasless unilateral axillobreast or axillary approach for papillary thyroid carcinoma: 2 years’ experience. Head Neck. 2012, 34, 617–625. [Google Scholar] [CrossRef]
- Aliyev, S.; Taskin, H.E.; Agcaoglu, O.; Aksoy, E.; Milas, M.; Siperstein, A.; Berber, E. Robotic transaxillary total thyroidectomy through a single axillary incision. Surgery 2013, 153, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.R.; Lee, J.; Park, J.H.; Kang, S.W.; Jeong, J.J.; Hong, J.Y.; Chung, W.Y. A comparison of postoperative pain after conventional open thyroidectomy and transaxillary single-incision robotic thyroidectomy: A prospective study. Ann. Surg. Oncol. 2013, 20, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Yi, O.; Yoon, J.H.; Lee, Y.M.; Sung, T.Y.; Chung, K.W.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Ryu, J.S.; Hong, S.J. Technical and oncologic safety of robotic thyroid surgery. Ann. Surg. Oncol. 2013, 20, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Chae, Y.J.; Cho, H.B.; Park, K.H.; Kim, J.S.; Lee, S.Y. Comparison of the incidence of postoperative nausea and vomiting between women undergoing open or robot-assisted thyroidectomy. Surg. Endosc. 2013, 27, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.W.; Kim, J.S.; Hur, S.M.; Kim, S.H.; Lee, S.K.; Choi, J.H.; Kim, S.; Lee, J.E.; Kim, J.H.; Nam, S.J.; et al. Is robotic surgery superior to endoscopic and open surgeries in thyroid cancer? World J. Surg. 2011, 35, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Koo, D.H.; Im, H.J.; Park, S.K.; Choi, J.Y.; Paeng, J.C.; Chung, J.K.; Oh, S.K.; Youn, Y.K. Surgical completeness of bilateral axillo-breast approach robotic thyroidectomy: comparison with conventional open thyroidectomy after propensity score matching. Surgery 2011, 150, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, J.S.; Chang, M.S.; Yoo, Y.K.; Kim, D.K. Influence of carbon dioxide insufflation of the neck on intraocular pressure during robot-assisted endoscopic thyroidectomy: a comparison with open thyroidectomy. Surg. Endosc. 2013, 27, 1587–1593. [Google Scholar] [CrossRef]
- Song, X.; Wang, D.; Sun, X.; Wang, J.; Liu, Z.; Liu, Q.; Gu, Y. Cumulative sum analysis of the learning curve for endoscopic resection of juvenile nasopharyngeal angiofibroma. Surg. Endosc. 2018, 32, 3181–3191. [Google Scholar] [CrossRef] [PubMed]
- Parisi, A.; Scrucca, L.; Desiderio, J.; Gemini, A.; Guarino, S.; Ricci, F.; Cirocchi, R.; Palazzini, G.; D’Andrea, V.; Minelli, L.; et al. Robotic right hemicolectomy: Analysis of 108 consecutive procedures and multidimensional assessment of the learning curve. Surg. Oncol. 2017, 26, 28–36. [Google Scholar] [CrossRef]
- Tomassini, F.; Scuderi, V.; Colman, R.; Vivarelli, M.; Montalti, R.; Troisi, R.I. The single surgeon learning curve of laparoscopic liver resection: A continuous evolving process through stepwise difficulties. Medicine (Baltimore) 2016, 95, e5138. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, H.J.; Huh, J.W.; Kim, Y.J.; Kim, H.R. Learning curve of laparoscopic low anterior resection in terms of local recurrence. J. Surg. Oncol. 2014, 110, 989–996. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology, Thyroid Carcinoma. 2009. Available online: http://www.sioechcf.it/allegati/lineeguida/NCCN_2009_Tumore_della_tiroide.pdf (accessed on May 15 2018).
| Characteristics | Overall (n = 194) | Before (n = 119) | After (n = 75) | p-Value |
|---|---|---|---|---|
| Age, year, mean ± SD | 41.8 ± 8.8 | 41.9 ± 8.4 | 41.6 ± 9.4 | 0.788 |
| Sex, n (%) | 0.045 | |||
| Male | 7 (3.6) | 7 (5.9) | 0 (0) | |
| Female | 187 (96.4) | 112 (94.1) | 75 (100.0) | |
| Primary tumor size, cm, mean ± SD | 1.0 ± 0.6 | 0.9 ± 0.5 | 1.1 ± 0.6 | 0.084 |
| Extrathyroidal extension, n (%) | 0.518 | |||
| None | 73 (37.6) | 42 (35.2) | 31 (41.3) | |
| Minimal | 77 (39.7) | 51 (42.9) | 26 (34.7) | |
| Extensive | 44 (22.7) | 26 (21.9) | 18 (24.0) | |
| Multiplicity, n (%) | 90 (46.4) | 42 (35.3) | 48 (64.0) | <0.001 |
| Bilaterality, n (%) | 70 (36.1) | 31 (26.1) | 39 (52.0) | <0.001 |
| Extent of LN dissection, n (%) | 0.005 | |||
| Ipsilateral CCND | 163 (84.0) | 107 (89.9) | 56 (74.7) | |
| Bilateral CCND | 31 (16.0) | 12 (10.1) | 19 (25.4) | |
| Lymph node metastasis, n (%) | 85 (43.8) | 48 (40.3) | 37 (49.3) | 0.219 |
| Postoperative hospital stay, day, mean ± SD | 3.8 ± 1.7 | 3.9 ± 1.8 | 3.7 ± 1.6 | 0.305 |
| Characteristics | Number of Patients (n = 194) |
|---|---|
| Operation time, min, mean ± SD | 174.9 ± 36.1 |
| Blood loss, ml, mean ± SD Postoperative hemorrhage, n (%) | 4.3 ± 3.2 0 (0) |
| Temporary hypoparathyroidism, n (%) | 71 (36.6) |
| Permanent hypoparathyroidism, n (%) | 2 (1.0) |
| RLN injury, n (%) | 3 (1.5) |
| Infection, n (%) | 2 (1.0) |
| Seroma, n (%) | 0 (0) |
| Chyle leakage, n (%) | 5 (2.6) |
| Tracheal injury, n (%) | 0 (0) |
| Characteristics | Overall (n = 194) | Before (n = 119) | After (n = 75) | p-Value |
|---|---|---|---|---|
| Retrieved LNs, n, median (range) | 7 (0–24) | 6 (0–24) | 8 (0–24) | 0.135 |
| RAI remnant ablation, n (%) | 159 (82.0) | 107 (89.9) | 52 (69.3) | <0.001 |
| Anti-Tg Ab (+), n (%) | 30 (15.5) | 21 (17.7) | 9 (12.0) | 0.289 |
| Ablation sTg, ng/mL *, median (range) | 0.55 (0.08~47.10) | 0.54 (0.08–47.10) | 0.76 (0.08–17.80) | 0.899 |
| Ablation sTg < 10 g/mL *, n (%) | 125/133 (94.0) | 81/88 (92.1) | 44/45 (97.8) | 0.259 |
| Control sTg, ng/mL, median (range) | 0.08 (0.08~16.50) | 0.08 (0.08–16.50) | 0.08 (0.08–6.30) | 0.872 |
| Control sTg < 1 ng/mL, n (%) | 99/108 (91.7) | 68/74 (91.9) | 31/34 (91.2) | 1.000 |
| Characteristics | Number of Patients (n = 194) | |
|---|---|---|
| Odds Ratio (95% CI) | p-Value | |
| Age, years | 0.98 (0.94–1.02) | 0.285 |
| Gender * | ||
| Female | Ref | |
| Male | 3.83 (0.70–20.87) | 0.121 |
| Primary tumor size, cm | 0.55 (0.29–1.07) | 0.078 |
| Multifocality | ||
| No | Ref | |
| Yes | 0.57 (0.30–1.08) | 0.083 |
| Lymph node metastasis | ||
| No | Ref | |
| Yes | 1.86 (0.93–3.73) | 0.078 |
| Thyroid weight, gram | 1.04 (0.99–1.09) | 0.136 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Lee, J.H.; Cho, J.W.; Yoon, J.H. The Learning Curve of Robotic Thyroid Surgery and the Avoidance of Temporary Hypoparathyroidism after Total Thyroidectomy and Concomitant Central Compartment Node Dissection: A Single Surgeon’s Experience. Appl. Sci. 2019, 9, 2594. https://doi.org/10.3390/app9132594
Park JH, Lee JH, Cho JW, Yoon JH. The Learning Curve of Robotic Thyroid Surgery and the Avoidance of Temporary Hypoparathyroidism after Total Thyroidectomy and Concomitant Central Compartment Node Dissection: A Single Surgeon’s Experience. Applied Sciences. 2019; 9(13):2594. https://doi.org/10.3390/app9132594
Chicago/Turabian StylePark, Jae Hyun, Jun Hyeok Lee, Jae Won Cho, and Jong Ho Yoon. 2019. "The Learning Curve of Robotic Thyroid Surgery and the Avoidance of Temporary Hypoparathyroidism after Total Thyroidectomy and Concomitant Central Compartment Node Dissection: A Single Surgeon’s Experience" Applied Sciences 9, no. 13: 2594. https://doi.org/10.3390/app9132594
APA StylePark, J. H., Lee, J. H., Cho, J. W., & Yoon, J. H. (2019). The Learning Curve of Robotic Thyroid Surgery and the Avoidance of Temporary Hypoparathyroidism after Total Thyroidectomy and Concomitant Central Compartment Node Dissection: A Single Surgeon’s Experience. Applied Sciences, 9(13), 2594. https://doi.org/10.3390/app9132594





