Developments in Contact Lens Imaging: New Applications of Optical Coherence Tomography
Abstract
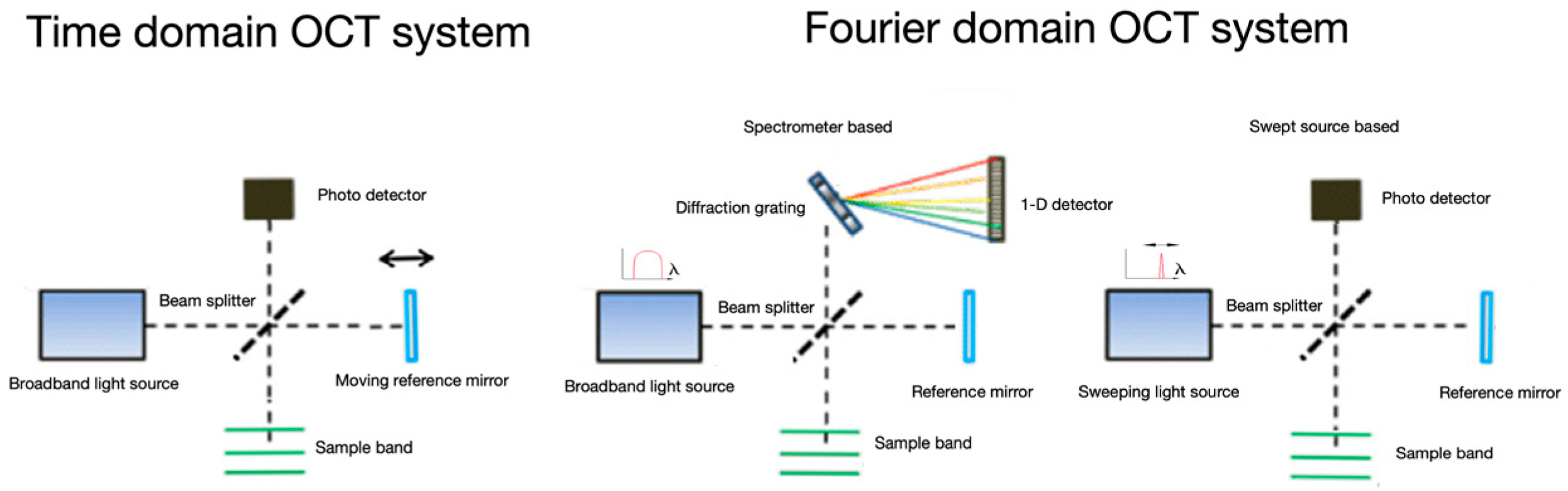
:1. Introduction
2. Clinical Uses in CL Practice
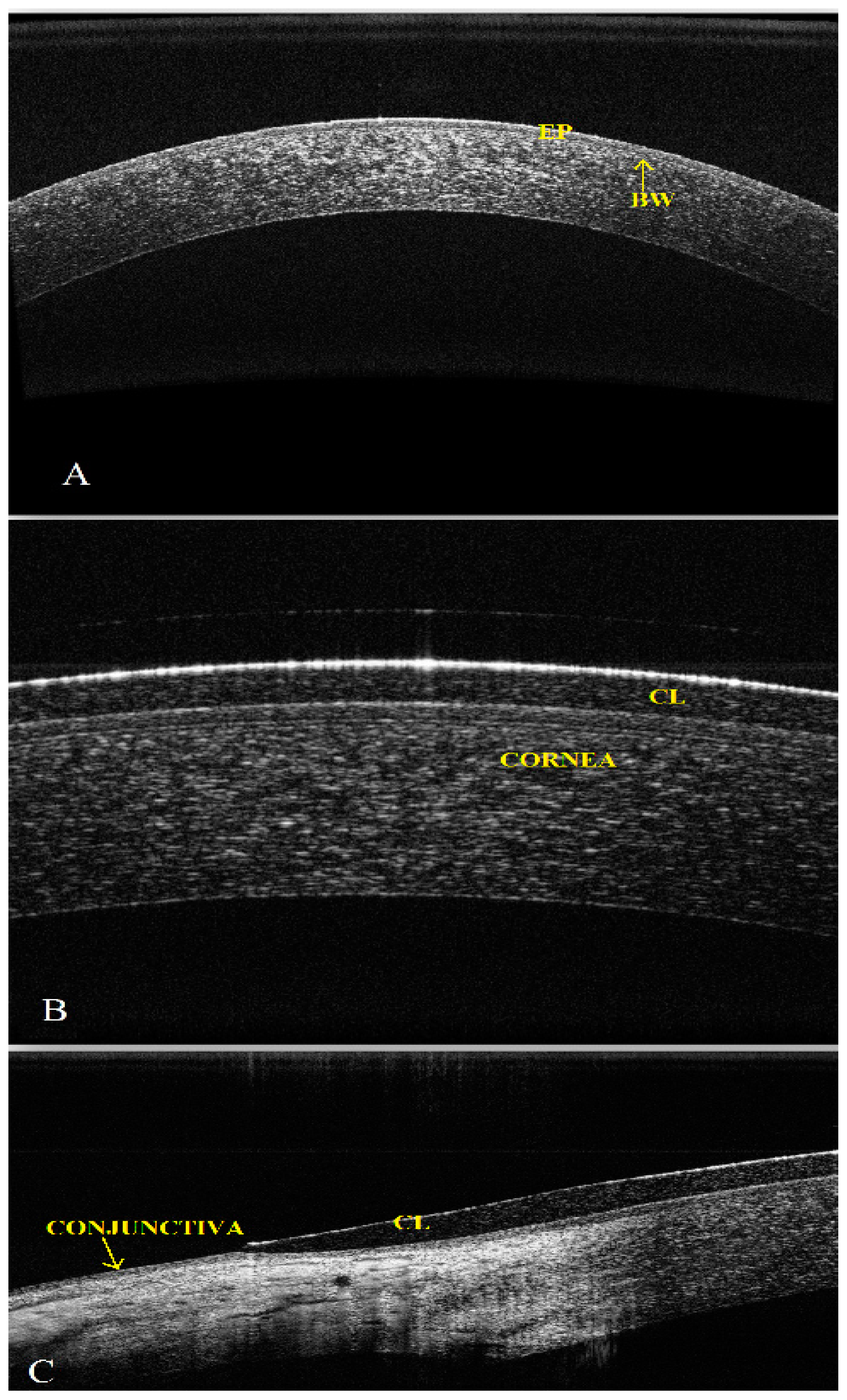
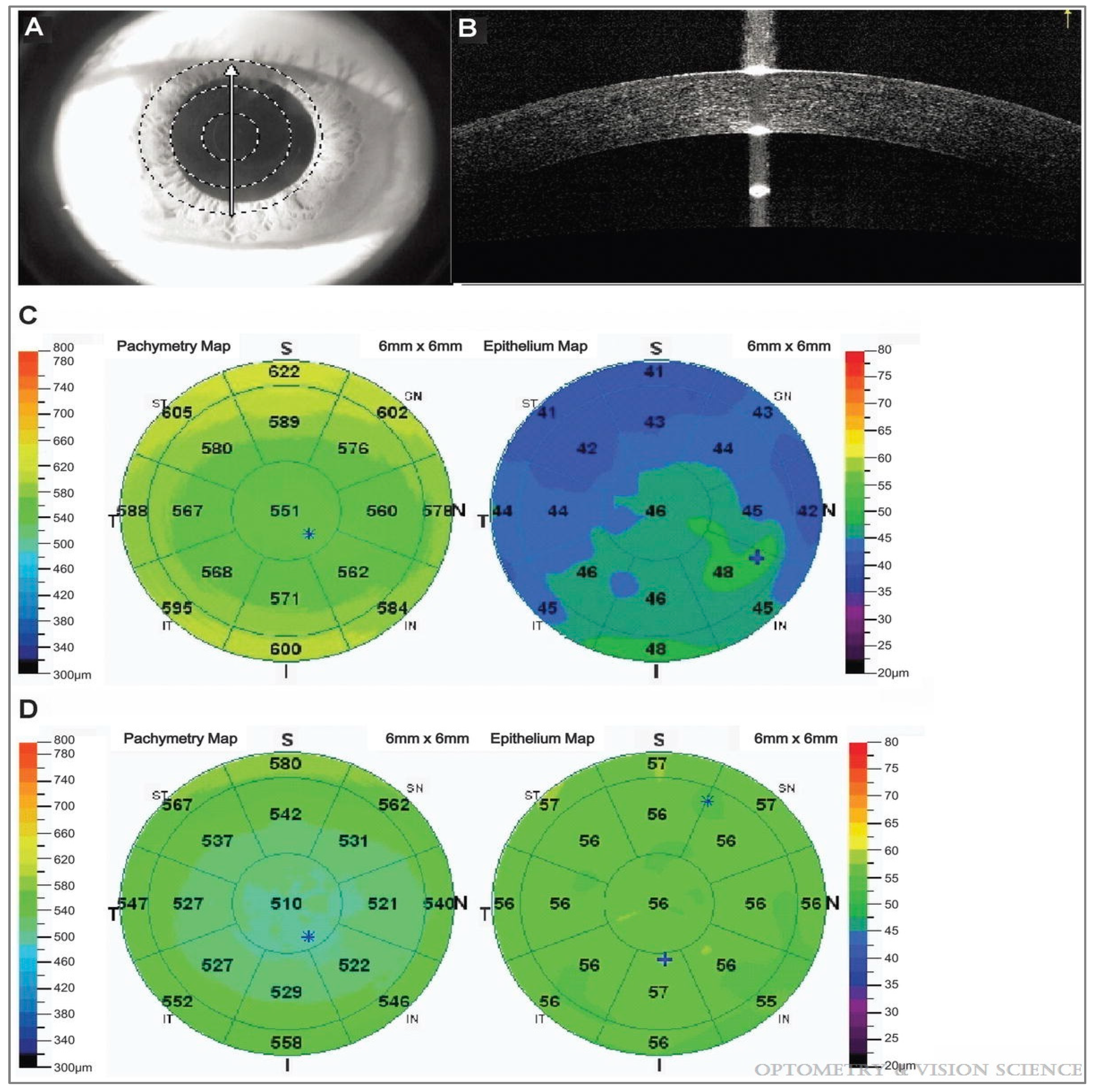
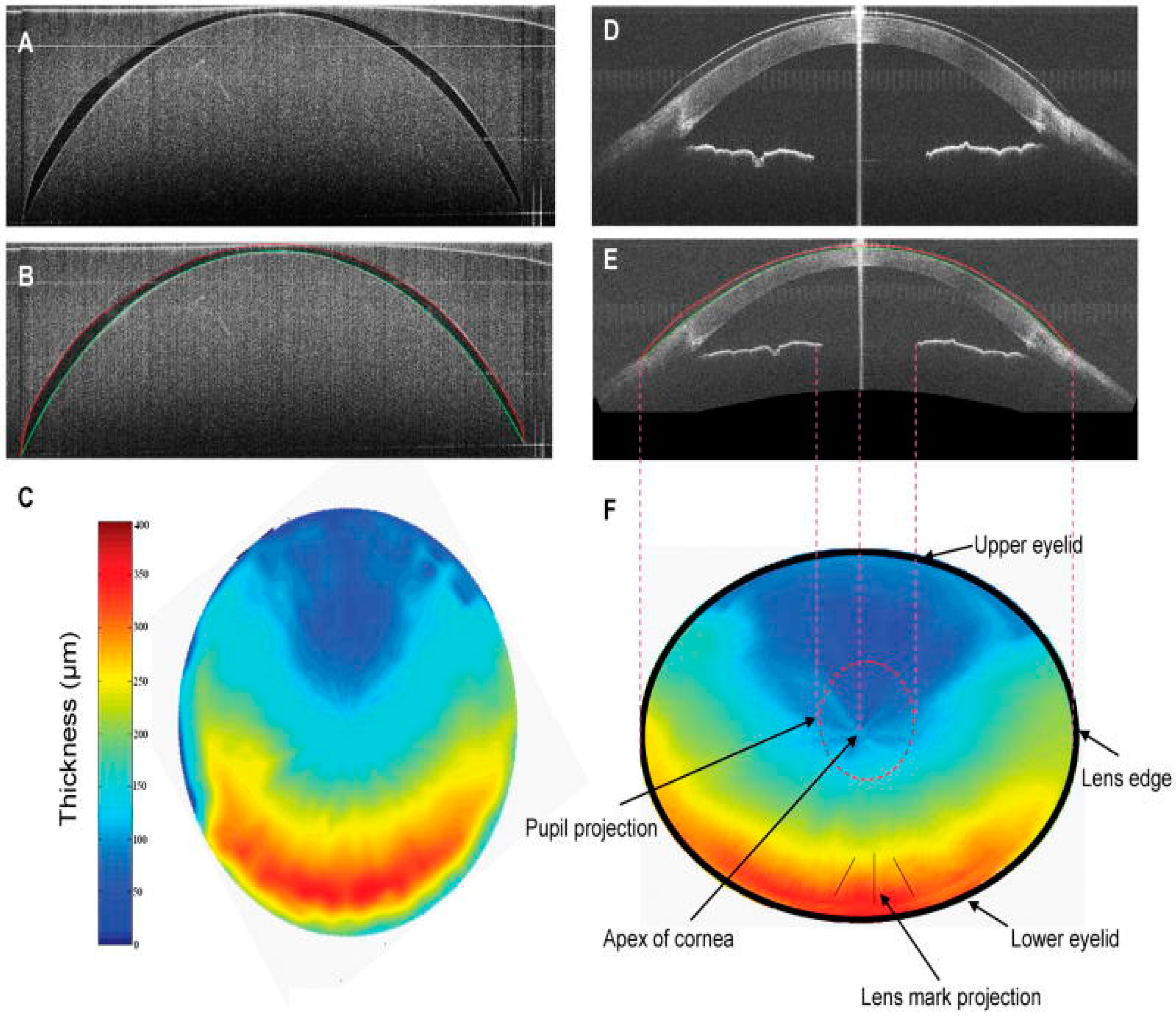
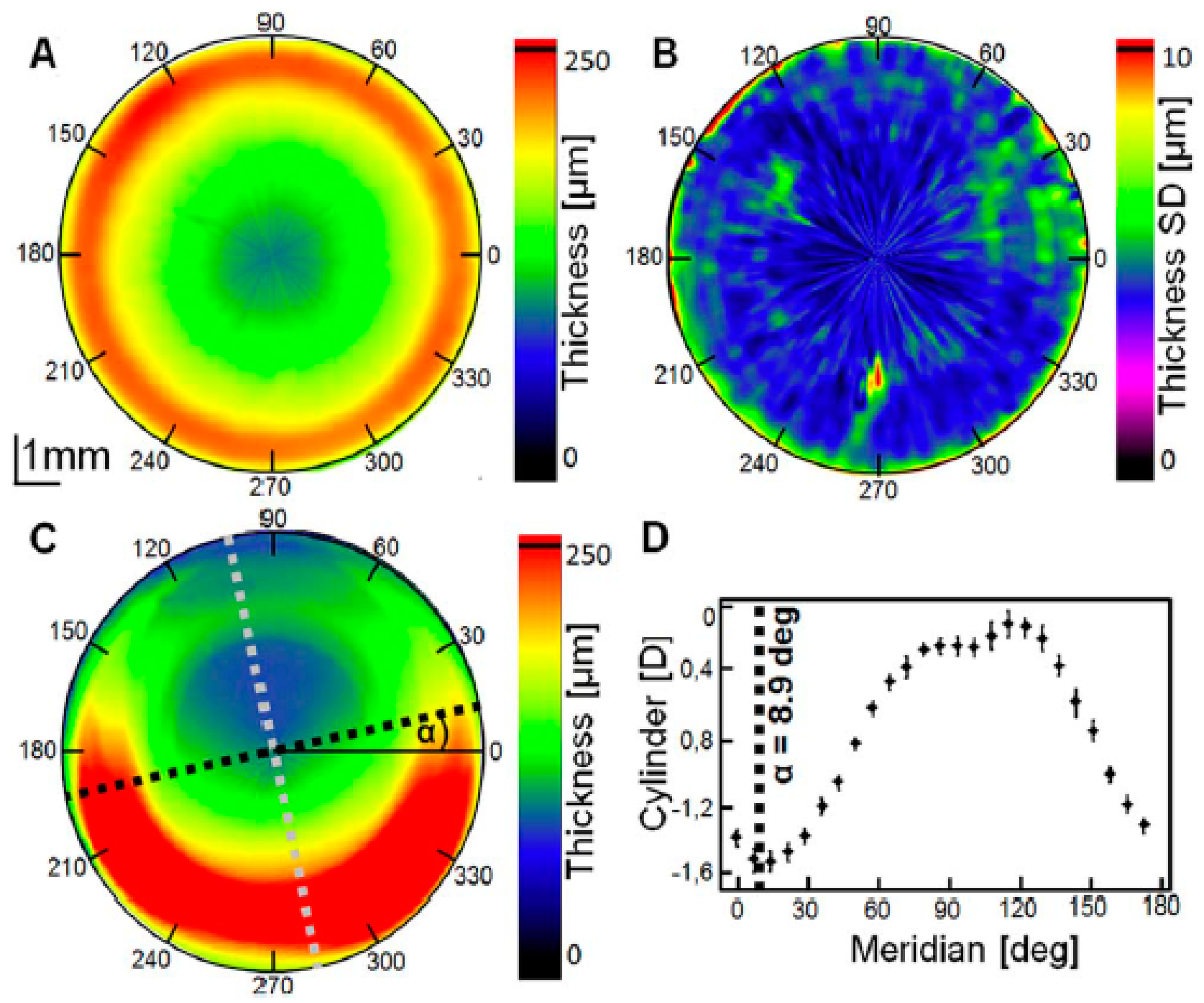
2.1. Qualitative and Quantitative Evaluation: Surface Topography and Biometric Data
2.2. Evaluation of Tear Fluid in CL Wearers
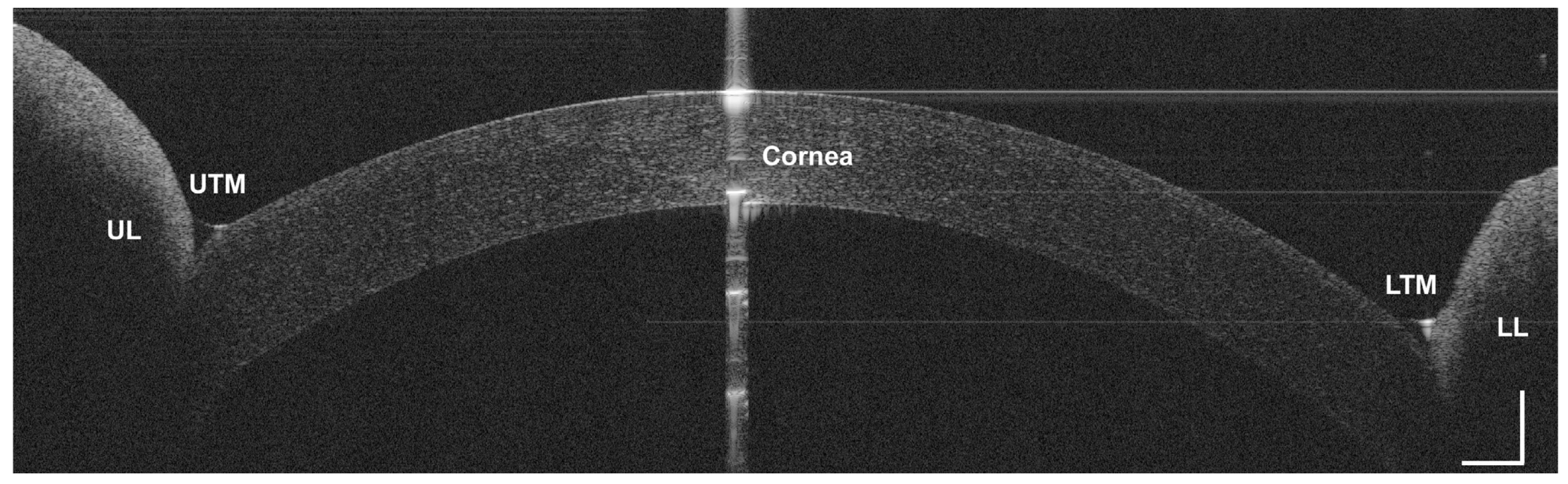
2.3. Static and Dynamic Assessment of CL Fitting
2.4. CL Complications
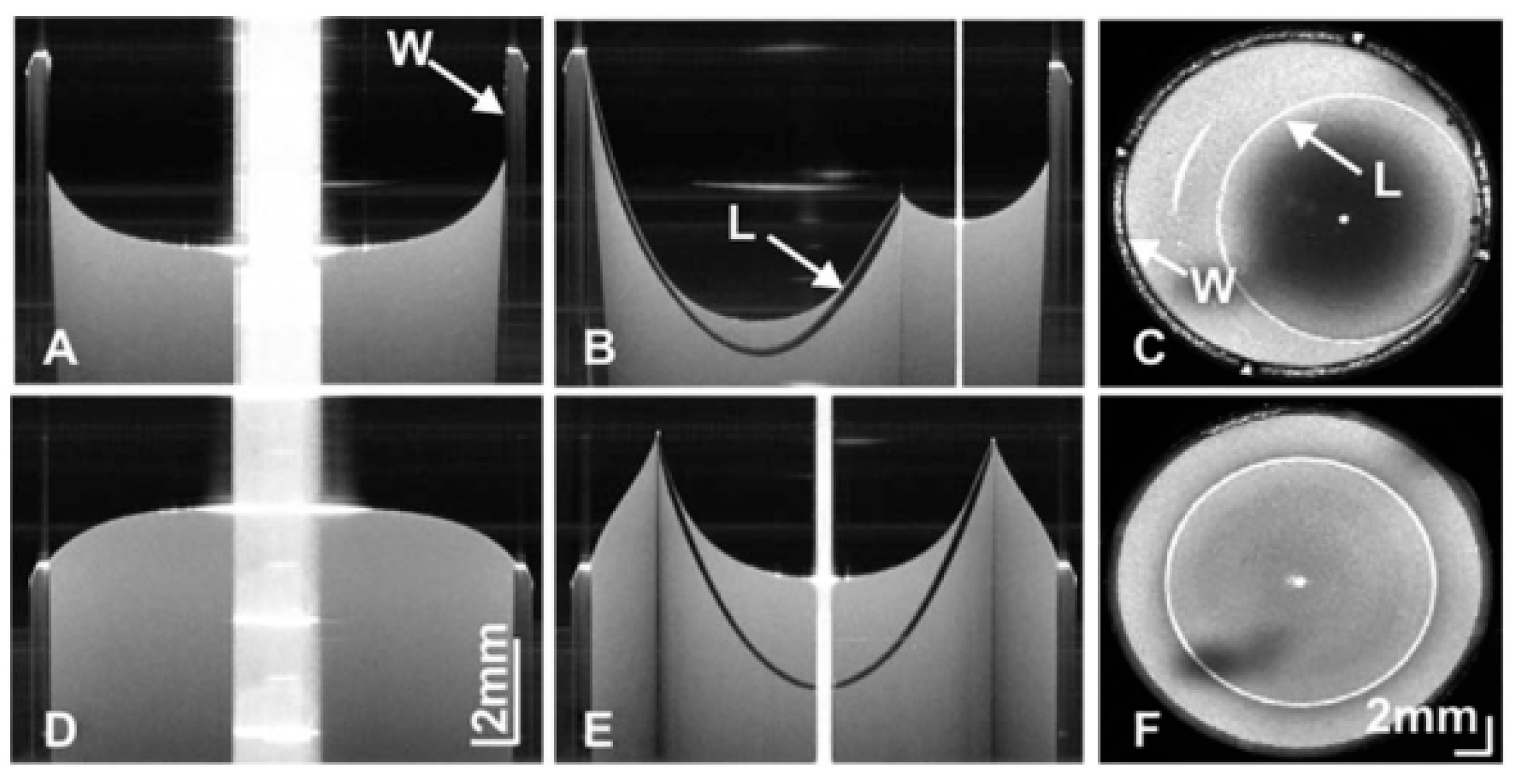
3. CL Metrology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luo, Z.K.; Jacobs, D.S. Current and Potential Applications of Anterior Segment Optical Coherence Tomography in Contact Lens Fitting. Semin. Ophthalmol. 2012, 27, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kałużny, B.J.; Kałużny, J.J.; Szkulmowska, A.; Górczynska, I.; Szkulmowski, M.; Bajraszewski, T.; Wojtkowski, M.; Targowski, P. Spectral Optical Coherence Tomography A Novel Technique for Cornea Imaging. Cornea 2006, 25, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fonn, D.; Simpson, T. Topographical Thickness of the Epithelium and Total Cornea after Hydrogel and PMMA Contact Lens Wear with Eye Closure. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1070–1074. [Google Scholar] [CrossRef] [Green Version]
- Pang, C.E.M.V.; Tan, D.T.H.; Mehta, J.S. Evaluation of Corneal Epithelial Healing Under Contact Lens Edge Fitting with Spectral-Domain Anterior Segment Optical Coherence Tomography (SD-OCT). Open Ophthalmol. J. 2011, 5, 51–54. [Google Scholar]
- Doors, M.; Berendschot, T.T.J.M.; de Brabander, J.; Webers, C.A.B.; Nuijts, R.M.M.A. Value of optical coherence tomography for anterior segment surgery. J. Ctataract Refract. Surg. 2010, 36, 1213–1229. [Google Scholar] [CrossRef] [Green Version]
- Izatt, J.A.; Hee, M.R.; Swanson, E.A.; Lin, C.P.; Huang, D.; Schuman, J.S.; Puliafito, C.A.; Fujimoto, J.G. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch. Ophthalmol. 1994, 112, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Rollins, A.M.; Roth, J.E.; Yazdanfar, S.; Westphal, V.; Bardenstein, D.S.; Izatt, J.A. Real-time optical coherence tomography of the anterior segment at 1310 nm. Arch. Ophthalmol. 2001, 119, 1179–1185. [Google Scholar] [CrossRef]
- Kałużny, J.J.; Wojtkowski, M.; Kowalczyk, A. Imaging of the anterior segment of the eye by Spectral Optical Coherence Tomography. Opt. Appl. 2002, 32, 581–589. [Google Scholar]
- Kałużny, B.J.; Kałużny, J.J.; Szkulmowska, A.; Górczynska, I.; Szkulmowski, M.; Bajraszewski, T.; Targowski, P.; Kowalczyk, A. Spectral optical coherence tomography: A new imaging technique in contact lens practice. Ophthalmic Physiol. Opt. 2006, 26, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Kałużny, B.J.; Fojt, W.; Szkulmowska, A.; Bajraszewski, T.; Wojtkowski, M.; Kowalczyk, A. Spectral Optical Coherence Tomography in Video-Rate and 3D Imaging of contact Lens Wear. Optom. Vis. Sci. 2007, 84, 1104–1109. [Google Scholar] [CrossRef]
- Wojtkowski, M. High-speed optical coherence tomography: Basics and applications. Appl. Opt. 2010, 49, D30–D61. [Google Scholar] [CrossRef]
- Rio-Cristobal, A.; Marin, R. Corneal assessment technologies: Current status. Surv. Ophthalmol. 2014, 30, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Grulkowski, I.; Liu, J.J.; Potsaid, B.; Jayaraman, V.; Lu, C.D.; Jiang, J.; Cable, A.E.; Duker, J.S.; Fujimoto, J.G. Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers. Biomed. Opt. Express 2012, 3, 2733–2751. [Google Scholar] [CrossRef] [Green Version]
- Englander, M.; Xu, D.; Kaiser, P.K. Optical Coherence Tomography. In Ophtalmology, 4th ed.; Yanoff, M., Duker, J.S., Eds.; Elsevier Saunders: Philadelphia, PA, USA; Springer: Berlin/Heidelberg, Germany, 2014; pp. 448–457. [Google Scholar]
- Fukuda, R.; Usui, T.; Miyai, T.; Yamagami, S.; Amano, S. Tear Meniscus Evaluation by Anterior Segment Swept-Source Optical Coherence Tomography. Am. J. Ophthalmol. 2013, 155, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Shao, Y.; Zhong, J.; Jiang, H.; Shen, M.; Wang, J. Versatile optical coherence tomography for imaging the human eye. Biomed. Opt. Express 2013, 4, 1031–1044. [Google Scholar] [CrossRef] [Green Version]
- Wojtkowski, M.; Kałużny, B.; Zawadzki, R.J. New Directions in Ophthalmic Optical Coherence Tomography. Optom. Vis. Sci. 2012, 89, 524–542. [Google Scholar] [CrossRef]
- Hassani, R.T.J.; Liang, H.; El Sanharawi, M.; Brasnu, E.; Kallel, S.; Labbe, A.; Baudouin, C. En-face Optical Coherence Tomography as a Novel Tool for Exploring the Ocular Surface: A Pilot Comparative Study to Conventional B-Scans and in Vivo Confocal Microscopy. Ocul. Surf. 2014, 12, 285–306. [Google Scholar] [CrossRef]
- Ang, M.; Cai, Y.; Tan, A.C.S. Swept Source Optical Coherence Tomography Angiography for Contact Lens-Related Corneal Vascularization. J. Ophthalmol. 2016, 2016, 9685297. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.J.; Fischer, D. Contact lenses 2018. Contact Lens Spectr. 2019, 34, 18–23. [Google Scholar]
- Morgan, P.B.; Woods, C.A.; Tranoudis, I.G.; Efron, N.; Jones, L.; Aighamdi, W.; Awasthi, S.; Belousov, V.; Bendoriene, J.; Chandrinos, A.; et al. International Contact Lens Prescribing in 2018. Contact Lens Spectr. 2019, 33, 26–32. [Google Scholar]
- Chisholm, C.; Woods, C.A. Contact Lens Assessment. In Clinical Procedures in Primary Eye Care, 4th ed.; Elliott, D.B., Ed.; Elsevier Saunders: Cambridge, UK, 2014; pp. 112–146. [Google Scholar]
- Van der Worp, E.; Bornman, D.; Lopes Ferreira, D.; Faria-Ribeiro, M.; Garcia-Porta, N.; González-Meijome, J.M. Modern scleral contact lens: A review. Cont. Lens Anterior Eye 2014, 37, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Shen, M.; Wang, M.R.; Wang, J. Micrometer-Scale Contact Lens Movements Imaged by Ultrahigh-resolution Optical Coherence Tomography. Am. J. Ophthalmol. 2012, 153, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Elbendary, A.M.; Samra, W.A. Evaluation of rigid gas permeable lens fitting in keratoconic patients with optical coherence tomography. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 1565–1570. [Google Scholar] [CrossRef]
- Ramdas, W.D.; Vervaet, C.J.W.C.; Bleyen, I. Corneal topography for pancorneal toric edge rigid gas-permeable contact lens fitting in patients with keratoconus, and differences in age and gender. Cont. Lens Anterior Eye 2014, 37, 20–25. [Google Scholar] [CrossRef]
- Hall, L.A.; Young, G.; Wolffsohn, J.S.; Riley, C. The Influence of Corneoscleral Topography on Soft Contact Lens Fit. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6801–6806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, M.; Wang, M.R.; Wang, J.; Yuan, Y.; Chen, F. Entire contact lens imaged in vivo and in vitro with spectral domain optical coherence tomography. Eye Contact Lens 2010, 36, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Caneiro, D.; Shaw, A.J.; Collins, M.J. Using Optical Coherence Tomography to Assess Corneoscleral Morphology After Soft Contact Lens Wear. Optom. Vis. Sci. 2012, 89, 1619–1626. [Google Scholar] [CrossRef] [Green Version]
- Tao, A.; Shao, Y.; Jiang, H.; Ye, Y.; Lu, F.; Shen, M.; Zhu, D.; Wang, J. Entire thickness profiles of the epithelium and contact lens in vivo imaged with high speed and high resolution optical coherence tomography. Eye Contact Lens 2013, 39, 329–334. [Google Scholar] [CrossRef]
- Shen, M.; Cui, L.; Riley, C.; Wang, M.R.; Wang, J. Characterization of Soft Contact Lens Edge Fitting Using Ultra-High Resolution and Ultra-Long Scan Depth Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4091–4097. [Google Scholar] [CrossRef] [Green Version]
- Morgan, P.B. Optimising contact lens wear for a lifetime of use. Optician 2013, 5, 33–37. [Google Scholar]
- Góra, M.; Karnowski, K.; Szkulmowski, M.; Kałużny, B.J.; Huber, R.; Kowalczyk, A.; Wojtkowski, M. Ultra high-speed swept source OCT imaging of the anterior segment of human eye at 200 kHz with adjustable imaging range. Opt. Express 2009, 17, 14880–14894. [Google Scholar] [CrossRef] [PubMed]
- Rathi, V.M.; Mandathara, P.S.; Dumpati, S.; Sangwan, V.S. Change in vault during scleral lens trials assessed with anterior segment optical coherence tomography. Cont. Lens Anterior Eye 2017, 40, 157–161. [Google Scholar] [CrossRef]
- Bandlitz, S.; Baumer, J.; Conrad, U.; Wolffsohn, J. Scleral topography analysed by optical coherence tomography. Cont. Lens Anterior Eye 2017, 40, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuczkowski, S.; Filipek, E.; Borkowska-Kuczkowska, A.; Barchanowska, D. Optical coherent tomography use in hybrid contact lenses application in keratoconus management in patients with RGP lenses intolerance—Case report. Okulistyka 2013, 4, 23–26. [Google Scholar]
- Sorbara, L.; Maram, J.; Fonn, D.; Woods, C.; Simpson, T. Metrics of the normal cornea: Anterior segment imaging with the Visante OCT. Clin. Exp. Optom. 2010, 93, 150–156. [Google Scholar] [CrossRef]
- Wang, J.; Simpson, T.; Fonn, D. Objective Measurements of Corneal Light-Backscatter during Corneal Swelling, by Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3493–3498. [Google Scholar] [CrossRef]
- Martin, R.; Izquierdo, M.; Saber, A. Investigation of posterior corneal curvature in CL-induced corneal swelling. Cont. Lens Anterior Eye 2009, 32, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Qian, T.; Yang, Y.; Jiang, C.; Sun, X.; Deng, S.X.; Xu, J. Corneal Epithelial Thickness Map in Long-Term Soft Contact Lenses Wearers. Optom. Vis. Sci. 2014, 91, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, O.; Brass, R.; Weiss, J.L.; Huang, D. Corneal Epithelial Thickness Mapping by Fourier-Domain Optical Coherence Tomography in Normal and Keratoconic Eyes. Ophthalmology 2012, 119, 2425–2433. [Google Scholar] [CrossRef] [Green Version]
- Karnowski, K.; Kałużny, B.J.; Szkulmowski, M.; Góra, M.; Wojtkowski, M. Corneal topography with high-speed swept source OCT in clinical examination. Biomed. Opt. Express 2011, 2, 2709–2720. [Google Scholar] [CrossRef] [Green Version]
- Tapasztó, B.; Veres, A.; Kosina-Hagyó, K.; Somfai, G.M.; Németh, J. OCT Imaging of Lid-Parallel Conjunctival Folds in Soft Contact Lens Wearers. Optom. Vis. Sci. 2011, 88, 1206–1213. [Google Scholar]
- Evans, K.; Pult, H. How important are surface properties for successful contact lens wear? Optician 2012, 5, 14–18. [Google Scholar]
- Chen, Q.; Wang, J.; Shen, M.; Cai, C.; Li, J.; Cui, L.; Qu, J.; Lu, F. Lower Volumes of Tear Menisci in Contact Lens Wearers with Dry Eye Symptoms. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3159–3163. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Wang, J.; Shen, M.; Cui, L.; Cai, C.; Li, M.; Li, K.; Lu, F. Tear Menisci and Ocular Discomfort during Daily Contact Lens Wear in Symptomatic Wearers. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2175–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Fonn, D.; Simpson, T.; Jones, L. Precorneal and Pre- and Postlens Tear Film Thickness Measured Indirectly with Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2524–2528. [Google Scholar] [CrossRef]
- Wang, J.; Aquavella, J.; Palakuru, J.; Chung, S. Repeated measurements of dynamic tear distribution on the ocular surface after instillation of artificial tears. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3325–3329. [Google Scholar] [CrossRef]
- Wang, J.; Aquavella, J.; Palakuru, J.; Chung, S.; Feng, C. Relationships between central tear film thickness and tear menisci of the upper and lower eyelids. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4349–4355. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cox, I.; Reindel, W.T. Upper and Lower Tear Menisci on Contact Lens. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, J.; Tao, A.; Shen, M.; Jiao, S.; Lu, F. Ultrahigh-Resolution Measurement by Optical Coherence Tomography of Dynamic Tear Film Changes on Contact Lenses. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1988–1993. [Google Scholar] [CrossRef]
- Wang, J.; Jiao, S.; Ruggeri, M.; Abou Shousha, M.; Chen, Q. In situ visualization of tears on contact lens using ultra high resolution optical coherence tomography. Eye Contact Lens 2009, 2, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Piotrowiak, I.; Kałużny, B.J.; Danek, B.; Chwiędacz, A.; Sikorski, B.Ł.; Malukiewicz, G. Spectral Optical Coherence Tomography vs. Fluorescein pattern for rigid gas-permeable lens fit. Med. Sci. Mon. 2014, 20, 1137–1141. [Google Scholar]
- Cui, L.; Ming, L.; Shen, M.; Lu, F.; Wang, J. Characterization of Soft Contact Lens Fitting Using Ultra-Long Scan Depth Optical Coherence Tomography. J. Ophthalmol. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Nixon, A.D.; Barr, J.T.; VanNasdale, D.A. Corneal epithelial bullae after short-term wear of small diameter scleral lenses. Cont. Lens Anterior Eye 2017, 40, 116–126. [Google Scholar] [CrossRef]
- Alabi, E.; Hutchings, N.; Bizheva, K.; Simpson, T. Relationship between vessel diameter and depth measurements within the limbus using ultra-high resolution optical coherence tomography. J. Optom. 2018, 11, 57–65. [Google Scholar] [CrossRef]
- Davidson, B.R.; Barton, J.K. Application of optical coherence tomography to automated contact lens metrology. J. Biomed. Opt. 2010, 15. [Google Scholar] [CrossRef]
- Karnowski, K.; Grulkowski, I.; Mohan, N.; Cox, I.; Wojtkowski, M. Quantitative optical inspection of contact lenses immersed in wet cell using swept source OCT. Opt. Lett. 2014, 39, 4727–4730. [Google Scholar] [CrossRef] [PubMed]
- Plainis, S.; Atchison, D.A.; Charman, W.N. Power Profiles of Multifocal Contact Lenses and Their Interpretation. Optom. Vis. Sci. 2013, 90, 1066–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tankam, P.; Won, J.; Canavesi, C.; Cox, I.; Rolland, J.P. Optical assessment of soft Contact Lens Edge-Thickness. Optom. Vis. Sci. 2016, 93, 987–996. [Google Scholar] [CrossRef]
- Coldrick, B.J.; Richards, C.; Sugden, K.; Wolffsohn, J.S.; Drew, T.E. Developments in contact lens measurements: A comparative study of industry standard geometric inspection and optical coherence tomography. Cont. Lens Anterior Eye 2016, 39, 270–276. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachura, J.; Seredyka-Burduk, M.; Piotrowiak-Słupska, I.; Kaszuba-Modrzejewska, M.; Rzeszewska-Zamiara, J.; Kałużny, B.J. Developments in Contact Lens Imaging: New Applications of Optical Coherence Tomography. Appl. Sci. 2019, 9, 2580. https://doi.org/10.3390/app9132580
Stachura J, Seredyka-Burduk M, Piotrowiak-Słupska I, Kaszuba-Modrzejewska M, Rzeszewska-Zamiara J, Kałużny BJ. Developments in Contact Lens Imaging: New Applications of Optical Coherence Tomography. Applied Sciences. 2019; 9(13):2580. https://doi.org/10.3390/app9132580
Chicago/Turabian StyleStachura, Joanna, Małgorzata Seredyka-Burduk, Ilona Piotrowiak-Słupska, Magdalena Kaszuba-Modrzejewska, Jagoda Rzeszewska-Zamiara, and Bartłomiej J. Kałużny. 2019. "Developments in Contact Lens Imaging: New Applications of Optical Coherence Tomography" Applied Sciences 9, no. 13: 2580. https://doi.org/10.3390/app9132580
APA StyleStachura, J., Seredyka-Burduk, M., Piotrowiak-Słupska, I., Kaszuba-Modrzejewska, M., Rzeszewska-Zamiara, J., & Kałużny, B. J. (2019). Developments in Contact Lens Imaging: New Applications of Optical Coherence Tomography. Applied Sciences, 9(13), 2580. https://doi.org/10.3390/app9132580




