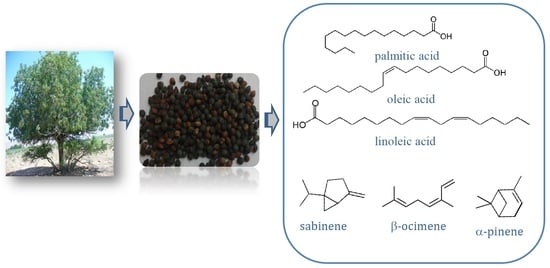
Pistacia Atlantica Desf., a Source of Healthy Vegetable Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Area of Seed Harvest and Plant Materials
2.2. Chemical Analysis
2.2.1. Oil Extraction
2.2.2. Fatty Acid Composition
2.2.3. Volatile Organic Compounds Identification
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boudy, B. Economie forestière Nord.-Africaine, Tome 1, Milieu Physique et Humain, 4th ed.; Larose Ed.: Paris, France, 1948; pp. 483–497. [Google Scholar]
- El Zerey-Belaskri, A.; Ribeiro, T.; Alcaraz, M.L.; EL Zerey, W.; Castro, S.; Loureiro, J.; Benhassaini, H.; Hormaz, J.I. Molecular characterization of Pistacia atlantica Desf. subsp. Atlantica (Anacardiaceae) in Algeria: Genome size determination, chromosome count and genetic diversity analysis using SSR markers. Sci. Hort. 2018, 227, 278–287. [Google Scholar] [CrossRef]
- Zohary, D. The genus Pistacia L. In Taxonomy, Distribution, Conservation and Uses of Pistacia Genetic Resources, Report of the IPGRI Workshop, Palermo, Italy, 29–30 June 1995; Padulosi, S., Caruso, T., Barone, E., Eds.; IPGRI: Rome, Italy, 2013. [Google Scholar]
- Chapagain, B.P.; Saharan, V.; Wiesman, Z. Larvicidal activity of saponins from Balanites aegyptiaca callus against Aedes aegypti mosquito. Bioresour. Technol. 2008, 99, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Obidah, W.; Nadro, M.S.; Tiyafo, G.O.; Wurochekke, A.U. Toxicity of crude Balanites aegyptiaca seed oil in rats. J. Am. Sci. 2009, 5, 13–165. [Google Scholar]
- Delazar, A.; Reid, R.G.; Sarker, S.D. GC-MS analysis of the essential oil from the oleoresin of Pistacia atlantica var. mutica. Chem. Nat. Comp. 2004, 40, 24–27. [Google Scholar] [CrossRef]
- Benhammou, N.; Bekkara, F.A.; Panovska, T.K. Antioxidant and antimicrobial activities of the Pistacia lentiscus and Pistacia atlantica extracts. Afr. J. Pharm. Pharmacol. 2008, 2, 22–28. [Google Scholar]
- Gourine, N.; Sifi, I.; Gaydou, E.M.; Yousfi, M. Chemical composition of the essential oil of unripe galls of Pistacia atlantica Desf. from Algeria. Nat. Prod. J. 2011, 1, 125–127. [Google Scholar] [CrossRef]
- Khallouki, F.; Breuer, A.; Merieme, E.; Ulrich, C.M.; Owen, R.W. Characterization and quantitation of the polyphenolic compounds detected in methanol extracts of Pistacia atlantica Desf. fruits from the Guelmim region of Morocco. J. Pharm. Biomed. Anal. 2017, 134, 310–318. [Google Scholar] [CrossRef]
- Martinez, J.J.I. Impact of a gall-inducing aphid on Pistacia atlantica Desf. trees. Arthr. Plant. Inter. 2008, 2, 147–151. [Google Scholar] [CrossRef]
- Talibi, I.; Askarne, L.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Ait Ben Aoumar, A. Antifungal activity of some Moroccan plants against Geotrichum candidum, causal agent of postharvest citrus sour rot. Crop Prot. 2012, 35, 41–46. [Google Scholar] [CrossRef]
- Sifi, I.; Gourine, N.; Gaydou, E.M.; Yousfi, M. Chemotypes of essential oil of unripe galls of Pistacia atlantica Desf. from Algeria. Nat. Prod. Res. 2015, 29, 1945–1949. [Google Scholar] [CrossRef]
- Memariani, Z.; Sharifzadeh, M.; Bozorgi, M.; Hajimahmoodi, M.; Farzaei, M.H.; Gholami, M.; Siavoshi, F.; Saniee, P. Protective effect of essential oil of Pistacia atlantica Desf. on peptic ulcer: Role of α-pinene. J. Tradit. Chin. Med. 2017, 37, 57–63. [Google Scholar] [CrossRef]
- Pourya, M.; Sadeghi, A.; Ghobari, H.; Nji Tizi Taning, C.; Smagghe, G. Bioactivity of Pistacia atlantica desf. Subsp. Kurdica (Zohary) Rech. F. and Pistacia khinjuk stocks essential oils against Callosobruchus maculatus (F, 1775) (Coloeptera: Bruchidae) under laboratory conditions. J. Stored Prod. Res. 2018, 77, 96–105. [Google Scholar] [CrossRef]
- Fabani, M.P.; Luna, L.; Baroni, M.V.; Monferran, M.V.; Ighani, M.; Tapia, A.; Wunderlin, D.A.; Feresin, G.E. Pistachio (Pistacia vera var Kerman) from Argentinean cultivars. A natural product with potential to improve human health. J. Funct. Foods. 2013, 5, 1347–1356. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, L.; Li, P.; Yu, L.; Mao, J.; Wang, X.; Zhang, Q. A review of chemical composition and nutritional properties of minor vegetable oils in China. Trend Food Sci. Technol. 2018, 74, 26–32. [Google Scholar] [CrossRef]
- Kushi, L.; Giovannucci, E. Dietary fat and cancer. Am. J. Med. 2002, 113, 63–70. [Google Scholar] [CrossRef]
- Ristić-Medić, D.; Vučić, V.; Takić, M.; Karadžić, I.; Glibetić, M. Polyunsaturated fatty acids in health and disease. J. Serb. Chem. Soc. 2013, 78, 1269–1289. [Google Scholar] [CrossRef]
- Khan, S.; Choudhary, S.; Pandey, A.; Khan, M.K.; Thomas, G. Sunflower Oil: Efficient Oil Source for Human Consumption. Emer. Life Sci. Res. 2015, 1, 1–3. [Google Scholar]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Thomas, J.; Thomas, C.J.; Radcliffe, J.; Itsiopoulos, C. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. Biomed Res. Int. 2015, 2015, 172801. [Google Scholar] [CrossRef]
- Sayed Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, H.; Cerny, M.; Chokr, A.; Kanaan, H.; Merah, O. Fennel seed oil and by-products characterization and their potential applications. Ind. Crops Prod. 2018, 111, 92–98. [Google Scholar] [CrossRef]
- Chaabani, E.; Abert Vian, M.; Dakhlaoui, S.; Bourgou, S.; Chemat, F.; Ksouri, R. Pistacia lentiscus L. edible oil: Green extraction with bio-based solvents, metabolite profiling and in vitro anti-inflammatory activity. OCL 2019, 26, 25. [Google Scholar] [CrossRef]
- Yingprasert, W.; Matan, N.; Chaowana, P. Fungal resistance and physico-mechanical properties of cinnamon oil and clove oil-treated rubberwood particleboards. J. Trop. For. Sci. 2015, 27, 69–79. [Google Scholar]
- Sayed Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, H.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Uitterhaegen, E.; Burianová, K.; Ballas, S.; Véronèse, T.; Merah, O.; Talou, T.; Stevens, C.V.; Evon, P.; Simon, V. Characterization of volatile organic compound emissions from self-bonded boards resulting from a coriander biorefinery. Ind. Crops Prod. 2018, 122, 57–65. [Google Scholar] [CrossRef]
- Mahjoub, F.; Rezayat, K.A.; Yousefi, M.; Mohebbi, M.; Salari, R. Pistacia atlantica Desf. A review of its traditional uses, phytochemicals and pharmacology. J. Med. Life 2018, 11, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ghalem, B.R.; Benhassaini, H. Etude des phytosterols et des acides gras de Pistacia atlantica. Afr. Sci. 2007, 3, 405–412. [Google Scholar]
- Yousfi, M.; Nedjmi, B.; Bellal, R.; Benbertal, D.; Palla, G. Fatty acids and sterols of Pistacia atlantica fruit oil. J. Am. Oil Chem. Soc. 2002, 79, 1049–1050. [Google Scholar] [CrossRef]
- Gourine, N.; Yousfi, M.; Bombarda, I.; Nadjemi, B.; Stocker, P.; Gaydou, E.M. Antioxidant activities and chemical composition of essential oil of Pistacia atlantica from Algeria. Ind. Crops Prod. 2010, 31, 203–208. [Google Scholar] [CrossRef]
- El Zerey-Belaskri, A.; Cavaleiro, C.; Romane, A.; Benhassaini, H.; Salgueiro, L. Intraspecific chemical variability of Pistacia atlantica Desf. subsp. atlantica essential oil from Northwest Algeria. J. Ess. Oil Res. 2017, 29, 32–41. [Google Scholar] [CrossRef]
- Chervin, J.; Talou, T.; Audonnet, M.; Dumas, B.; Camborde, L.; Esquerré-Tugayé, M.-T.; Roux, C.; Cabanac, G.; Marti, G. Deciphering the phylogeny of violets based on multiplexed genetic and metabolomic approaches. Phytochemistry 2019, 163, 99–110. [Google Scholar] [CrossRef]
- Waddell, K.L. Sampling coarse woody debris for multiple attributes in extensive resource inventories. Ecol. Ind. 2002, 1, 139–153. [Google Scholar] [CrossRef]
- Benhassaini, H.; Bendahmane, M.; Benchalgo, N. The chemical composition of fruits of Pistacia atlantica desf. subsp atlantica from Algeria. Chem. Nat. Compd. 2007, 43, 121–124. [Google Scholar] [CrossRef]
- Roche, J.; Bouniols, A.; Cerny, M.; Mouloungui, Z.; Merah, O. Fatty acid and phytosterol accumulation during seed ripening in three oilseed species. Intern. J. Food Sci. Technol. 2016, 51, 1820–1826. [Google Scholar] [CrossRef]
- Merah, O.; Langlade, N.; Alignan, M.; Roche, J.; Pouilly, N.; Lippi, Y.; Bouniols, A.; Vear, F.; Cerny, M.; Mouloungui, Z. Genetic control of phytosterol content in sunflower seeds. Theor. Appl. Genet. 2012, 125, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Alignan, M.; Bouniols, A.; Cerny, M.; Vear, F.; Mouloungui, Z.; Merah, O. Sterol content in sunflower seeds (Helianthus annuus L.) as affected by genotypes and environmental conditions. Food Chem. 2010, 121, 990–995. [Google Scholar] [CrossRef]
- Roche, J.; Alignan, M.; Bouniols, A.; Cerny, M.; Mouloungui, Z.; Merah, O. Sterol concentration and distribution in sunflower seeds (Helianthus annuus L.) during seed development. Food Chem. 2010, 119, 1451–1456. [Google Scholar] [CrossRef]
- Dobravalskytė, D.; Venskutonis, P.R.; Zebib, B.; Merah, O.; Talou, T. Essential Oil Composition of Myrrhis odorata (L.) Scop. Leaves Grown in Lithuania and France. J. Essent. Oil Res. 2013, 25, 44–48. [Google Scholar] [CrossRef]
- Barragan-Ferrer, D.; Venskutonis, P.R.; Talou, T.; Zebib, B.; Barragan-Ferrer, M.J.; Merah, O. Bioactive compounds and antioxidant properties of Myrrhis odorata deodorized residue leaves extracts from Lithuania and France origins. Pharm. Chem. J. 2016, 3, 43–48. [Google Scholar]
- Alignan, M.; Roche, J.; Bouniols, A.; Cerny, M.; Mouloungui, Z.; Merah, O. Effects of genotype and sowing date on—phytostanols-phytosterols content and agronomic traits in wheat under organic agriculture. Food Chem. 2009, 117, 219–225. [Google Scholar] [CrossRef]
- Santoso, H.; Iryanto, I.; Inggrid, M. Effects of temperature, pressure, preheating time and pressing time on rubber seed oil extraction using hydraulic press. Proc. Chem. 2014, 9, 248–256. [Google Scholar] [CrossRef]
- Ayerza, R.; Coates, W. Influence of environment on growing period and yield, protein, oil and α-linolenic content of three chia (Salvia hispanica L.) selections. Ind. Crops Prod. 2009, 30, 321–324. [Google Scholar] [CrossRef]
- Rondanini, D.; Mantese, A.; Savin, R.; Hall, A.J. Responses of sunflower yield and grain quality to alternating day/night high temperature regimes during grain filling: Effects of timing, duration and intensity of exposure to stress. Field Crops Res. 2006, 96, 48–62. [Google Scholar] [CrossRef]
- Merah, O. Genetic variability in glucosinolates in seed of Brassica juncea: Interest for mustard condiment. J. Chem. 2015, 2015, 606142. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Talou, T.; Cerny, M.; Merah, O. Oil and fatty acid accumulation during coriander (Coriandrum sativum L.) fruit ripening under organic agriculture. Crop. J. 2015, 3, 366–369. [Google Scholar] [CrossRef]
- Lecerf, J.M.; de Lorgeril, M. Dietary cholesterol: From physiology to cardiovascular risk. Brit. J. Nutr. 2011, 106, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Kratz, M.; Cullen, P.; Kannenberg, F.; Kassner, A.; Fobker, M.; Abuja, P.M.; Assmann, G.; Wahrburg, U. Effects of dietary fatty acids on the composition and oxidizability of low-density lipoprotein. Eur. J. Clin. Nutr. 2002, 56, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kondjoyan, N.; Berdagué, J.L. A Compilation of Relative Retention Indices for the Analysis of Volatile Compounds; Edition du Laboratoire Flaveur: Theix, France, 1996; pp. 12–43. [Google Scholar]
- Dwivedy, A.K.; Prakash, B.; Chanotiya, C.S.; Bisht, D.; Dubey, N.K. Chemically characterized Mentha cardiaca L. essential oil as plant based preservative in view of efficacy against biodeteriorating fungi of dry fruits, aflatoxin secretion, lipid peroxidation and safety profile assessment. Food Chem. Toxicol. 2017, 106, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Morshedloo, M.R.; Maggi, F.; Neko, H.T.; Aghdam, M.S. Sumac (Rhus coriaria L.) fruit: Essential oil variability in Iranian populations. Ind. Crops Prod. 2018, 111, 1–7. [Google Scholar] [CrossRef]
- Araújo, F.M.; Dantas, M.C.S.M.; Silva, L.S.; Aona, L.Y.S.; de Souza-Neta, L.C. Antibacterial activity and chemical composition of the essential oil of Croton heliotropiifolius Kunth from Amargosa, Bahia, Brazil. Ind. Crops Prod. 2017, 105, 203–206. [Google Scholar] [CrossRef]
- Stewart, C.D.; Jones, C.; Setzer, W.N. Essential oil compositions of Juniperus virginiana and Pinus virginiana, two important trees in Cherokee traditional medicine. Am. J. Essent. Oil Nat. Prod. 2014, 2, 17–24. [Google Scholar]
- Bilia, A.R.; Flamini, G.; Taglioli, V.; Morelli, I.; Vincieri, F.F. GC–MS analysis of essential oil of some commercial Fennel teas. Food Chem. 2002, 76, 307–310. [Google Scholar] [CrossRef]
- Khanavi, M.; Ahmadi, R.; Rajabi, A.; Jabbari, S.; Gholamreza, A.; Khademi, H.R.; Hadjiakhoondi, A.; Beyer, C.; Sharifzadeh, M. Pharmacological and histological effects of Centaurea bruguierana ssp. belangerana on indomethacin-induced peptic ulcer in rats. J. Nat. Med. 2012, 66, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, M.; Farhoosh, R.; Sharif, A.; Asili, J.; Iranshahi, M. Chemical composition, antioxidant and antibacterial properties of Bene (Pistacia atlantica subsp. mutica) hull essential oil. J. Food Sci. Technol. 2015, 52, 6784–6790. [Google Scholar] [CrossRef] [PubMed]
- Hamelian, M.; Hemmati, S.; Varmira, K.; Veisi, H. Green synthesis, antibacterial, antioxidant and cytotoxic effect of gold nanoparticles using Pistacia atlantica extract. J. Taiw. Inst. Chem. Engin. 2018, 93, 21–30. [Google Scholar] [CrossRef]
- Khodavaisy, S.; Rezaie, S.; Noorbakhsh, F.; Baghdadi, E.; Sharifynia, S.; Aala, F. Effects of Pistacia atlantica subsp. kurdica on Growth and Aflatoxin Production by Aspergillus parasiticus. Jundishapur J. Microbiol. 2016, 9, e35452. [Google Scholar] [CrossRef] [PubMed]
- Tanideh, N.; Masoumi, S.; Hosseinzadeh, M.; Safarpour, A.R.; Erjaee, H.; Koohi-Hosseinabadi, O.; Rahimikazerooni, S. Healing effect of Pistacia atlantica fruit oil extract in acetic Acid-induced colitis in rats. Iran. J. Med. Sci. 2014, 39, 522–528. [Google Scholar]
- Taghizadeh, S.F.; Davarynejad, G.; Asili, J.; Riahi-Zanjani, B.; Nemati, S.H.; Karim, G. Chemical composition, antibacterial, antioxidant and cytotoxic evaluation of the essential oil from pistachio (Pistacia khinjuk) hull. Microb. Path. 2018, 124, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Igarashi, J.; Ohtuka, Y.; Oguchi, S.; Kaneko, K.; Yamashiro, Y. Effects of n-3 polyunsaturated fatty acids and vitamin E on colonic mucosal leukotriene generation, lipid peroxidation, and microcirculation in rats with experimental colitis. Digestion 2001, 63, 49–54. [Google Scholar] [CrossRef]
- Rezaei, P.F.; Fouladdel, S.; Ghaffari, S.M.; Amin, G.; Azizi, E. Induction of G1 cell cycle arrest and cyclin D1 down-regulation in response to pericarp extract of Baneh in human breast cancer T47D cells. DARU J. Pharm. Sci. 2012, 20, 101. [Google Scholar] [CrossRef]
- Nazifi, S.; Saeb, M.; Sepehrimanesh, M.; Poorgonabadi, S. The effects of wild pistachio oil on serum leptin, thyroid hormones, and lipid profile in female rats with experimental hypothyroidism. Comp. Clin. Pathol. 2012, 21, 851–857. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Stathopoulou, M.G.; Triantafillidis, J.K.; Dedoussis, G.V.; Andrikopoulos, N.K. Chios mastic treatment of patients with active Crohn’s disease. World J. Gastroenterol. 2007, 13, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Derwich, E.; Manar, A.; Benziane, Z.; Boukir, A. GC/MS analysis and in vitro antibacterial activity of the essential oil isolated from leaf of Pistacia lentiscus growing in Morocoo. World Appl. Sci. J. 2010, 8, 1267–1276. [Google Scholar]
- Kamal, F.; Shahzad, M.; Ahmad, T.; Ahmed, Z.; Tareen, R.B.; Naz, R.; Ahmad, A. Antihyperlipidemic effect of Pistacia khinjuk. Biomed. Pharmacother. 2017, 96, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, A.; Sakvand, T.; Hajialyani, M.; Shahbazi, B.; Shakiba, M.; Tajehmiri, A.; Shakiba, E. Preparation and characterization of Pistacia khinjuk gum nanoparticles using response surface method: Evaluation of its anti-bacterial performance and cytotoxicity. Adv. Pharmaceut. Bull. 2017, 7, 159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smeriglio, A.; Denaro, M.; Barreca, D.; Calderaro, A.; Bisignano, C.; Ginestra, G.; Bellocco, E.; Trombetta, D. In vitro evaluation of the antioxidant, cytoprotective, and antimicrobial properties of essential oil from Pistacia vera L. Variety Bronte hull. Int. J. Mol. Sci. 2017, 18, 1212. [Google Scholar] [CrossRef] [PubMed]
- Bellakhder, J. La Pharmacopée Marocaine Traditionnelle, 1st ed.; Ibis Press: Paris, France, 1997; pp. 140–141. [Google Scholar]
- Ghalem, B.R.; Mohamed, B. Antimicrobial activity determination of the gum of Pistacia atlantica Desf. Oil. Afr. J. Microbiol. Res. 2010, 4, 2457–2460. [Google Scholar]
- Orhan, I.; Kupeli, E.; Aslan, M.; Kartal, M.; Yesilada, E. Bioassay-guided evaluation of anti-inflammatory and antinociceptive activities of pistachio, Pistacia vera L. J. Ethnopharmacol. 2006, 105, 235–240. [Google Scholar] [CrossRef]
- Mecherara-Idjeri, S.; Hassani, A.; Castola, V.; Casanova, J. Composition of leaf, fruit and gall essential oils of Algerian Pistacia atlantica Desf. J. Essent. Oil Res. 2008, 20, 215–219. [Google Scholar] [CrossRef]
| Month | Rainfall (mm) | Temperature (°C) | Relative Humidity (%) |
|---|---|---|---|
| January | 6 | 7.8 | 67 |
| February | 24 | 7.5 | 71 |
| March | 30 | 8.4 | 68 |
| April | 30 | 13.8 | 53 |
| May | 7 | 18.6 | 44 |
| June | 1 | 22.8 | 36 |
| July | 6 | 27.1 | 35 |
| August | 4 | 25.6 | 39 |
| September | 18 | 20.5 | 51 |
| October | 13 | 17.8 | 55 |
| November | 24 | 9.4 | 71 |
| December | 23 | 6 | 87 |
| Total | 185 | ||
| Mean | 15.4 | 56 |
| Fatty Acids | Djelfa | Unkown [29] | Sfisef [28] | Tlemcen [34] |
|---|---|---|---|---|
| Saturated fatty acid (SFA) | ||||
| C16:0 (Palmitic acid) | 26.7 ± 0.7 | 24.0 | 12.2 | 12.2 |
| C18:0 (Stearic acid) | 2.1 ± 0.0 | 1.8 | 2.4 | 2.4 |
| C20:0 (Arachidic acid) | 0.1 ± 0.1 | - | 0.1 | 0.1 |
| Total SFA | 28.8 ± 0.9 | 25.8 | 14.8 | 14.8 |
| Monounsaturated fatty acid (MUFA) | ||||
| ²²C16:1n-7 (Palmitoleic acid) | 1.0 ± 0.0 | 1.2 | 1.8 | 1.8 |
| C18:1n-9 (Oleic acid) | 40.9 ± 0.2 | 46.0 | 54.2 | 54.2 |
| C18:1n-7 (Vaccenic acid) | 1.2 ± 0.0 | - | - | |
| C20:1n-9 (Eicosenoic acid) | 0.2 ± 0.0 | - | - | |
| Total MUFA | 43.4 ±0.2 | 47.2 | 55.9 | 55.9 |
| Polyunsaturated fatty acid (PUFA) | ||||
| C18:2n-6 (Linoleic acid) | 26.8 ± 0.7 | 27.4 | 28.8 | 28.8 |
| C18:3n-3 (Linolenic acid) | 1.1 ± 0.0 | - | 0.4 | 0.4 |
| Total PUFA | 27.9 ± 0.7 | 27.4 | 29.3 | 29.3 |
| Total MUFA+PUFA | 71.3 ± 0.9 | 74.6 | 85.2 | 85.2 |
| Oil yield (%) | 40.4 ± 2.2 | 45.0 | 40 | 39.8 |
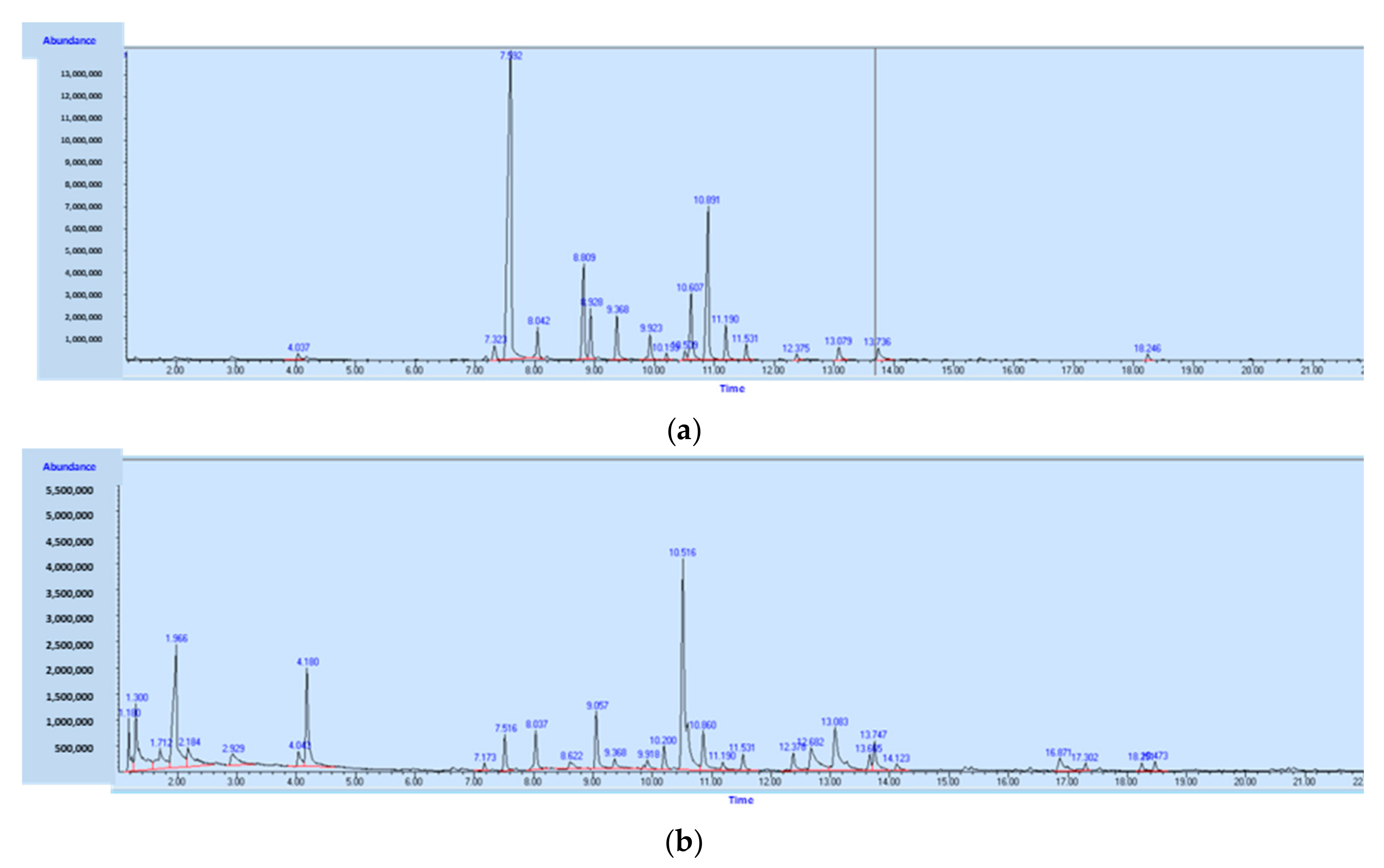
| tR (min) | Compound | RIlitterature | RIexperimental |
|---|---|---|---|
| 4.03 | hexanal | 799(a) | 805 |
| 7.17 | tricyclene | 920(b) | 922 |
| 7.32 | α-thujene | 925(a) | 926 |
| 7.59 | α-pinene | 934(a) | 935 |
| 8.04 | camphene | 946(a) | 950 |
| 8.21 | 2,4(10)-thujadiene | 961(c) | 955 |
| 8.8 | sabinene | 973(a) | 974 |
| 8.92 | β-pinene | 982(a) | 978 |
| 9.36 | β-myrcene | 989(a) | 992 |
| 9.86 | α-phellandrene | 1005(a) | 1008 |
| 9.92 | Δ3-carene | 1013(a) | 1010 |
| 10.19 | α-terpinene | 1034(a) | 1019 |
| 10.51 | p-cymene | 1029(a) | 1029 |
| 10.61 | limonene | 1031(a) | 1032 |
| 10.89 | Z-β-ocimene | 1037(a) | 1041 |
| 11.19 | E-β-ocimene | 1048(a) | 1050 |
| 11.53 | γ-terpinene | 1061(a) | 1061 |
| 12.1 | cis-sabinene hydrate | 1069(a) | 1080 |
| 12.37 | terpinolene | 1091(a) | 1088 |
| 12.55 | fenchone | 1094(f) | 1094 |
| 12.6 | C10H12 | 1096 | |
| 13.07 | nonanal | 1104(a) | 1111 |
| 13.73 | allocimene | 1130(a) | 1133 |
| 14.48 | trans-verbenol | 1148(b) | 1158 |
| 14.85 | pinocarvone | 1162(c) | 1171 |
| 15.27 | borneol | 1173(a) | 1185 |
| 15.44 | terpinen-4-ol | 1181(b) | 1190 |
| 16.32 | verbenone | 1211(c) | 1221 |
| 16.36 | C10H14O | 1223 | |
| 18.24 | bornyl acetate | 1286(d) | 1290 |
| 19.98 | α-terpinyl acetate | 1348(e) | 1355 |
| 20.44 | α-ylangene | 1368(e) | 1373 |
| 20.6 | α-copaene | 1376(c) | 1379 |
| 20.82 | β-bourbonene | 1392(b) | 1387 |
| 21.78 | β-caryophyllene | 1437(a) | 1425 |
| 23.18 | γ-muurolene | 1486(a) | 1481 |
| 23.33 | D-germacrene | 1480(c) | 1487 |
| 23.77 | α-muurolene | 1501(c) | 1505 |
| 24.12 | γ-cadinene | 1521(e) | 1519 |
| 24.24 | cardina-1(10),4-diene | 1531(e) | 1524 |
| 34.00 | palmitic acid | ||
| 36.10 | oleic acid |
| Species | Plant Part | Extract | Component | Activity | References |
|---|---|---|---|---|---|
| P. atlantica | Hull of fruit | Essential oil | Myrcene, α-Pinene, Limonene and α-Humulene | Antibacterial | Rezaie et al. [56] |
| Leaves | Essential oil, resin | Antibacterial | Hamelian et al. [57] | ||
| gum | Reduce growth and aflatoxin production of Aspergillus parasiticus | Khodavaisy et al. [58] | |||
| gum, fruit and leaves | Essential oil | fumigant activity against Callosobruchus maculatus | Pourya et al. [14] | ||
| Leaves | antifungal Geotrichum candidum | Talibi et al. [11] | |||
| leaves | Healing effect | Tanideh et al. [59] | |||
| Hull | Essential oil | gastroprotective anti-Helicobacter pylori anti-Staphylococcus | Taghizadeh et al. [60] | ||
| seed | Oil and fatty acids | Decrease of colonic mucosal blood flow | Shimizu et al. [61] | ||
| Fresh unripe fruits | Anti-breast cancer | Rezaei et al. [62] | |||
| Seed | Oil and fatty acids | Anticholesterolemic | Nazifi et al. [63] | ||
| Pistacia lentiscus | Gum | Management of inflammatory bowel diseases | Kaliora et al. [64] | ||
| fruits | Lipids | Anti-inflammatory | Chaabani et al. [23] | ||
| Leaves | Essential oil | α-Pinene | Antibacterial | Derwich et al. [65] | |
| Pistacia khinjuk | Leaves | Antihyperlipidemic | Kamal et al. [66] | ||
| Gum | Antibacterial | Fattahi et al. [67] | |||
| Pistacia vera | Hull | Essential oil | Antibacterial | Smeriglio et al. [68] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labdelli, A.; Zemour, K.; Simon, V.; Cerny, M.; Adda, A.; Merah, O. Pistacia Atlantica Desf., a Source of Healthy Vegetable Oil. Appl. Sci. 2019, 9, 2552. https://doi.org/10.3390/app9122552
Labdelli A, Zemour K, Simon V, Cerny M, Adda A, Merah O. Pistacia Atlantica Desf., a Source of Healthy Vegetable Oil. Applied Sciences. 2019; 9(12):2552. https://doi.org/10.3390/app9122552
Chicago/Turabian StyleLabdelli, Amina, Kamel Zemour, Valérie Simon, Muriel Cerny, Ahmed Adda, and Othmane Merah. 2019. "Pistacia Atlantica Desf., a Source of Healthy Vegetable Oil" Applied Sciences 9, no. 12: 2552. https://doi.org/10.3390/app9122552
APA StyleLabdelli, A., Zemour, K., Simon, V., Cerny, M., Adda, A., & Merah, O. (2019). Pistacia Atlantica Desf., a Source of Healthy Vegetable Oil. Applied Sciences, 9(12), 2552. https://doi.org/10.3390/app9122552