A Picture of Modern Tc-99m Radiopharmaceuticals: Production, Chemistry, and Applications in Molecular Imaging
Abstract
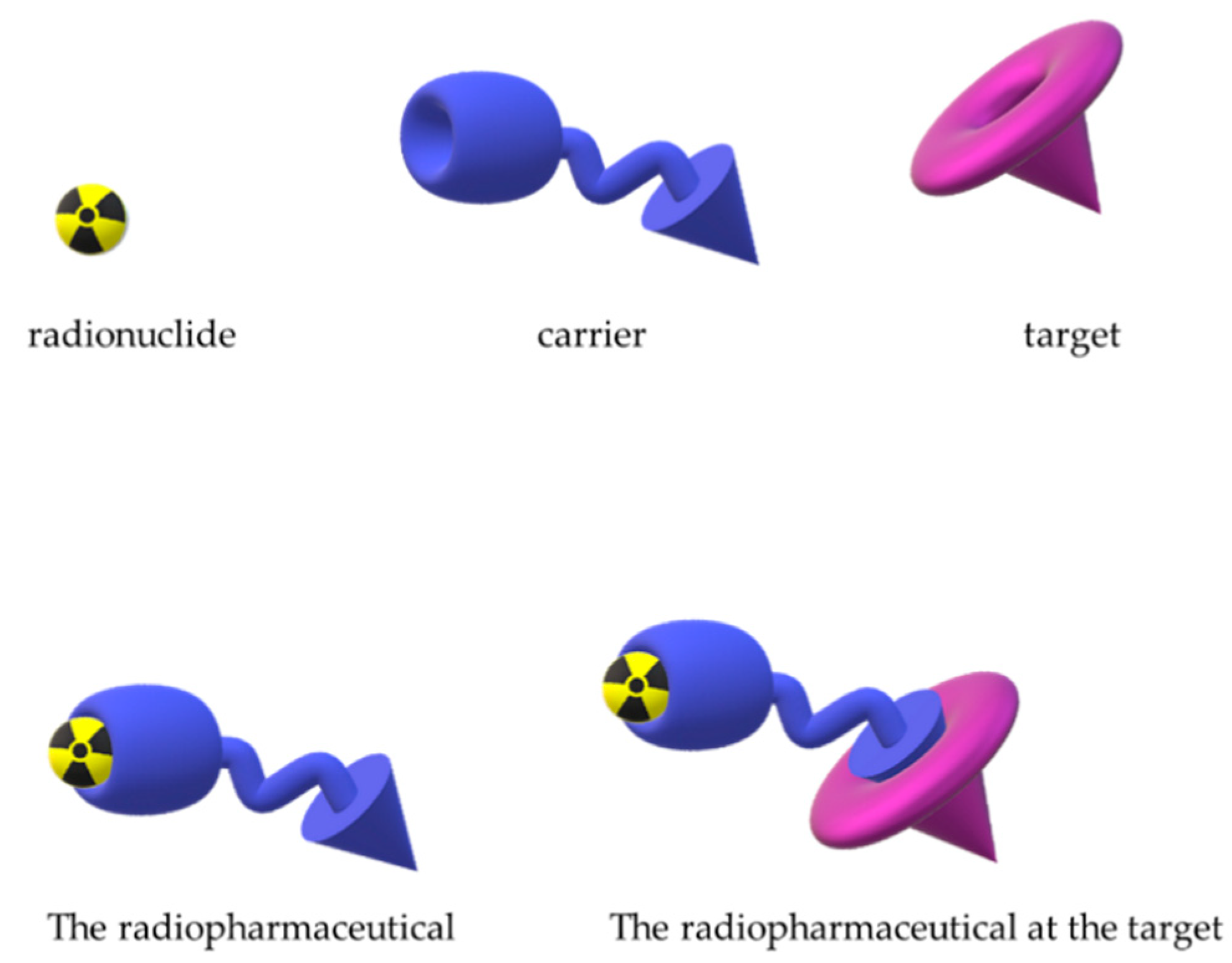
1. Introduction
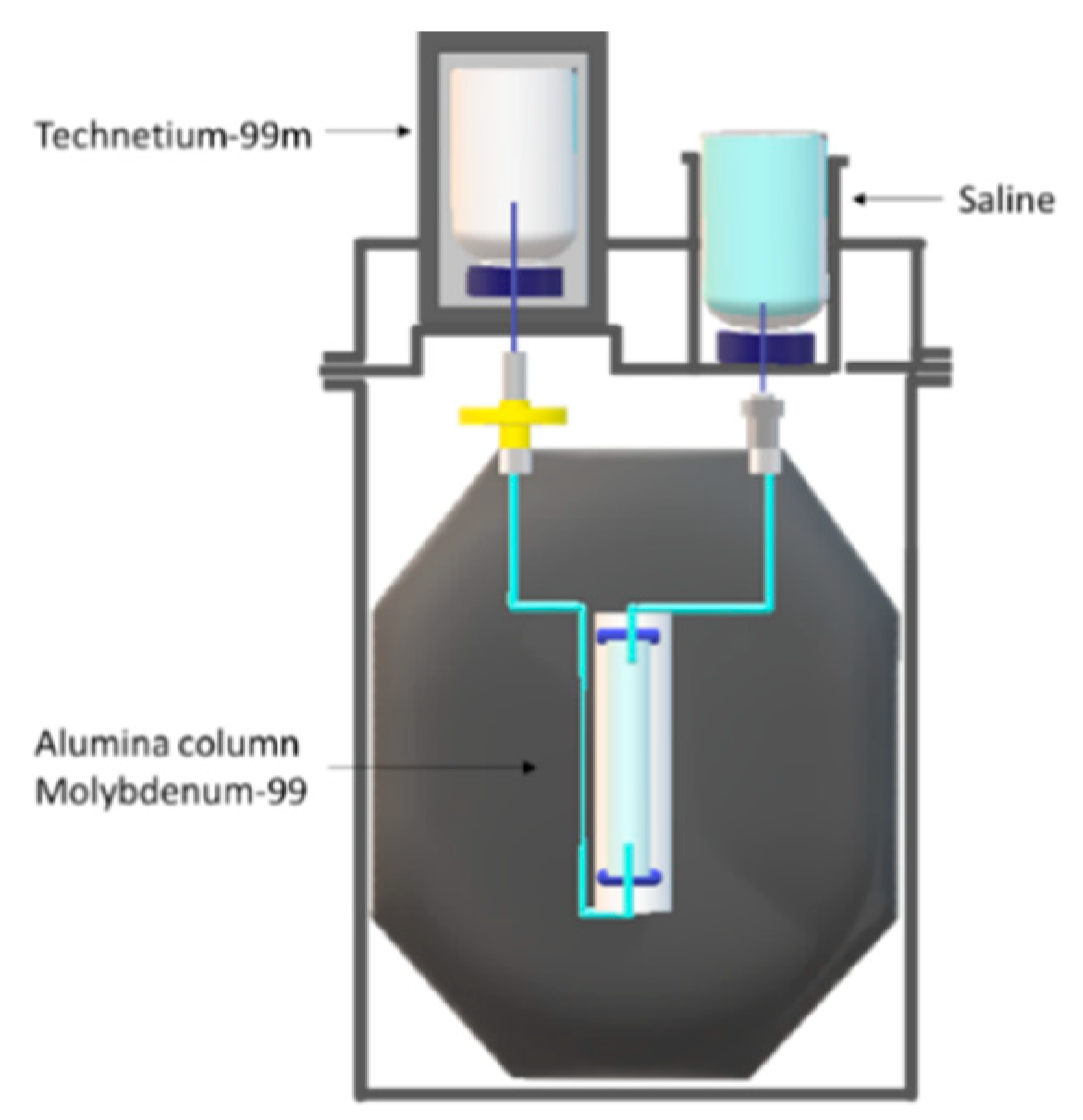
2. Techentium-99m Production Routes
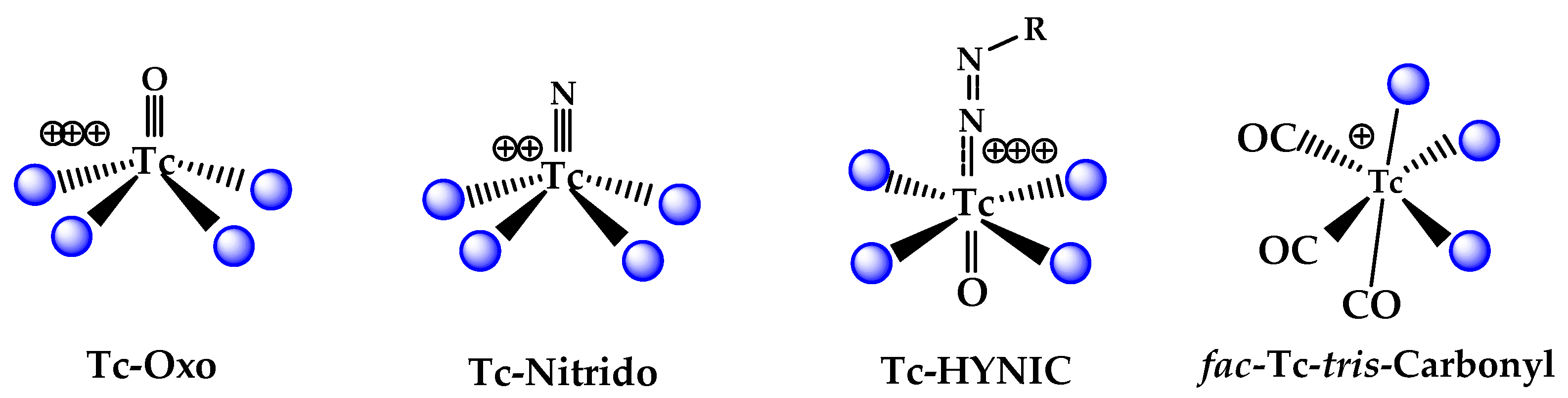
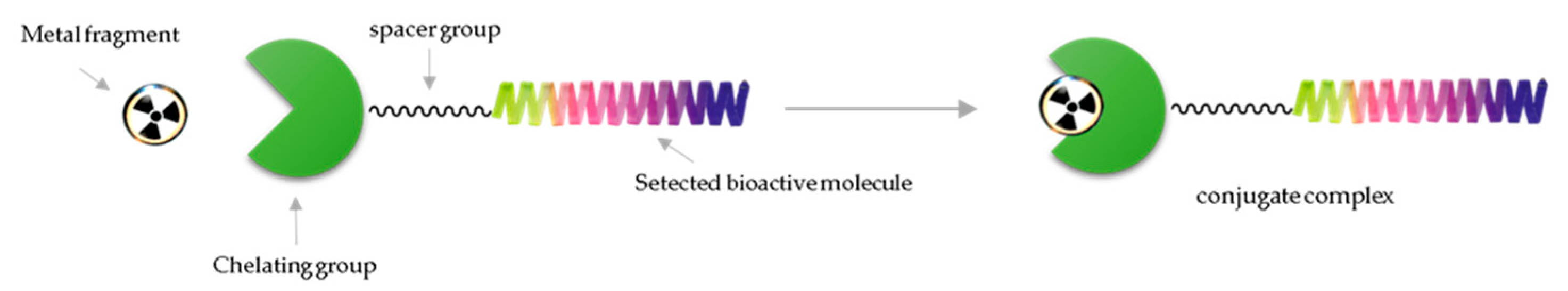
3. Chemical Approaches to Bioactive Molecule Labelling with Technetium-99m
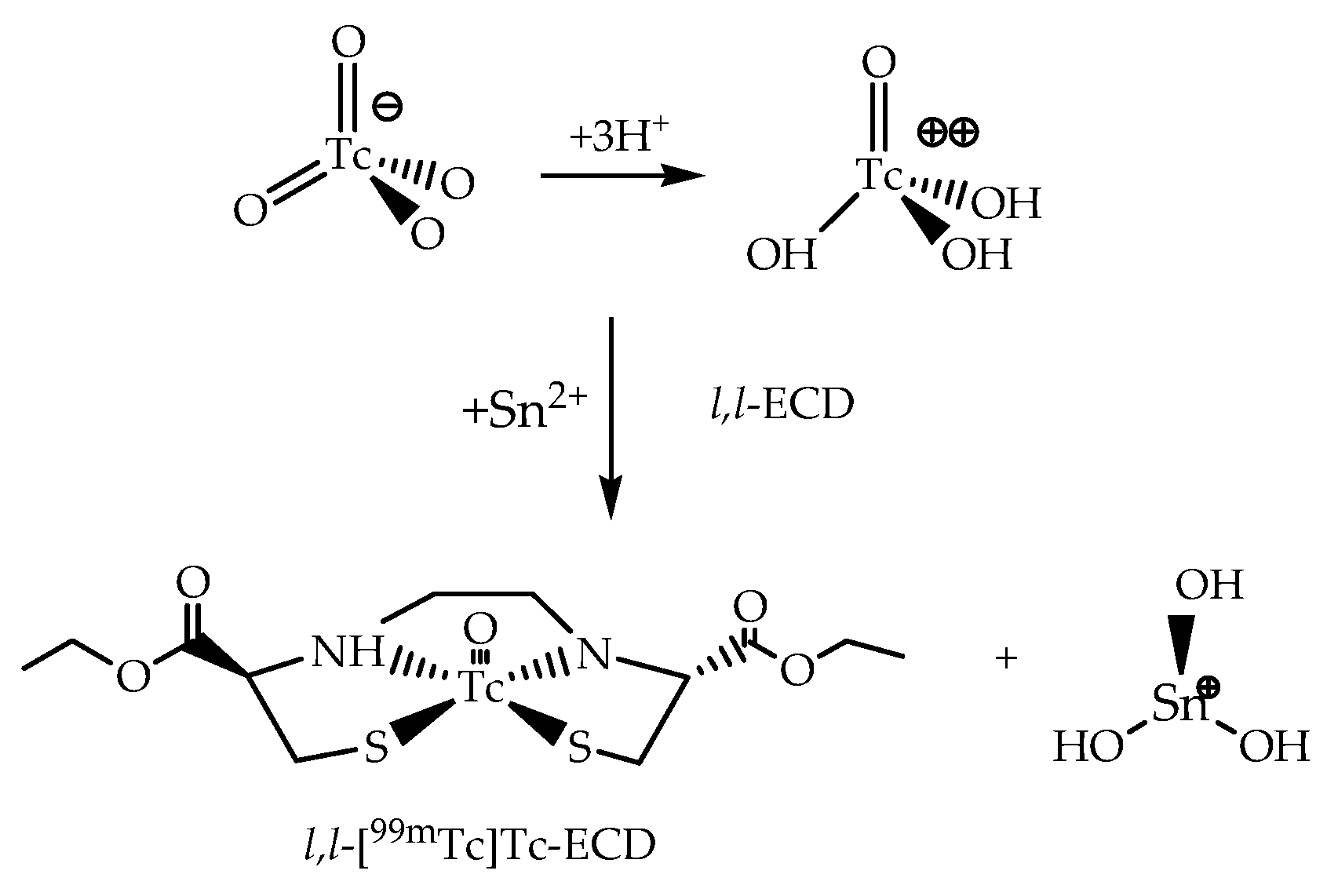
3.1. The 99mTc(V)-Oxo Core
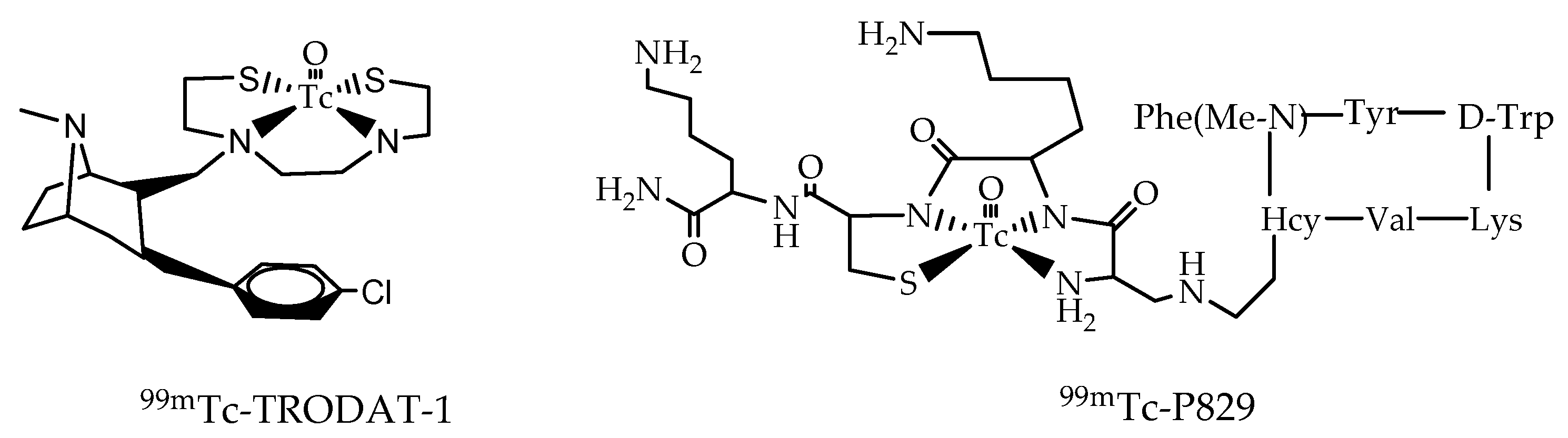
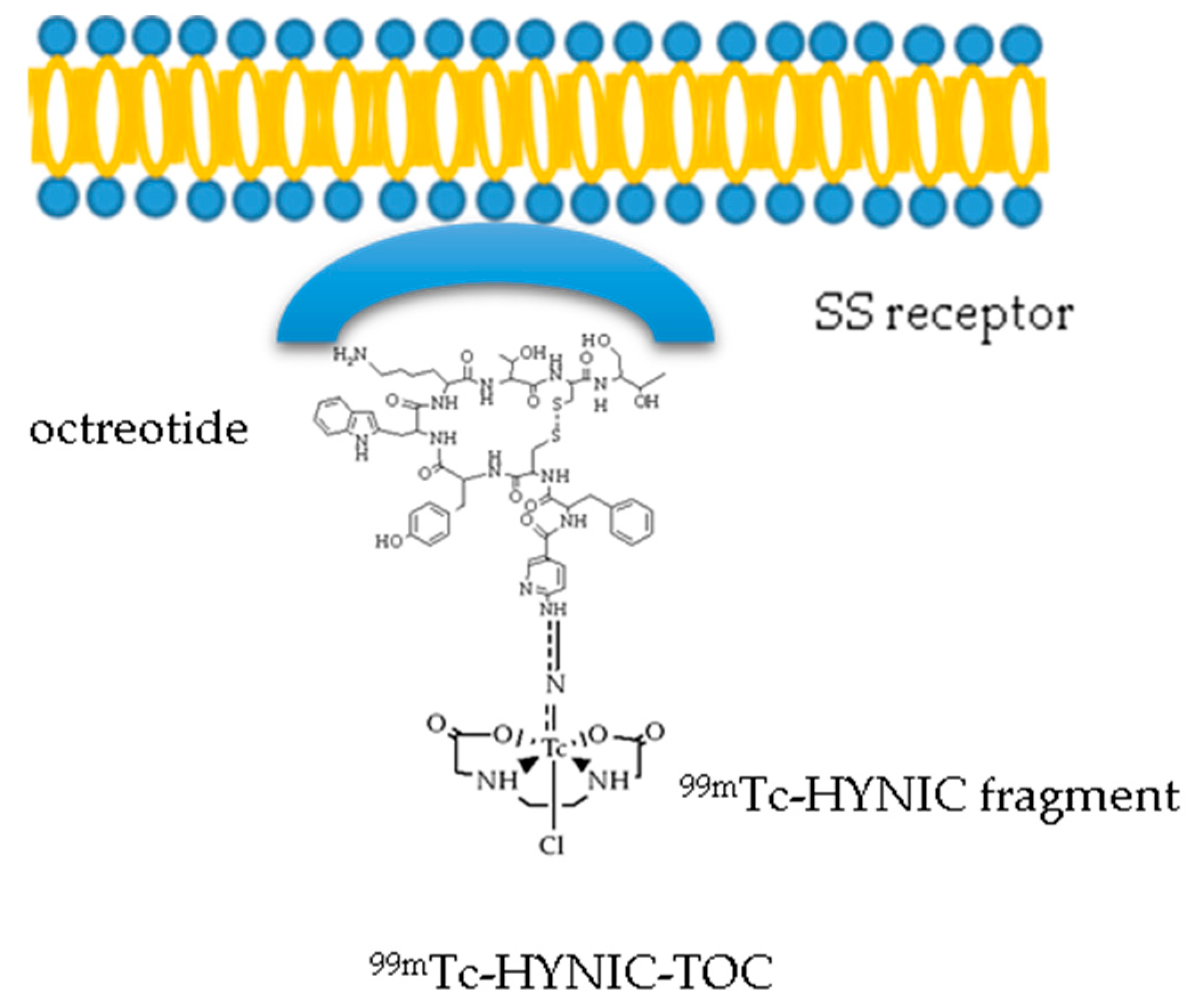
3.2. The 99mTc(V)- Hydrazido Metal Fragment
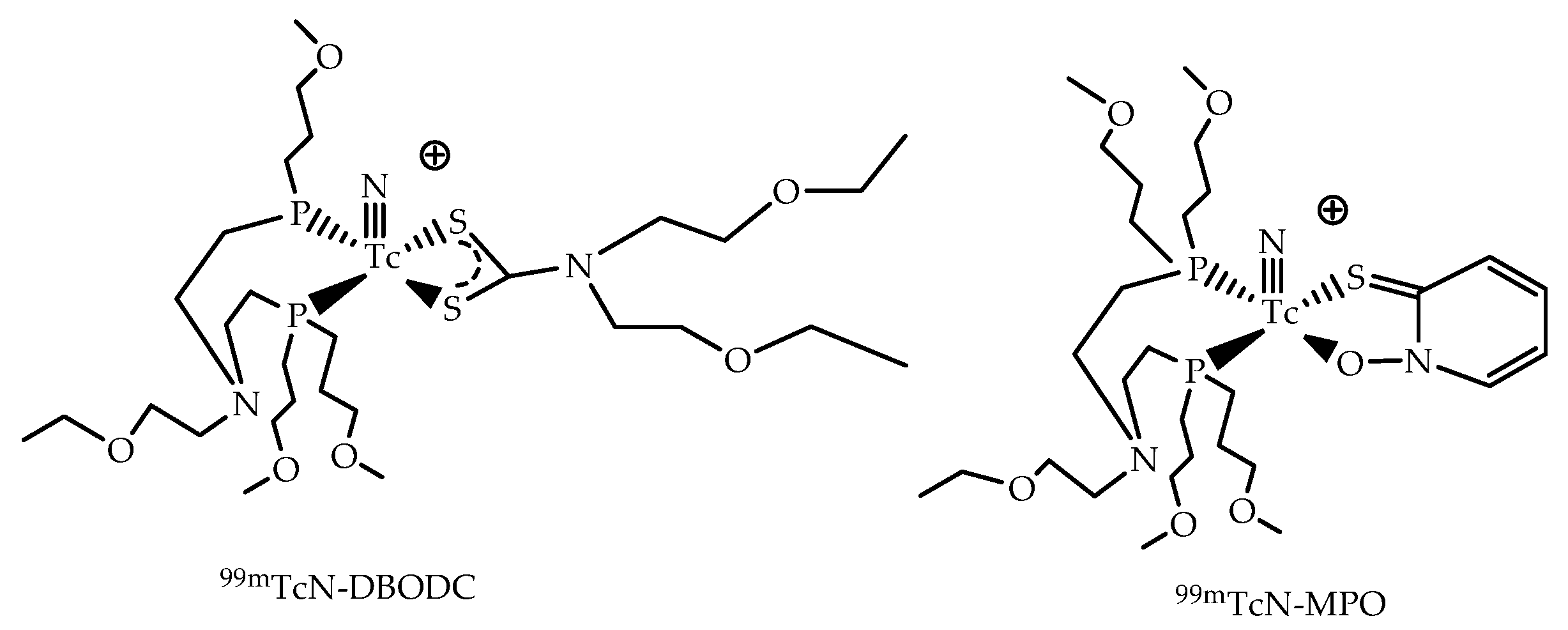
3.3. The [99mTc(N)PNP]2+ Metal Fragment
3.4. The [99mTc][Tc(CO)3]+ Metal Fragment
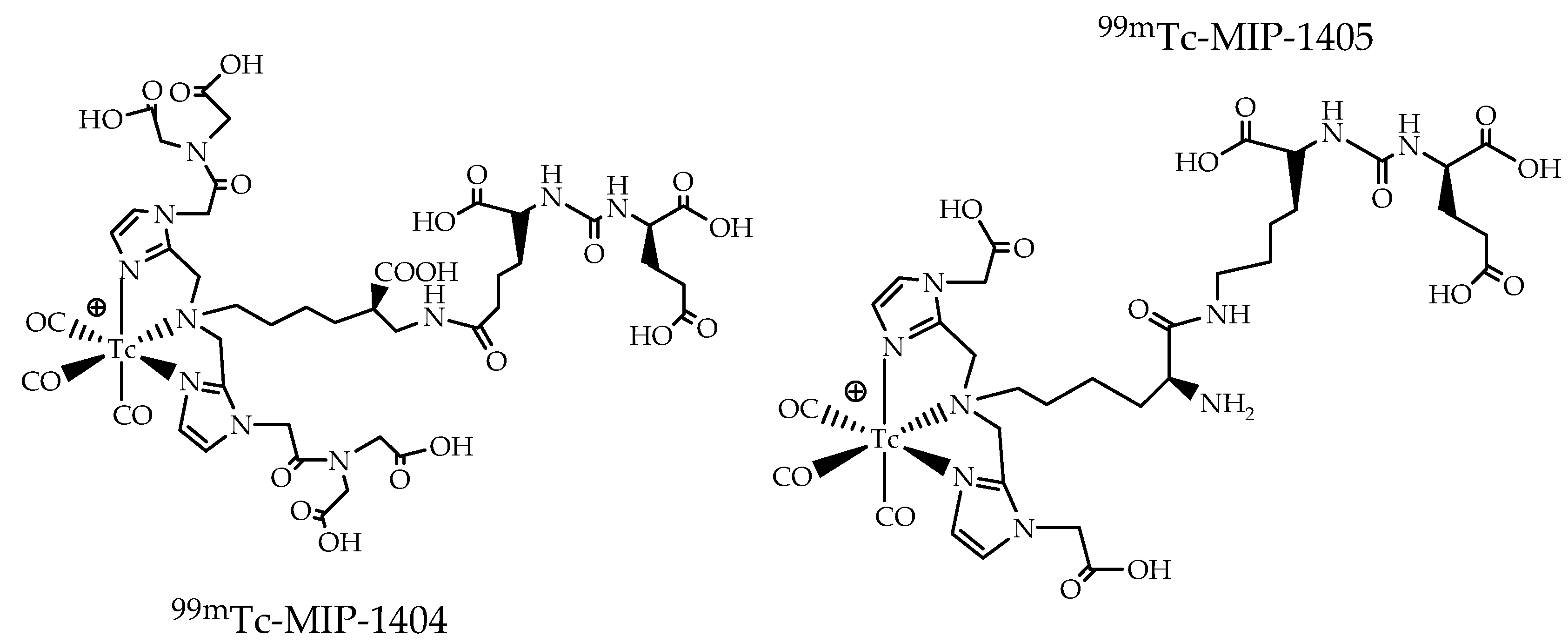
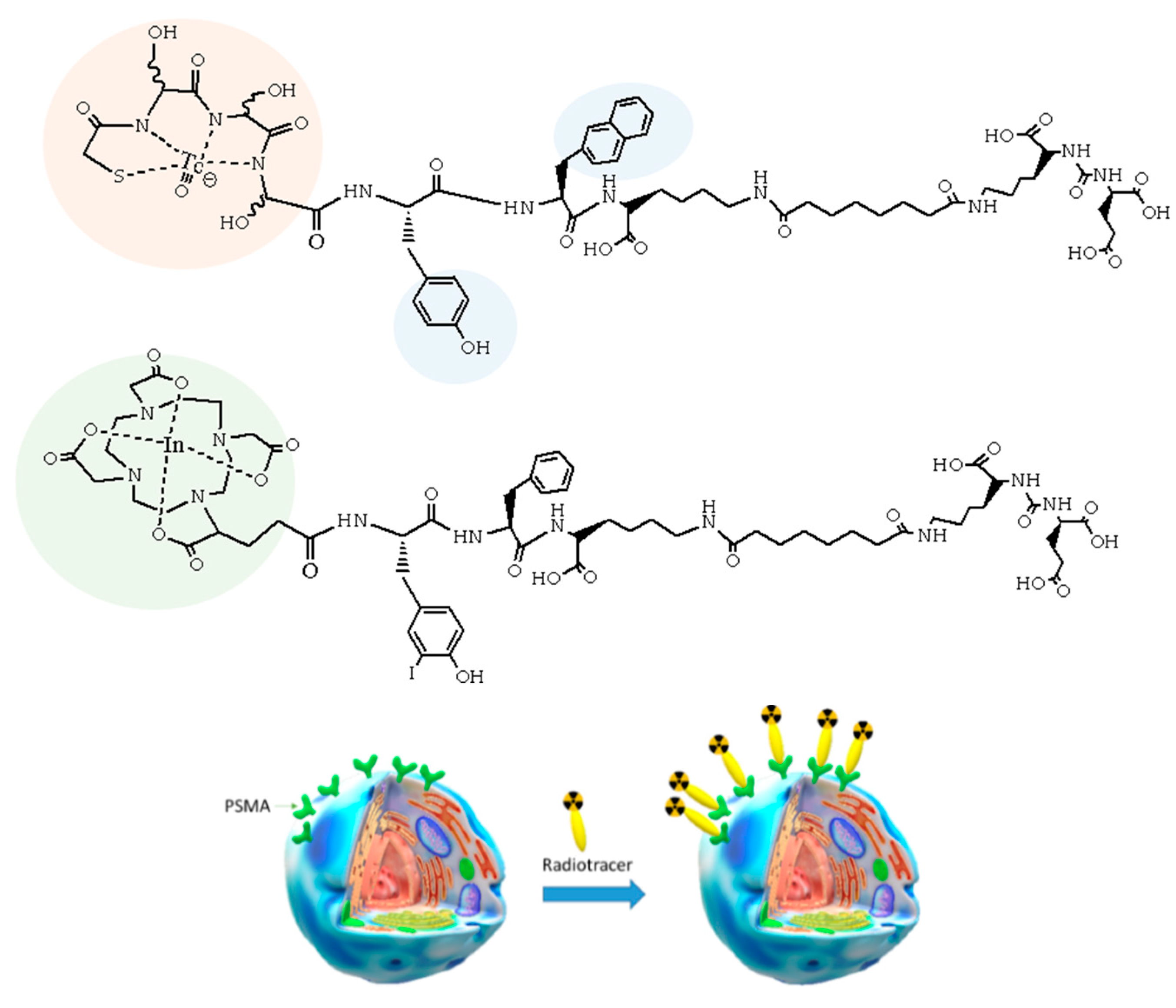
4. The Metal Fragments Approach in the Development of New 99mTc-Based Radiopharmaceuticals for Prostate Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mankoff, D.A. A definition of molecular imaging. J. Nucl. Med. 2007, 48, 18N–21N. [Google Scholar] [PubMed]
- Pysz, M.A.; Gambhir, S.S.; Willmann, J.K. Molecular Imaging: Current Status and Emerging Strategies. Clin. Radiol. 2010, 65, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Qaim, S.M.; Spahn, I. Development of novel radionuclides for medical applications. J. Label. Comp. Radiopharm. 2018, 61, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Boschi, A.; Martini, P.; Uccelli, L. 188Re(V) Nitrido Radiopharmaceuticals for Radionuclide Therapy. Pharmaceuticals 2017, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Mottaghy, F.M. Current and future aspects of molecular imaging. Methods 2009, 48, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, L.; Martini, M.; Cittanti, C.; Carnevale, A.; Missiroli, L.; Giganti, M.; Bartolomei, M.; Boschi, A. Therapeutic Radiometals: Worldwide Scientific Literature Trend Analysis (2008–2018). Molecules 2019, 24, 640. [Google Scholar] [CrossRef] [PubMed]
- Qaim, S.M. Nuclear data for medical applications: An overview. Radiochim. Acta 2001, 89, 189–196. [Google Scholar] [CrossRef]
- Boschi, A.; Martini, P.; Janevik-Ivanovska, E.; Duatti, A. The emerging role of copper-64 radiopharmaceuticals as cancer theranostics. Drug Discov. Today 2018, 23, 1489–1501. [Google Scholar] [CrossRef]
- Bartholoma, M.D.; Louie, A.S.; Valliant, J.F.; Zubieta, J. Technetium and Gallium Derived Radiopharmaceuticals: Comparing and Contrasting the Chemistry of Two Important Radiometals for the Molecular Imaging Era. Chem. Rev. 2010, 110, 2903–2920. [Google Scholar] [CrossRef]
- Uccelli, L.; Martini, P.; Pasquali, M.; Boschi, A. Monoclonal Antibodies Radiolabeling with Rhenium-188 for Radioimmunotherapy. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Rathmann, S.M.; Ahmad, Z.; Slikboer, S.; Bilton, H.A.; Snider, D.P.; Valliant, J.F. The Radiopharmaceutical Chemistry of Technetium-99m. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 311–333. [Google Scholar]
- Liu, S. Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Adv. Drug Deliv. Rev. 2008, 60, 1347–1370. [Google Scholar] [CrossRef] [PubMed]
- Jurisson, S.S.; Lydon, J.D. Potential Technetium Small Molecule Radiopharmaceuticals. Chem. Rev. 1999, 99, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Herschman, H.R. Molecular imaging: Looking at problems, seeing solutions. Science 2003, 302, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.V.; Faber, T.L. Advances in nuclear cardiology instrumentation: Clinical potential of SPECT and PET. Curr. Cardiovasc. Imaging Rep. 2009, 2, 230–237. [Google Scholar] [CrossRef]
- IAEA. Technetium-99m Radiopharmaceuticals: Status and Trends; IAEA Radioisotopes and Radiopharmaceuticals Series; International Atomic Energy Agency: Vienna, Austria, 2009; No. 1. [Google Scholar]
- Alenazy, A.B.; Wells, R.G.; Ruddy, T.D. New solid state cadmium-zinc-telluride technology for cardiac single photon emission computed tomographic myocardial perfusion imaging. Expert Rev. Med. Devices 2017, 14, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Molinski, V.J. A review of 99mTc generator technology. Int. J. Appl. Radiat. Isot. 1982, 33, 811–819. [Google Scholar] [CrossRef]
- Uccelli, L.; Martini, P.; Pasquali, M.; Boschi, A. Radiochemical purity and stability of 99mTc-HMPAO in routine preparations. J. Radioanal. Nucl. Chem. 2017, 314, 1177–1181. [Google Scholar] [CrossRef]
- Ross, C.A.; Diamond, W.T. Predictions regarding the supply of 99Mo and 99mTc when NRU ceases production in 2018. Phys. Can. 2015, 71, 131–138. [Google Scholar]
- Opportunities and Approaches for Supplying Molybdenum-99 and Associated Medical Isotopes to Global Markets Proceedings of a Symposium 2018. Available online: http://www.nap.edu/24909 (accessed on 15 May 2019).
- Boschi, A.; Martini, P.; Pasquali, M.; Uccelli, L. Recent achievements in Tc-99m radiopharmaceutical direct production by medical cyclotrons. Drug Dev. Ind. Pharm. 2017, 43, 1402–1412. [Google Scholar] [CrossRef]
- Uzunov, N.; Melendez-Alafort, L.; Bello, M.; Cicoria, G.; Zagni, F.; De Nardo, L.; Selva, A.; Mou, L.; Rossi-Alvarez, C.; Pupillo, G.; et al. Radioisotopic purity and imaging properties of cyclotron-produced. 99mTc using direct 100Mo(p,2n) reaction. Phys. Med. Biol. 2018, 63, 185021. [Google Scholar] [CrossRef]
- Capogni, M.; Pietropaolo, A.; Quintieri, L.; Angelone, M.; Boschi, A.; Capone, M.; Cherubini, N.; De Felice, P.; Dodaro, A.; Duatti, A. 14 MeV Neutrons for 99Mo/99mTc Production: Experiments, Simulations and Perspectives. Molecules 2018, 23, 1872. [Google Scholar] [CrossRef] [PubMed]
- Esposito, J.; Bettoni, D.; Boschi, A.; Calderolla, M.; Cisternino, S.; Fiorentini, G.; Keppel, G.; Martini, P.; Maggiore, M.; Mou, L.; et al. LARAMED: A Laboratory for Radioisotopes of Medical Interest. Molecules 2019, 24, 20. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yamamoto, M.; Uesaka, M. Design of an X-band electron linear accelerator dedicated to decentralized 99Mo/99mTc supply: From beam energy selection to yield estimation. Phys. Rev. Accel. Beams 2017, 20, 104701. [Google Scholar] [CrossRef]
- Martini, P.; Boschi, A.; Cicoria, G.; Zagni, F.; Corazza, A.; Uccelli, L.; Pasquali, M.; Pupillo, G.; Marengo, M.; Loriggiola, M.; et al. In-House Cyclotron Production of High-Purity Tc-99m and Tc-99m Radiopharmaceuticals. Appl. Radiat. Isot. 2018, 139, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Rovaisn, M.R.A.; Aardaneh, K.; Aslani, G.; Rahiminejad, A.; Yousefi, K.; Boulou, F. Assessment of the direct cyclotron production of 99mTc: An approach to crisis management of 99mTc shortage. Appl. Radiat. Isot. 2016, 112, 55–61. [Google Scholar] [CrossRef]
- Lei, K.; Rusckowski, M.; Chang, F.; Qu, T.; Mardirossian, G.; Hnatowich, D.J. Technetium-99m antibodies labeled with MAG3 and SHNH: An In Vitro and animal In Vivo comparison. Nucl. Med. Biol. 1996, 23, 917–922. [Google Scholar] [CrossRef]
- Goodbody, A.; Pollak, A. Peptide-Chelator Conjugates for Diagnostic Imaging. PCT International Application W09603427, 8 February 1996. [Google Scholar]
- Van Domselaar, G.H.; Okarvi, S.M.; Fanta, M.; Suresh, M.R.; Wishart, D.S. Synthesis and 99mTc-labelling of bz-MAG3-triprolinyl-peptides, their radiochemical evaluation and in vitro receptor-binding. J. Label. Compd. Radiopharm. 2000, 43, 1193–1204. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Y.; Zhang, Y.; Liu, G.; Liu, N.; Rusckowski, M.; Hnatowich, D.J. A novel and simplified route to the synthesis of N3S chelators for 99mTc labeling. Nucl. Med. Biol. 2001, 28, 703–708. [Google Scholar] [CrossRef]
- Abram, U.I.; Alberto, R. Technetium and rhenium-coordination chemistry and nuclear medical applications. J. Braz. Chem. Soc. 2006, 17, 1486–1500. [Google Scholar] [CrossRef]
- Skaddan, M.B.; Wust, F.R.; Jonson, S.; Syhre, R.; Welch, M.J.; Spies, H.; Katzenellenbogen, J.A. Radiochemical synthesis and tissue distribution of Tc-99m-labeled 7alpha-substituted estradiol complexes. Nucl. Med. Biol. 2000, 27, 269–278. [Google Scholar] [CrossRef]
- Shunichi, O.; Ploessl, K.; Kung, M.P.; Stevenson, D.A. Small and Neutral TcvO BAT, Bisaminoethanethiol (N2S2) Complexes for Developing New Brain Imaging Agents. Nucl. Med. Biol. 1998, 25, 135–140. [Google Scholar]
- Hnatowich, D.J.; Qu, T.; Chang, F.; Ley, A.C.; Ladner, R.C.; Rusckowski, M. Labeling peptides with technetium-99m using a bifunctional chelator of a N-hydroxysuccinimide ester of mercaptoacetyltriglycine. J. Nucl. Med. 1998, 39, 56–64. [Google Scholar] [PubMed]
- Hom, K.R.; Chi, D.Y.; Katzenellenbogen, J.A. Heterodimeric Bis (Amino Thiol) Complexes of Oxorhenium (V) That Mimic the Structure of Steroid Hormones. Synthesis and Stereochemical Issues. J. Org. Chem. 1996, 61, 2624–2631. [Google Scholar] [CrossRef] [PubMed]
- Mozley, P.D.; Schneider, J.S.; Acton, P.D.; Plossl, K.; Stern, M.B.; Siderowf, A.; Leopold, N.A.; Li, P.Y.; Alavi, A.; Kung, H.F. Binding of [99mTc]TRODAT-1 to dopamine transporters in patients with Parkinson’s disease and in healthy volunteers. J. Nucl. Med. 2000, 41, 584–589. [Google Scholar] [PubMed]
- Kung, H.F.; Kung, M.P.; Wey, S.P.; Lin, K.J.; Yen, T.C. Clinical acceptance of a molecular imaging agent: A long march with [99mTc]TRODAT. Nucl. Med. Biol. 2007, 34, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Kung, H.F.; Kim, H.J.; Kung, M.P.; Meegalla, S.K.; Plössl, K.; Lee, H.K. Imaging of dopamine transporters in humans with technetium-99m TRODAT-1. Eur. J. Nucl. Med. 1996, 23, 1527–1530. [Google Scholar] [CrossRef] [PubMed]
- Menda, Y.; Kahn, D. Somatostatin receptor imaging of non-small cell lung cancer with 99mTc depreotide. Semin. Nucl. Med. 2002, 32, 92–96. [Google Scholar] [CrossRef]
- Bååth, M.; Kölbeck, K.G.; Danielsson, R. Somatostatin receptor scintigraphy with 99mTc-Depreotide (NeoSpect) in discriminating between malignant and benign lesions in the diagnosis of lung cancer: A pilot study. Acta Radiol. 2004, 45, 833–839. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Abrams, M.J.; Hauser, M.M.; Gaul, F.E.; Larsen, S.K.; Rauh, D.; Zubieta, J. Preparation of hydrazino-modified proteins and their use for the synthesis of technetium-99m-protein conjugates. Bioconjug. Chem. 1991, 2, 333–336. [Google Scholar] [CrossRef]
- Rose, D.J.; Maresca, K.P.; Nicholson, T.; Davison, A.; Jones, A.G.; Babich, J.; Fischman, A.; Graham, W.; De Bord, J.R.D.; Zubieta, J. Synthesis and Characterization of Organohydrazino Complexes of Technetium, Rhenium, and Molybdenum with the {M(η1-HxNNR)(η2-HyNNR)} Core and Their Relationship to Radiolabeled Organohydrazine-Derivatized Chemotactic Peptides with Diagnostic Applications. Inorg. Chem. 1998, 37, 2701–2716. [Google Scholar] [CrossRef]
- Liu, S. 6-Hydrazinonicotinamide Derivatives as Bifunctional Coupling Agents for 99mTc-Labeling of Small Biomolecules. Top. Curr. Chem. 2008, 252, 117–153. [Google Scholar]
- Liepe, K.; Becker, A. 99mTc-Hynic-TOC imaging in the diagnostic of neuroendocrine tumors. World J. Nucl. Med. 2018, 17, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Trogrlic, M.; Težak, S. Incremental value of 99mTc-HYNIC-TOC SPECT/CT over whole-body planar scintigraphy and SPECT in patients with neuroendocrine tumours. Nuklearmedizin 2017, 56, 97–107. [Google Scholar]
- Czepczyński, R.; Gryczyńska, M.; Ruchała, M. 99mTc-EDDA/HYNIC-TOC in the diagnosis of differentiated thyroid carcinoma refractory to radioiodine treatment. Nucl. Med. Rev. Cent. East. Eur. 2016, 19, 67–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bangard, M.; Béhé, M.; Guhlke, S.; Otte, R.; Bender, H.; Maecke, H.R.; Biersack, H.J. Detection of somatostatin receptor-positive tumours using the new 99mTc-tricine-HYNIC-D-Phe1-Tyr3-octreotide: First results in patients and comparison with 111In-DTPA-D-Phe1-octreotide. Eur. J. Nucl. Med. 2000, 27, 628–637. [Google Scholar] [CrossRef]
- Boschi, A.; Duatti, A.; Uccelli, L. Development of Technetium-99m and Rhenium-188 Radiopharmaceuticals Containing a Terminal Metal–Nitrido Multiple Bond for Diagnosis and Therapy. Top. Curr. Chem. 2005, 252, 85–115. [Google Scholar]
- Cazzola, E.; Benini, E.; Pasquali, M.; Mirtschink, P.; Walther, M.; Pietzsch, H.J.; Uccelli, L.; Boschi, A.; Bolzati, C.; Duatti, A. Labeling of fatty acid ligands with the strong electrophilic metal fragment [99mTc(N)(PNP)]2+ (PNP = diphosphane ligand). Bioconjug. Chem. 2008, 19, 450–460. [Google Scholar] [CrossRef]
- Bu, L.; Li, R.; Jin, Z.; Wen, X.; Liu, S.; Yang, B.; Shen, B.; Chen, X. Evaluation of (99)(m)TcN-MPO as a new myocardial perfusion imaging agent in normal dogs and in an acute myocardial infarction canine model: Comparison with (99)(m)Tc-sestamibi. Mol. Imaging Biol. 2011, 13, 121–127. [Google Scholar] [CrossRef]
- Zheng, Y.; Ji, S.; Tomaselli, E.; Liu, S. Development of kit formulations for (99m)TcN-MPO: A cationic radiotracer for myocardial perfusion imaging. J. Label. Comp. Radiopharm. 2014, 57, 584–592. [Google Scholar] [CrossRef]
- Boschi, A.; Uccelli, L.; Duatti, A.; Bolzati, C.; Refosco, F.; Tisato, F.; Romagnoli, R.; Baraldi, P.G.; Varani, K.; Borea, P.A. Asymmetrical Nitrido Tc-99m Heterocomplexes as Potential Imaging Agents for Benzodiazepine Receptors. Bioconjug. Chem. 2003, 14, 1279–1288. [Google Scholar]
- Bolzati, C.; Mahmood, A.; Malago, E.; Uccelli, L.; Boschi, A.; Jones, A.G.; Refosco, F.; Duatti, A.; Tisato, F. The [99mTc(N)(PNP)]2+ Metal Fragment: A Technetium-Nitrido Synthon for Use with Biologically Active Molecules. The N-(2-Methoxyphenyl)piperazyl-cysteine Analogues as Examples. Bioconjug. Chem. 2003, 14, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Alberto, R.; Schibli, R.; Schubiger, A.P.; Abram, U.; Pietzsch, H.J.; Johannsen, B. First application of fac-[99mTc(OH2)3(CO)3]+ in bioorganometallic chemistry: Design, structure, and In Vitro affinity of a 5-HT1A receptor ligand labeled with 99mTc. J. Am. Chem. Soc. 1999, 121, 6076–6077. [Google Scholar] [CrossRef]
- Bilton, H.A.; Ahmad, Z.; Janzen, N.; Czorny, S.; Valliant, J.F. Preparation and evaluation of 99mTc-labeled tridentate chelates for pre-targeting using bioorthogonal chemistry. J. Vis. Exp. 2017, 120, e55188. [Google Scholar]
- Goffin, K.E.; Joniau, S.; Tenke, P.; Slawin, K.; Klein, E.A.; Stambler, N.; Strack, T.; Babich, J.; Armor, T.; Wong, V. Phase 2 study of 99mTc-trofolastat SPECT/CT to identify and localize prostate cancer in intermediate- and high-risk patients undergoing radical prostatectomy and extended pelvic LN dissection. J. Nucl. Med. 2017, 58, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Alberto, R.; Schibli, R.; Egli, A.; Schubiger, A.P.; Abram, U.; Kaden, T.A. A Novel organometallic aqua complex of technetium for the labeling of biomolecules: Synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4]− in aqueous solution and its reaction with a bifunctional ligand. J. Am. Chem. Soc. 1998, 120, 7987–7988. [Google Scholar] [CrossRef]
- Lipowska, M.; Klenc, J.; Taylor, A.T.; Marzilli, L.G. fac-99mTc/Re-tricarbonyl complexes with tridentate aminocarboxyphosphonate ligands: Suitability of the phosphonate group in chelate ligand design of new imaging agents. Inorganica Chim. Acta 2019, 486, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Mindt, T.L.; Struthers, H.; Brans, L.; Anguelov, T.; Schweinsberg, C.; Maes, V.; Tourwé, D.; Schibli, R. “Click to chelate”: Synthesis and installation of metal chelates into biomolecules in a single step. J. Am. Chem. Soc. 2006, 128, 15096–15097. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P.; et al. Dosimetry of 177Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.J. Lutetium-177 PSMA radioligand therapy of metastatic castration-resistant prostate cancer: Safety and efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef]
- Rahbar, K.; Schmidt, M.; Heinzel, A.; Eppard, E.; Bode, A.; Yordanova, A.; Claesener, M.; Ahmadzadehfar, H. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: A multicenter retrospective analysis. J. Nucl. Med. 2016, 57, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with Lu-177 labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Neels, O.; Müller, M.; Bauder-Wüst, U.; Remde, Y.; Schäfer, M.; Hennrich, U.; Eisenhut, M.; Afshar-Oromieh, A.; Haberkorn, U.; et al. Novel preclinical and radiopharmaceutical aspects of [68Ga] Ga-PSMA-HBED-CC: A new PET tracer for imaging of prostate cancer. Pharmaceuticals 2014, 7, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Haberkorn, U.; Schlemmer, H.; Fenchel, M.; Eder, M.; Eisenhut, M.; Hadaschik, B.A.; Kopp-Schneider, A.; Röthke, M. Comparison of PET/CT and PET/MRI hybrid systems using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: Initial experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.M.; Maresca, K.P.; Lu, G.; Merkin, R.D.; Marquis, J.C.; Zimmerman, C.N.; Eckelman, W.C.; Joyal, J.L.; Babich, J.W. 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen for molecular imaging of prostate cancer. J. Nucl. Med. 2013, 54, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Ferro-Flores, G.; Luna-Gutiérrez, M.; Ocampo-García, B.; Santos-Cuevas, C.; Azorín-Vega, E.; Jiménez-Mancilla, N.; Orocio-Rodríguez, E.; Davanzo, J.; García-Pérez, F.O. Clinical translation of a PSMA inhibitor for 99mTc-based SPECT. Nucl. Med. Biol. 2017, 48, 36–44. [Google Scholar] [CrossRef] [PubMed]
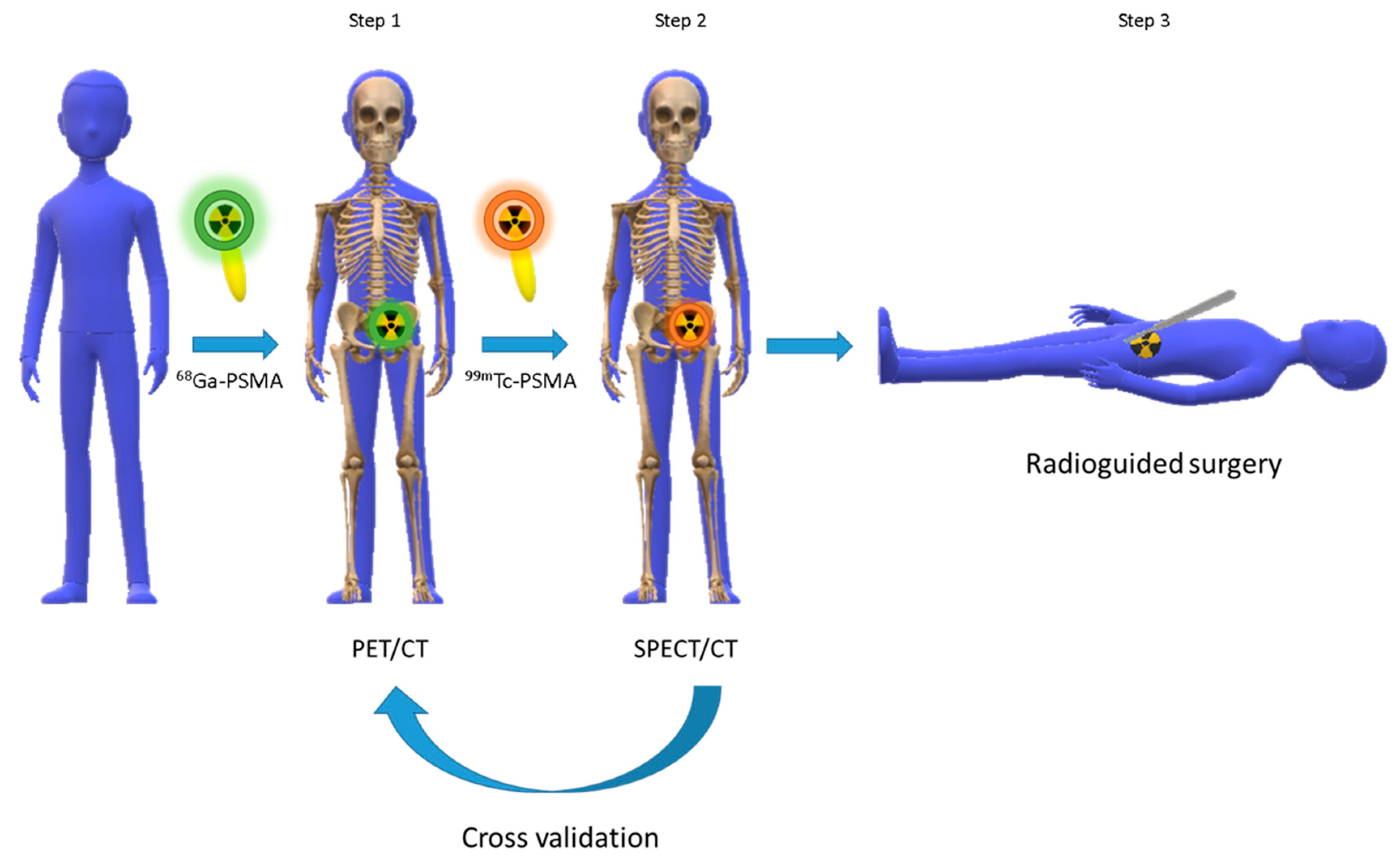
- Robu, S.; Schottelius, M.; Eiber, M.; Maurer, T.; Gschwend, J.; Schwaiger, M.; Wester, H.J. Preclinical Evaluation and First Patient Application of 99mTc-PSMA-I&S for SPECT Imaging and Radioguided Surgery in Prostate Cancer. J. Nucl. Med. 2017, 58, 235–242. [Google Scholar]
- Maurer, T.; Robu, S.; Schottelius, M.; Schwamborn, K.; Rauscher, I.; van den Berg, N.S.; van Leeuwen, F.W.B.; Haller, B.; Horn, T.; Heck, M.M.; et al. 99mTechnetium-Based Prostate-Specific Membrane Antigen-Radioguided Surgery in Recurrent Prostate Cancer. Eur. Urol. 2019, 75, 659–666. [Google Scholar] [CrossRef]
| Reactors | Countries | Six-Day GBq/Mo-99 GBq/year | Start of Service | End of Service |
|---|---|---|---|---|
| LVR-15 | Czech Republic | 3330000 | 1957 | 2028 |
| MARIA | Poland | 3515000 | 1974 | 2030 |
| OPAL | Australia | 3420650 | 2007 | 2055 |
| HFR | The Netherlands | 8946600 | 1961 | 2024 |
| BR2 | Belgium | 6060600 | 1961 | >2026 |
| RA-3 | Argentina | 680800 | 1967 | 2027 |
| SAFARI-1 | South Africa | 4835900 | 1965 | 2030 |
| Reactor Based | Accelerator Based |
|---|---|
| LEU-235U(n,f)99Mo | 238U(γ,f)99Mo |
| nat,98Mo(n,γ)99Mo | 96Zr(α,n)99Mo |
| 100Mo(γ,n)99Mo | |
| 100Mo(p,pn)99Mo | |
| 100Mo(p,2n)99mTc |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschi, A.; Uccelli, L.; Martini, P. A Picture of Modern Tc-99m Radiopharmaceuticals: Production, Chemistry, and Applications in Molecular Imaging. Appl. Sci. 2019, 9, 2526. https://doi.org/10.3390/app9122526
Boschi A, Uccelli L, Martini P. A Picture of Modern Tc-99m Radiopharmaceuticals: Production, Chemistry, and Applications in Molecular Imaging. Applied Sciences. 2019; 9(12):2526. https://doi.org/10.3390/app9122526
Chicago/Turabian StyleBoschi, Alessandra, Licia Uccelli, and Petra Martini. 2019. "A Picture of Modern Tc-99m Radiopharmaceuticals: Production, Chemistry, and Applications in Molecular Imaging" Applied Sciences 9, no. 12: 2526. https://doi.org/10.3390/app9122526
APA StyleBoschi, A., Uccelli, L., & Martini, P. (2019). A Picture of Modern Tc-99m Radiopharmaceuticals: Production, Chemistry, and Applications in Molecular Imaging. Applied Sciences, 9(12), 2526. https://doi.org/10.3390/app9122526







