Unobtrusive Respiratory Flow Monitoring Using a Thermopile Array: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Background and Challenges
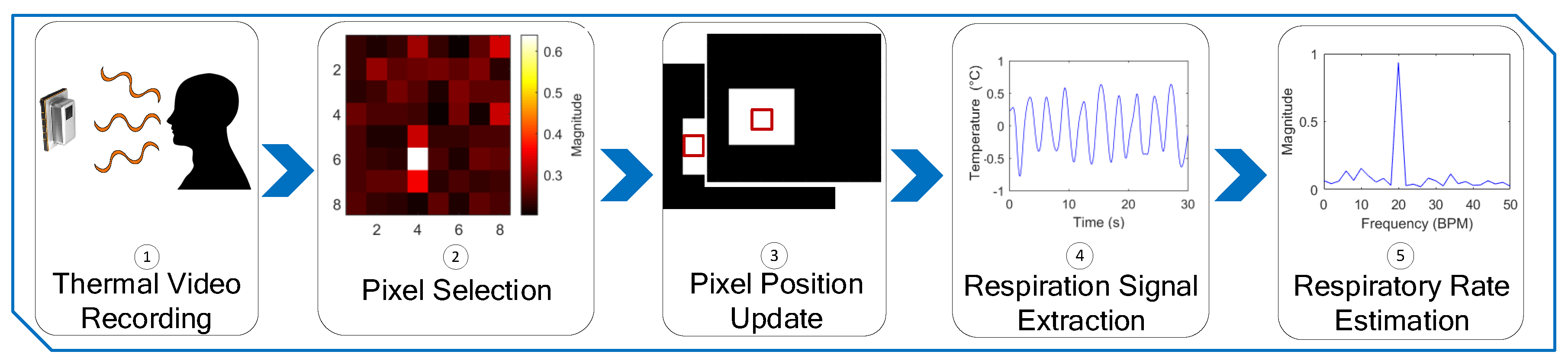
2.2. Method
2.2.1. Thermal Video Collection
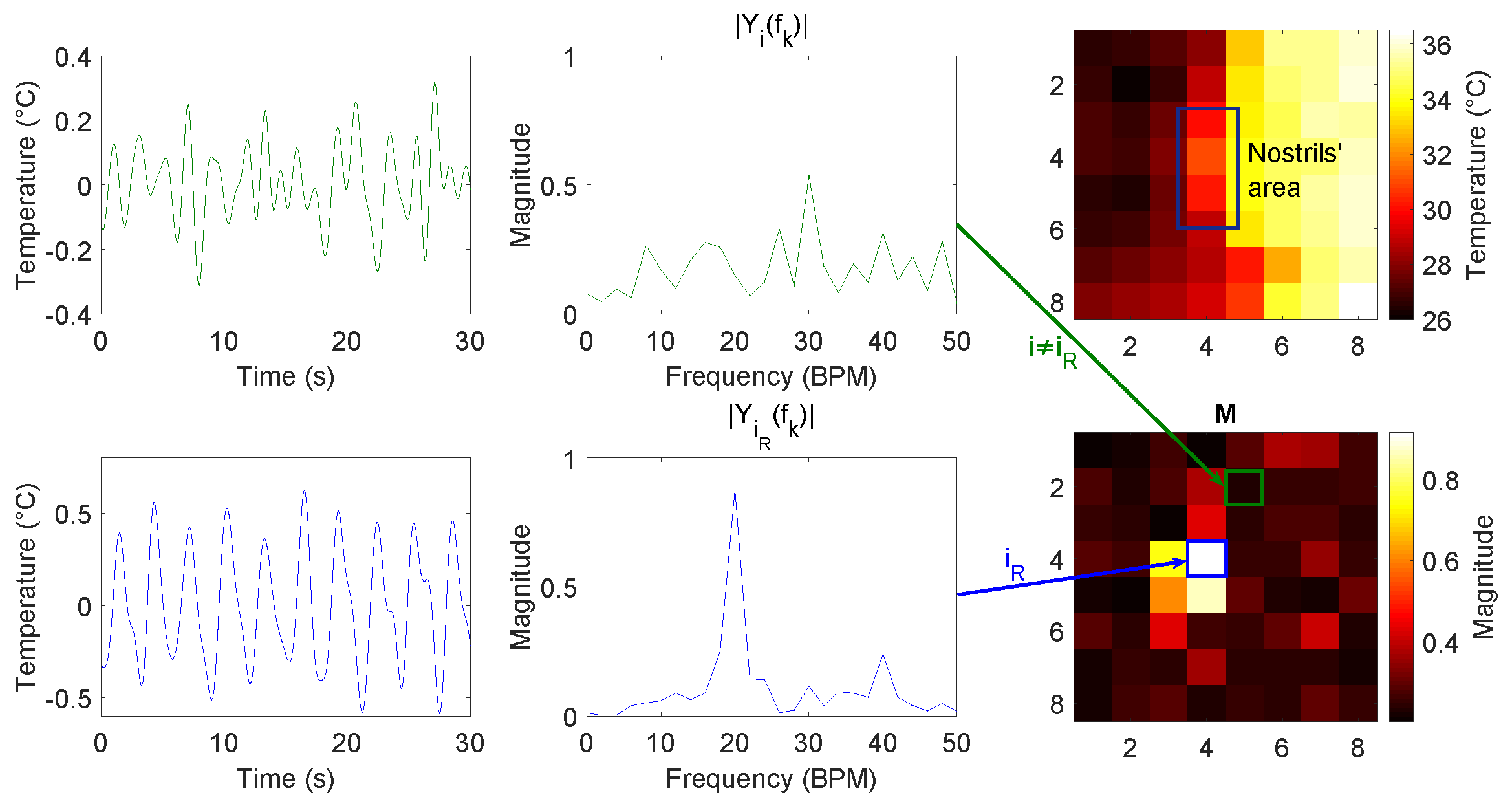
2.2.2. Pixel Selection and Pixel Update
2.2.3. Respiration Signal Extraction and RR Estimation
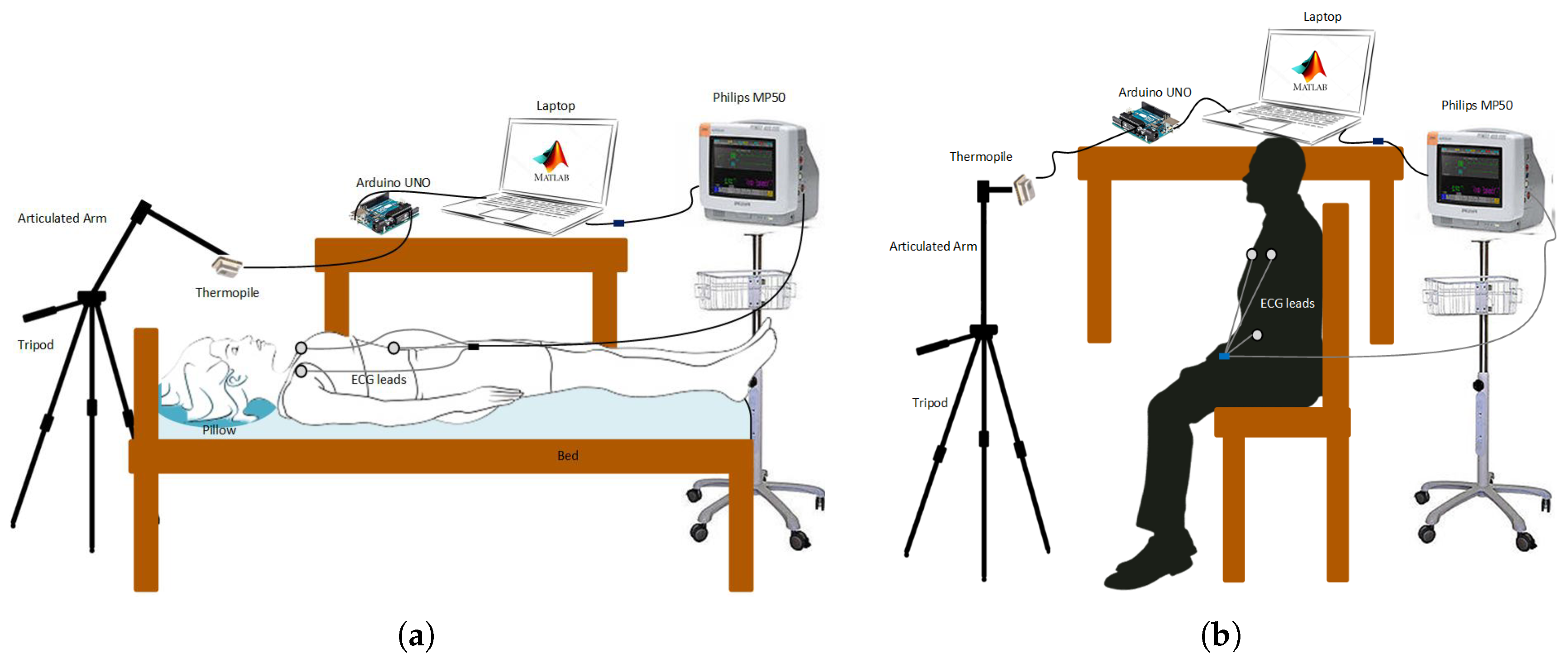
2.3. Experimental Setup
2.4. Datasets
2.4.1. Dataset A: Constant Guided Breathing in Different Settings
- Distance: signals were collected in side view with different distances, i.e., from 10–50 cm, and with constant RR of 20 BPM.
- Respiration rate: different RRs were used as the breathing pattern, i.e., from 10–30 BPM; signals were collected in side view with a constant distance of 20 cm.
- Orientation and oral/nasal respiration: the signals with constant RR of 20 BPM and distance of 20 cm were collected in side and frontal orientations during both nasal and oral breathing.
- Shoulder motion: the signals were collected in a seated position, with a constant RR of 20 BPM and a variable distance, i.e., 50 and 100 cm.
2.4.2. Dataset B: Guided Breathing with Different Patterns
2.5. Evaluation
3. Results
3.1. Dataset A
3.2. Dataset B
4. Discussion
4.1. Dataset A
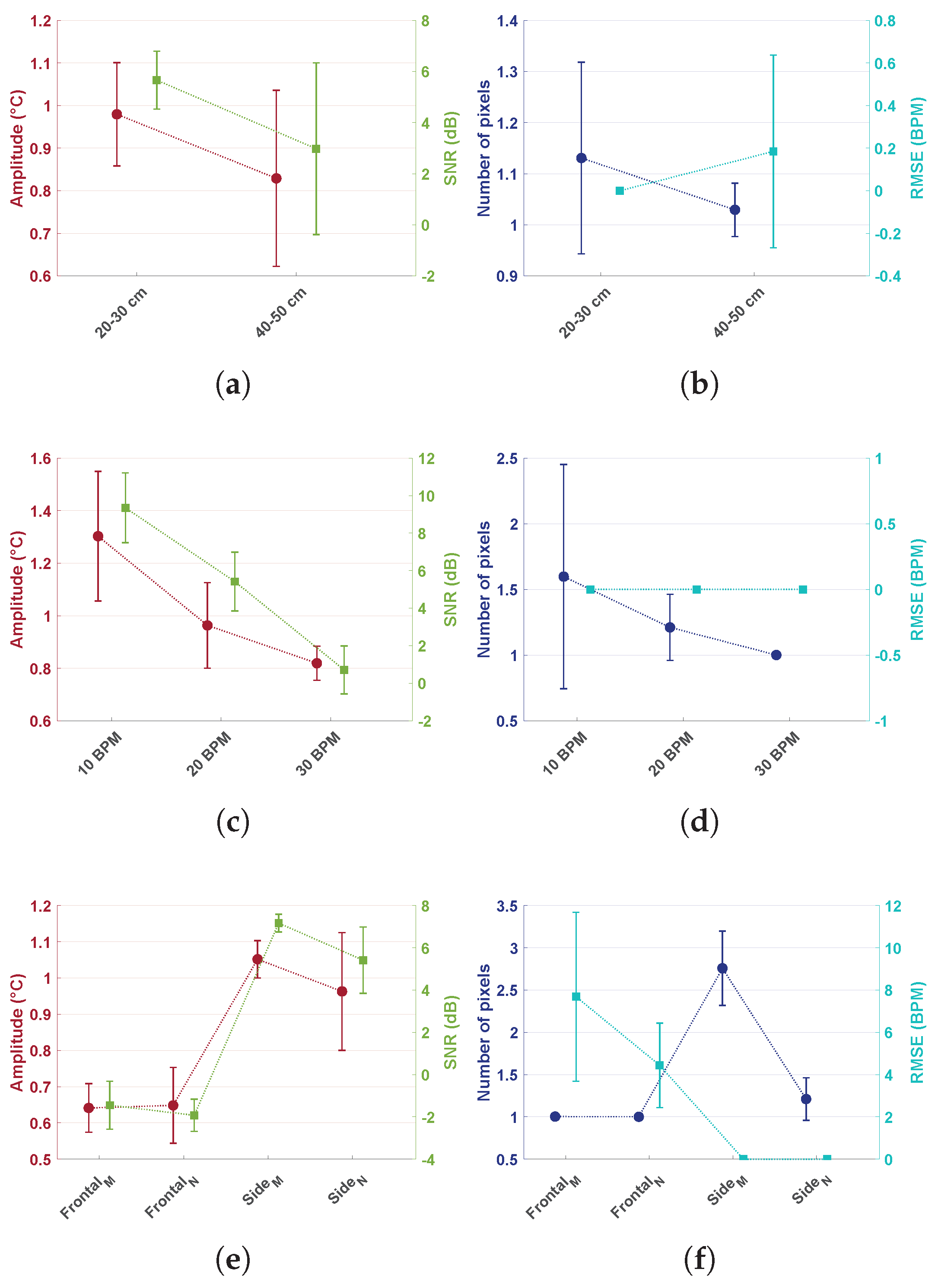
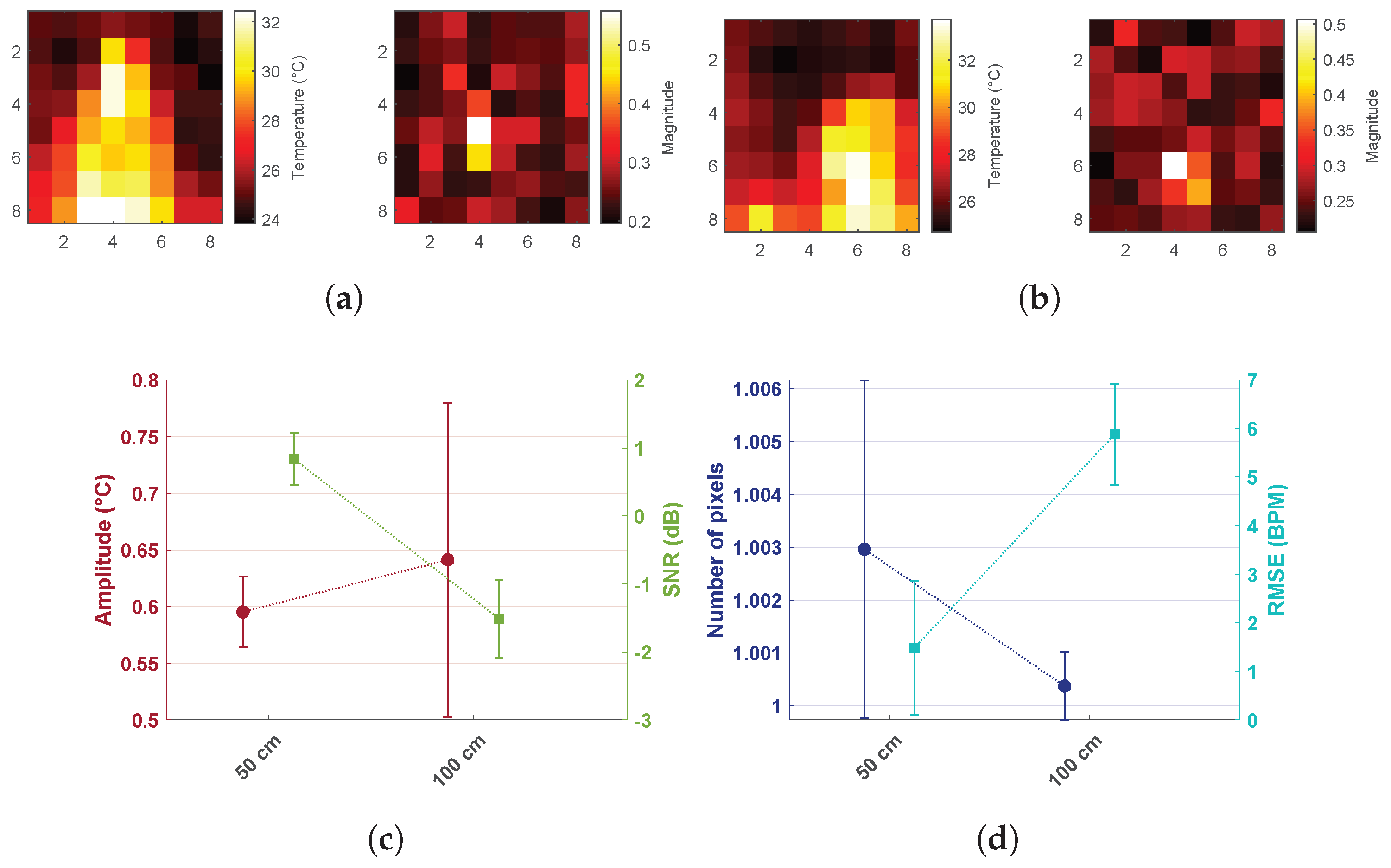
- Distance: Comparison of the performance between different distances proved that the thermopile would lose accuracy at larger distances. In particular, amplitude, SNR, and the number of pixels would decrease with larger distances, and the RMSE would increase. Amplitude decreased from an average of 0.98 °C to 0.83 °C. The SNR reduced from dB to dB, whereas RMSE went from BPM to BPM with a standard deviation of BPM. The number of pixels did not result in a big change on average, but it can be noticed that the standard deviation for the 20–30 cm case was much higher compared to the 40–50 cm case. This behavior was mainly due to the partial area effect. As already expected, the low resolution combined with the broad field of view limited the distance at which the sensor could be used.
- Increasing RR: Breathing with higher RR resulted also in a reduction of the amplitude from 1.30 °C to 0.82 °C, and the SNR drastically reduced from to dB, while the number of pixels from to . The RMSE remained constant. These variations can be considered to be caused by the reduced flow during higher RR. The flow applied to the surroundings would be imposed for less time, due to the higher frequencies, reducing the heat exchanged.
- Orientation and oral/nasal respiration: In this dataset, both orientation and nasal/oral respiration were compared. Frontal measurements were expected to produce a reduced variation in temperature due to the small surface for heat exchanges available. Frontal nasal respiration resulted in performing particularly worse compared to the other settings. That is because the heat would be exchanged mostly with the nasal area around the nostrils. Frontal oral respiration performed better compared to the nasal case, but the RMSE was still much higher compared to the side cases. The improved performance in the frontal oral breathing could be due to the increased surface for heat exchanges, i.e., nose and lower lip, and to the higher temperature of oral expired air. Side oral respiration resulted in causing a bigger temperature variation and higher SNRs. This behavior was expected since in the side case, the pillow’s textile material would be involved in heat exchanges as well, increasing the surface. This setting involved many more pixels, i.e., on average compared to side nasal breathing, which resulted in pixels on average; whereas, the variation in amplitude and SNR was not as big. The differences can be explained by considering the differences in expired air temperature as already indicated in Section 2.1 and differences in the flow direction.
- Shoulder motion: Measurements were collected in a seated position at 50 and 100 cm when the shoulders were included in the field of view. Figure 5a,b shows the two different situations: in the first case, the signal resulting in the maximum peak magnitude was coming from the shoulder area, whereas in the second case (side view with the subject laying down), the signal was coming from the nose area. This was due to the fact that the temperature variation due to respiratory shoulder motion had a higher amplitude compared to the variation due to the heat exchanged because of respiratory flow in the frontal view at the same distance. Shoulder and head motion due to respiration can be suppressed by performing the measurement with the subject lying down. Moreover, due to the partial area effect of the sensor, the error in the detection of respiration through motion increased drastically already at 100 cm.
4.2. Dataset B
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fieselmann, J.F.; Hendryx, M.S.; Helms, C.M.; Wakefield, D.S. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J. Gen. Intern. Med. 1993, 8, 354–360. [Google Scholar] [CrossRef] [PubMed]
- AL-Khalidi, F.Q.; Saatchi, R.; Burke, D.; Elphick, H.; Tan, S. Respiration rate monitoring methods: A review. Pediatr. Pulmonol. 2011, 46, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.B.; Yu, X.; Czaplik, M.; Rossaint, R.; Blazek, V.; Leonhardt, S. Remote monitoring of breathing dynamics using infrared thermography. Biomed. Opt. Express 2015, 6, 4378–4394. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, J.M. Neonatal cardiorespiratory monitoring techniques. Semin. Neonatol. 2004, 9, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Pavlidis, I. Virtual thermistor. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–23 August 2007; pp. 250–253. [Google Scholar]
- Lewis, G.F.; Gatto, R.G.; Porges, S.W. A novel method for extracting respiration rate and relative tidal volume from infrared thermography. Psychophysiology 2011, 48, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Murthy, J.N.; van Jaarsveld, J.; Fei, J.; Pavlidis, I.; Harrykissoon, R.I.; Lucke, J.F.; Faiz, S.; Castriotta, R.J. Thermal infrared imaging: A novel method to monitor airflow during polysomnography. Sleep 2009, 32, 1521–1527. [Google Scholar] [CrossRef][Green Version]
- Fei, J.; Pavlidis, I. Thermistor at a distance: Unobtrusive measurement of breathing. IEEE Trans. Biomed. Eng. 2010, 57, 988–998. [Google Scholar]
- Abbas, A.K.; Heimann, K.; Jergus, K.; Orlikowsky, T.; Leonhardt, S. Neonatal non-contact respiratory monitoring based on real-time infrared thermography. Biomed. Eng. Online 2011, 10, 93. [Google Scholar] [CrossRef]
- Cho, Y.; Julier, S.J.; Marquardt, N.; Bianchi-Berthouze, N. Robust tracking of respiratory rate in high-dynamic range scenes using mobile thermal imaging. Biomed. Opt. Express 2017, 8, 4480–4503. [Google Scholar] [CrossRef] [PubMed]
- Scebba, G.; Tüshaus, L.; Karlen, W. Multispectral camera fusion increases robustness of ROI detection for biosignal estimation with nearables in real-world scenarios. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 5672–5675. [Google Scholar]
- Fraden, J. Handbook of Modern Sensors: Physics, Designs, and Applications; Springer Science & Business Media: New York, NY, USA, 2004. [Google Scholar]
- Mariani, G.; Kenyon, M. Room-temperature remote sensing: Far-infrared imaging based on thermopile technology. In Proceedings of the 2015 40th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW–THz), Hong Kong, China, 23–28 August 2015; pp. 1–2. [Google Scholar]
- Chen, W.H.; Ma, H.P. A fall detection system based on infrared array sensors with tracking capability for the elderly at home. In Proceedings of the 2015 17th International Conference on E-Health Networking, Application & Services (HealthCom), Boston, MA, USA, 14–17 October 2015; pp. 428–434. [Google Scholar]
- Tanaka, J.; Shiozaki, M.; Aita, F.; Seki, T.; Oba, M. Thermopile infrared array sensor for human detector application. In Proceedings of the 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014; pp. 1213–1216. [Google Scholar]
- Deckers, N.; Yildirim, M.; Reulke, R. Sensor Fusion-Based Learning for the Improvement of Person Segmentation by Means of a Low-Resolution Thermal Infrared Array Sensor. In Proceedings of the 2017 International Conference on Computer Graphics and Digital Image Processing, Prague, Czech Republic, 2–4 July 2017; p. 9. [Google Scholar]
- Shetty, A.D.; Shubha, B.; Suryanarayana, K. Detection and tracking of a human using the infrared thermopile array sensor—“Grid-EYE”. In Proceedings of the 2017 International Conference on Intelligent Computing, Instrumentation and Control Technologies (ICICICT), Kannur, India, 6–7 July 2017; pp. 1490–1495. [Google Scholar]
- Sun, G.; Saga, T.; Shimizu, T.; Hakozaki, Y.; Matsui, T. Fever screening of seasonal influenza patients using a cost-effective thermopile array with small pixels for close-range thermometry. Int. J. Infect. Dis. 2014, 25, 56–58. [Google Scholar] [CrossRef]
- Lee, F.F.; Chen, F.; Liu, J. Infrared thermal imaging system on a mobile phone. Sensors 2015, 15, 10166–10179. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.B.; Yu, X.; Goos, T.; Reiss, I.; Orlikowsky, T.; Heimann, K.; Venema, B.; Blazek, V.; Leonhardt, S.; Teichmann, D. Noncontact Monitoring of Respiratory Rate in Newborn Infants Using Thermal Imaging. IEEE Trans. Biomed. Eng. 2018, 66, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.B.; Yu, X.; Czaplik, M.; Blazek, V.; Venema, B.; Leonhardt, S. Estimation of breathing rate in thermal imaging videos: a pilot study on healthy human subjects. J. Clin. Monit. Comput. 2017, 31, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Gade, R.; Moeslund, T.B. Thermal cameras and applications: A survey. Mach. Vis. Appl. 2014, 25, 245–262. [Google Scholar] [CrossRef]
- Abbas, A.K.; Heiman, K.; Orlikowsky, T.; Leonhardt, S. Non-contact respiratory monitoring based on real-time IR–thermography. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009; Springer: Berlin, Germany; pp. 1306–1309. [Google Scholar]
- Troost, M. Presence Detection and Activity Recognition Using Low-Resolution Passive IR Sensors. Master’s Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 3 April 2013. [Google Scholar]
- Höppe, P. Temperatures of expired air under varying climatic conditions. Int. J. Biometeorol. 1981, 25, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.; Mackay, J.; Fahrid, N.; Krucheck, D. Respiratory rate measurement: A comparison of methods. Br. J. Healthc. Assist. 2011, 5, 18–23. [Google Scholar] [CrossRef]
- van Gastel, M.; Stuijk, S.; de Haan, G. Robust respiration detection from remote photoplethysmography. Biomed. Opt. Express 2016, 7, 4941–4957. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.G.; Tarassenko, L. A comparison of signal processing techniques for the extraction of breathing rate from the photoplethysmogram. Int. J. Biol. Med. Sci. 2007, 2, 232–236. [Google Scholar]
- Pardey, J.; Roberts, S.; Tarassenko, L. A review of parametric modelling techniques for EEG analysis. Med. Eng. Phys. 1996, 18, 2–11. [Google Scholar] [CrossRef]
- Salinet, J.L.; Masca, N.; Stafford, P.J.; Ng, G.A.; Schlindwein, F.S. Three-dimensional dominant frequency mapping using autoregressive spectral analysis of atrial electrograms of patients in persistent atrial fibrillation. Biomed. Eng. Online 2016, 15, 28. [Google Scholar] [CrossRef]
- Aydin, S. Determination of autoregressive model orders for seizure detection. Turk. J. Electr. Eng. Comput. Sci. 2010, 18, 23–30. [Google Scholar]
- Chaichulee, S.; Villarroel, M.; Jorge, J.; Arteta, C.; Green, G.; McCormick, K.; Zisserman, A.; Tarassenko, L. Localised photoplethysmography imaging for heart rate estimation of pre-term infants in the clinic. In Proceedings of the Optical Diagnostics and Sensing XVIII: Toward Point-of-Care Diagnostics. International Society for Optics and Photonics, San Francisco, CA, USA, 29–30 January 2018; Volume 10501, p. 105010R. [Google Scholar]
- De Haan, G.; Jeanne, V. Robust pulse rate from chrominance-based rPPG. IEEE Trans. Biomed. Eng. 2013, 60, 2878–2886. [Google Scholar] [CrossRef] [PubMed]
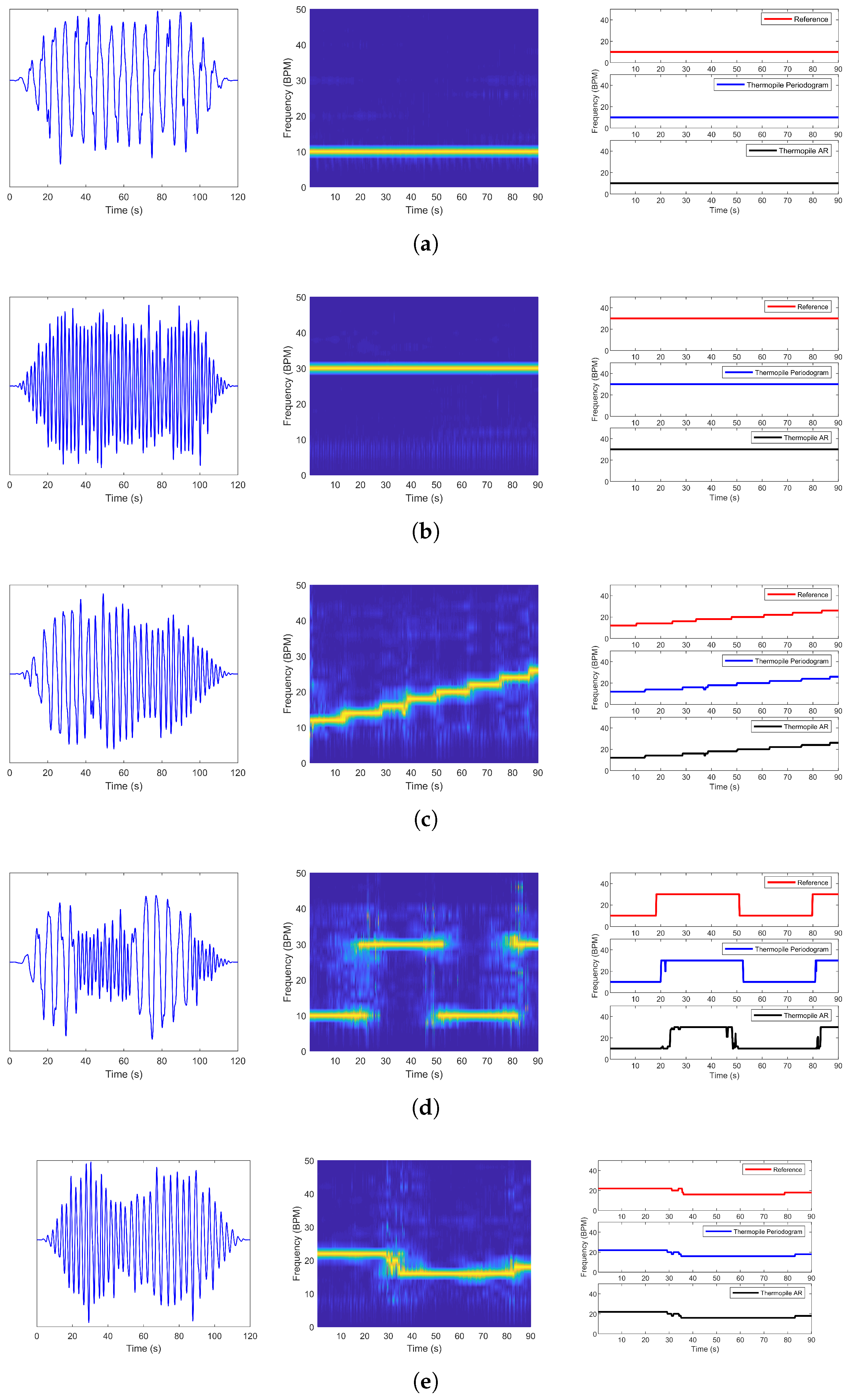

| Breathing Pattern | Periodogram | AR | ||||
|---|---|---|---|---|---|---|
| RMSE (BPM) | RE (%) | SNR (dB) | RMSE (BPM) | RE (%) | SNR (dB) | |
| 10 BPM | 0.00 | 0.00 | 8.71 | 0.00 | 0.00 | 13.59 |
| 30 BPM | 0.00 | 0.00 | 5.15 | 0.00 | 0.00 | 8.15 |
| Chirp | 1.41 | 1.19 | 4.09 | 1.41 | 1.21 | 5.87 |
| Sudden Change | 5.57 | 7.79 | 2.19 | 6.57 | 9.61 | 7.90 |
| Spontaneous | 0.98 | 1.59 | 4.27 | 0.99 | 1.72 | 5.81 |
| Average | 1.59 | 2.11 | 4.88 | 1.79 | 2.51 | 8.26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorato, I.; Bakkes, T.; Stuijk, S.; Meftah, M.; de Haan, G. Unobtrusive Respiratory Flow Monitoring Using a Thermopile Array: A Feasibility Study. Appl. Sci. 2019, 9, 2449. https://doi.org/10.3390/app9122449
Lorato I, Bakkes T, Stuijk S, Meftah M, de Haan G. Unobtrusive Respiratory Flow Monitoring Using a Thermopile Array: A Feasibility Study. Applied Sciences. 2019; 9(12):2449. https://doi.org/10.3390/app9122449
Chicago/Turabian StyleLorato, Ilde, Tom Bakkes, Sander Stuijk, Mohammed Meftah, and Gerard de Haan. 2019. "Unobtrusive Respiratory Flow Monitoring Using a Thermopile Array: A Feasibility Study" Applied Sciences 9, no. 12: 2449. https://doi.org/10.3390/app9122449
APA StyleLorato, I., Bakkes, T., Stuijk, S., Meftah, M., & de Haan, G. (2019). Unobtrusive Respiratory Flow Monitoring Using a Thermopile Array: A Feasibility Study. Applied Sciences, 9(12), 2449. https://doi.org/10.3390/app9122449





