Metal–Organic Frameworks Toward Electrocatalytic Applications
Abstract
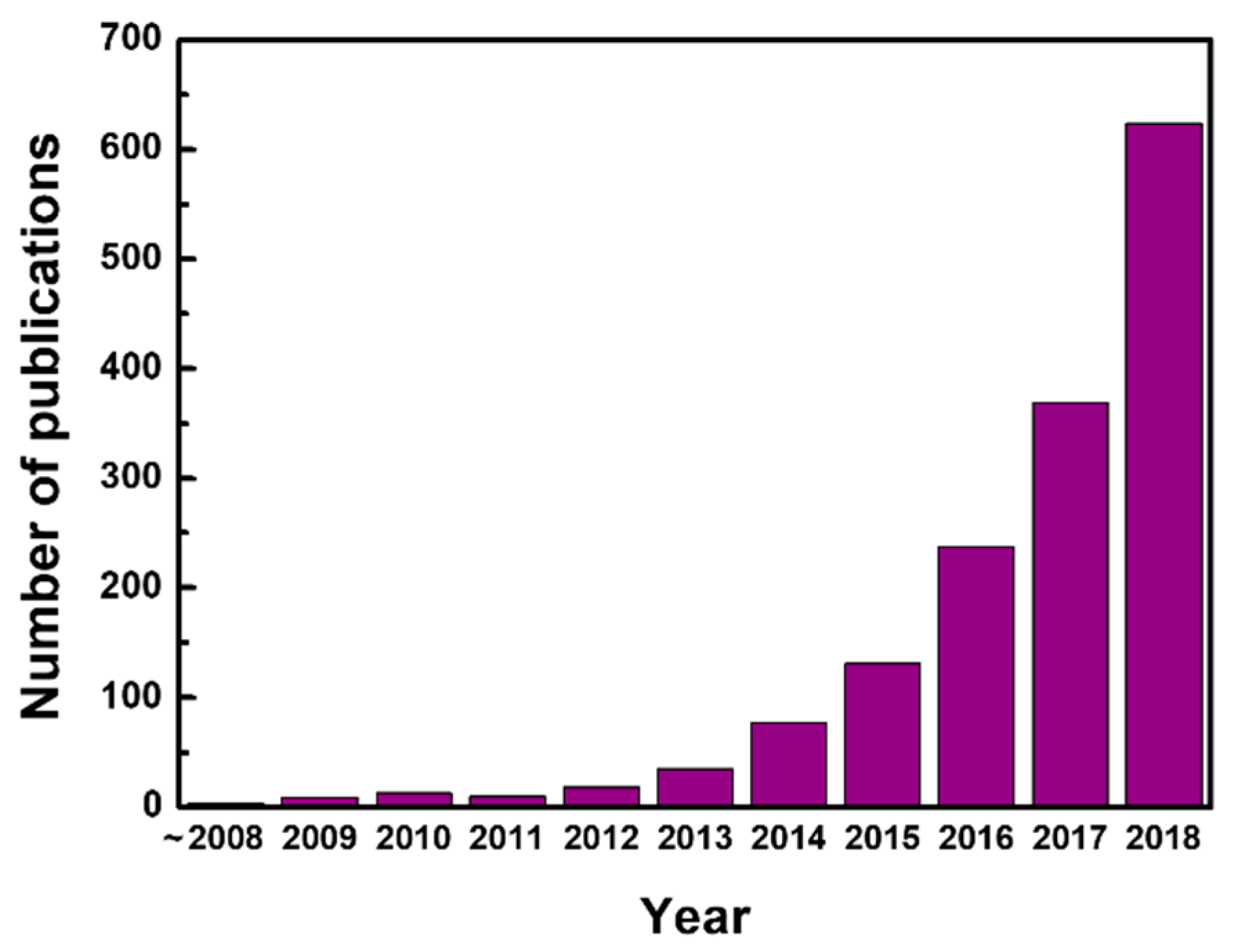
:1. Introduction
2. Charge Transport in Metal–Organic Frameworks (MOFs)
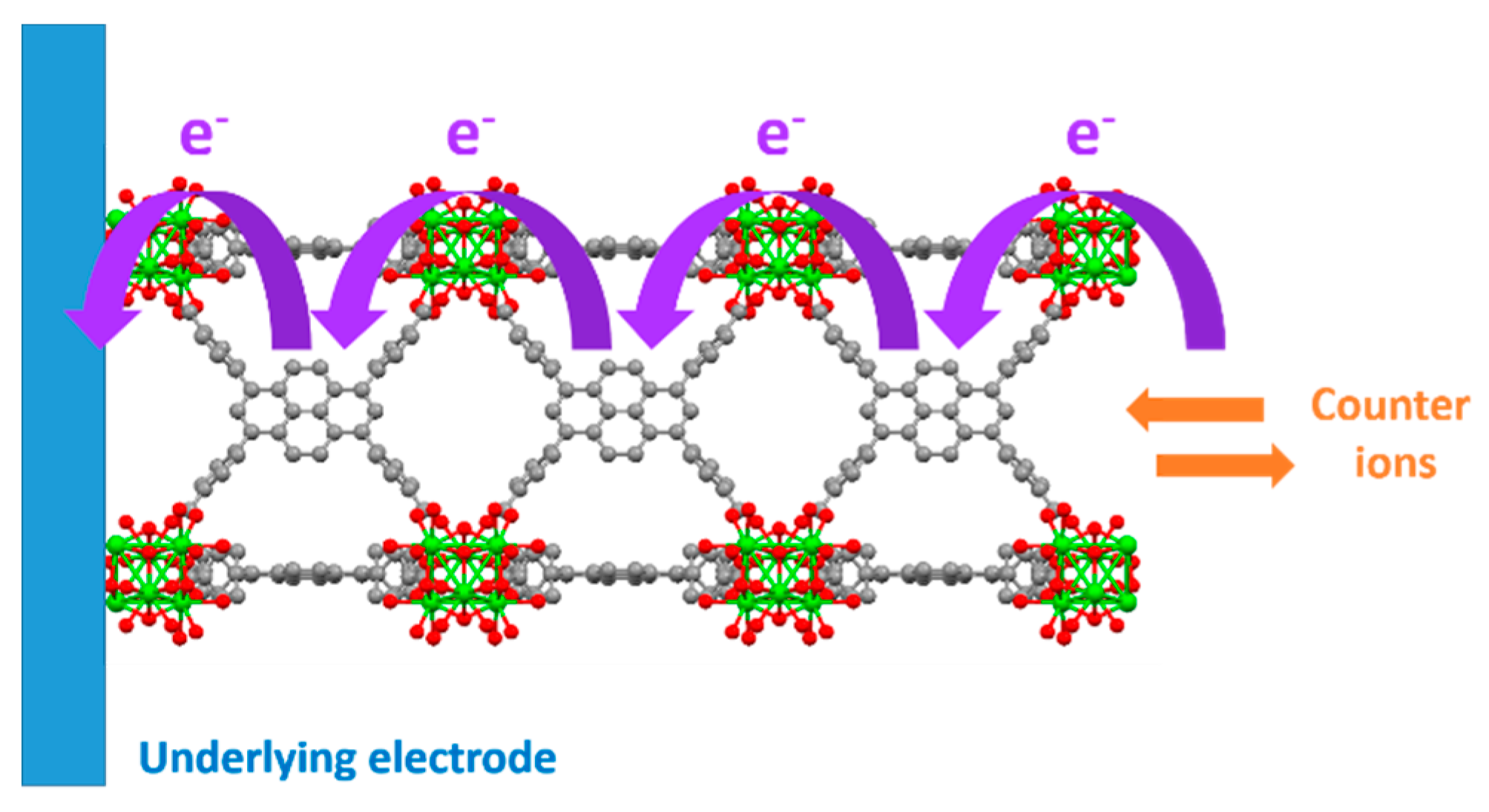
2.1. Redox Hopping
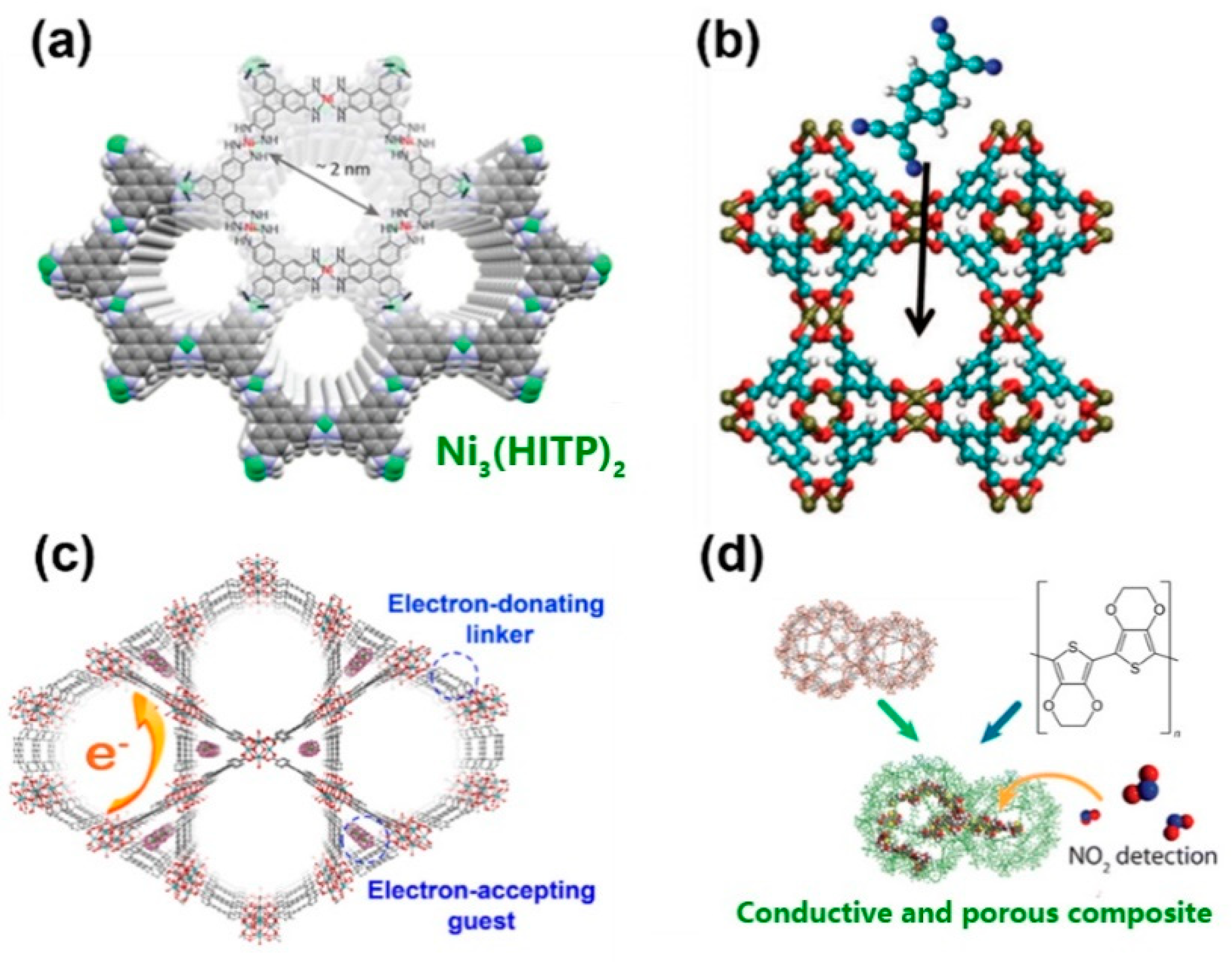
2.2. Band Transport
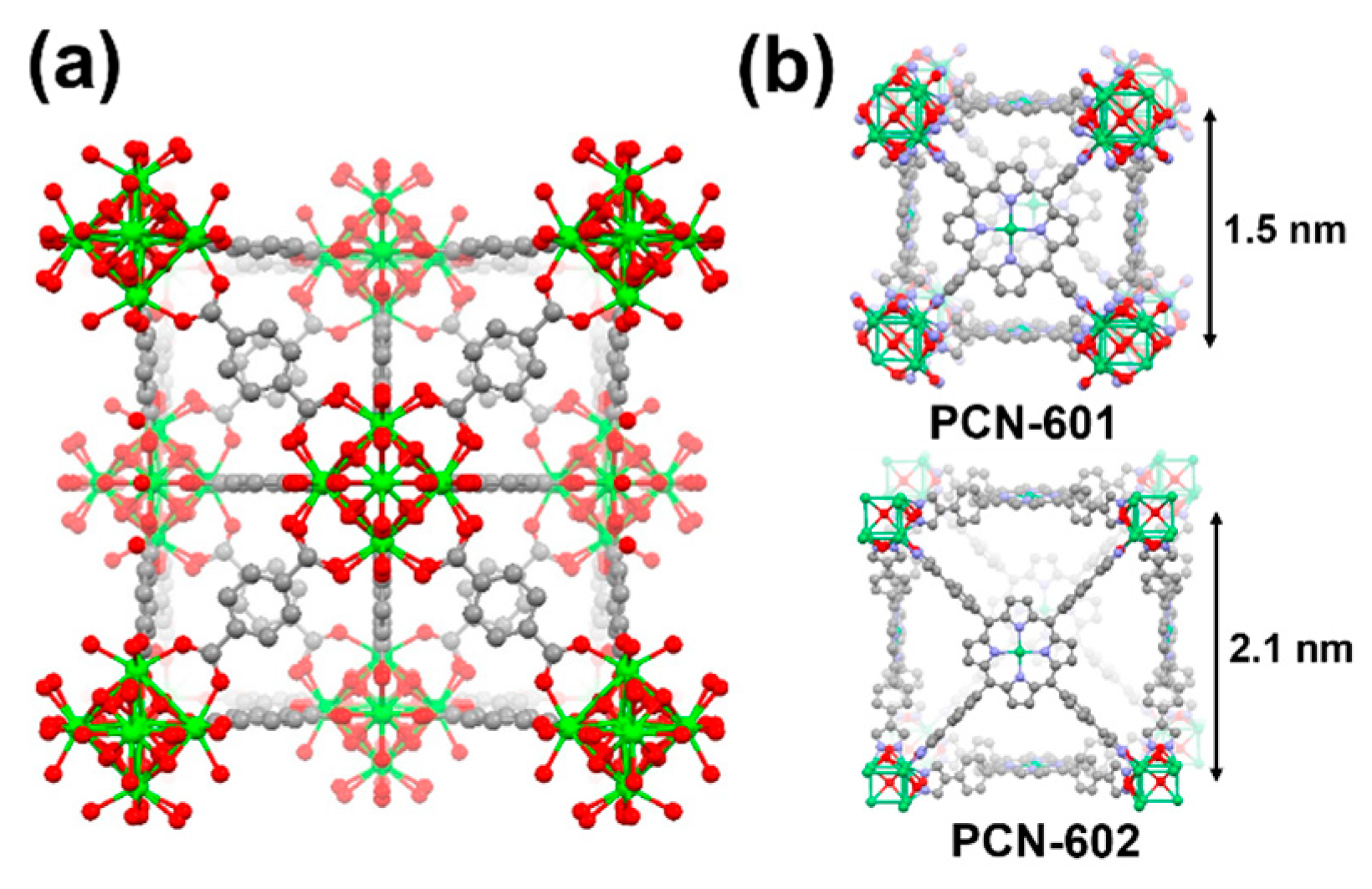
3. The Stability of Metal–Organic Frameworks (MOFs) under Electrochemical Environments
4. Recent Progress in Utilizing MOFs for Electrocatalysis and Relevant Applications
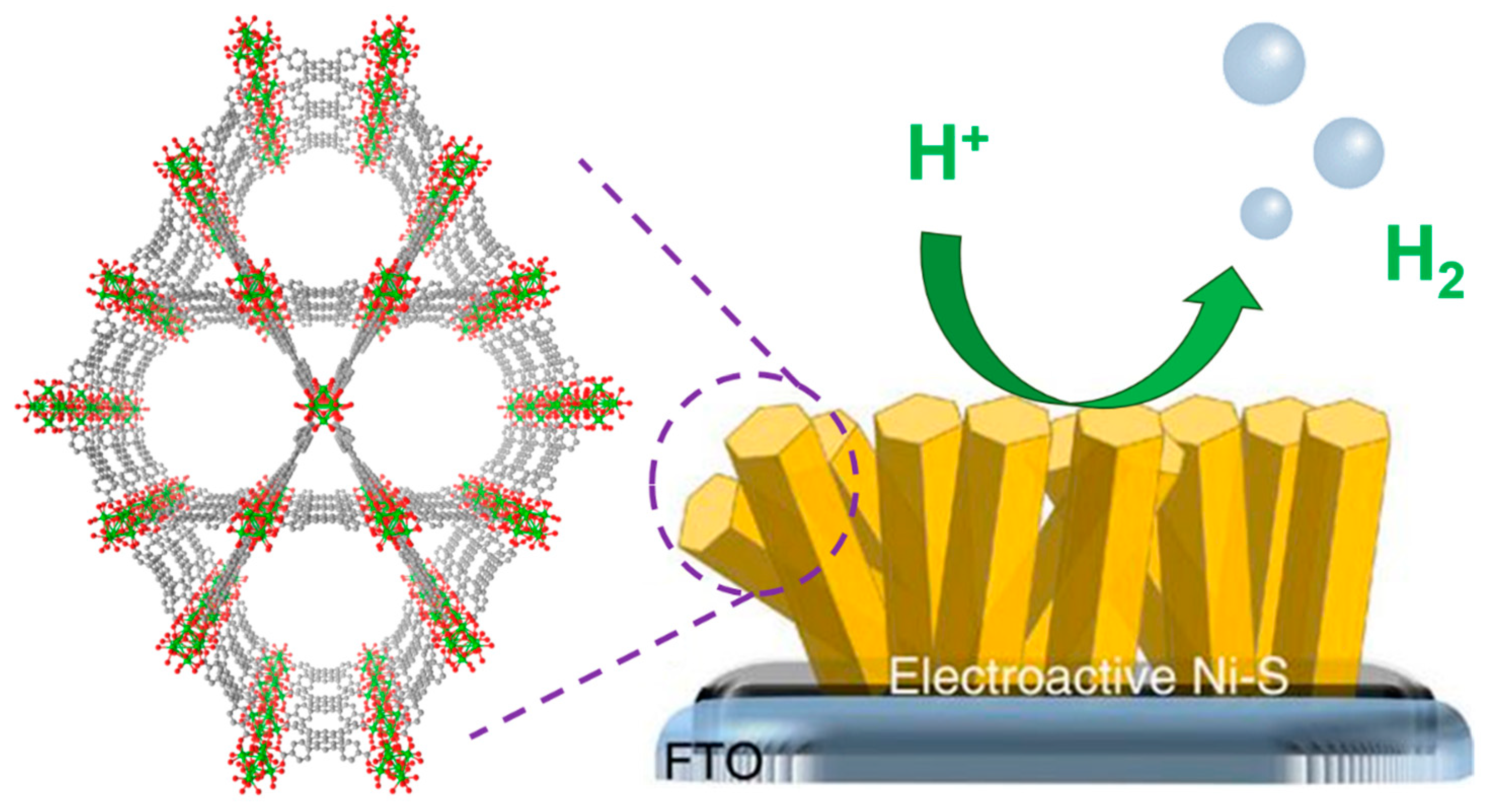
4.1. Hydrogen Evolution
4.2. Oxyben Evolution
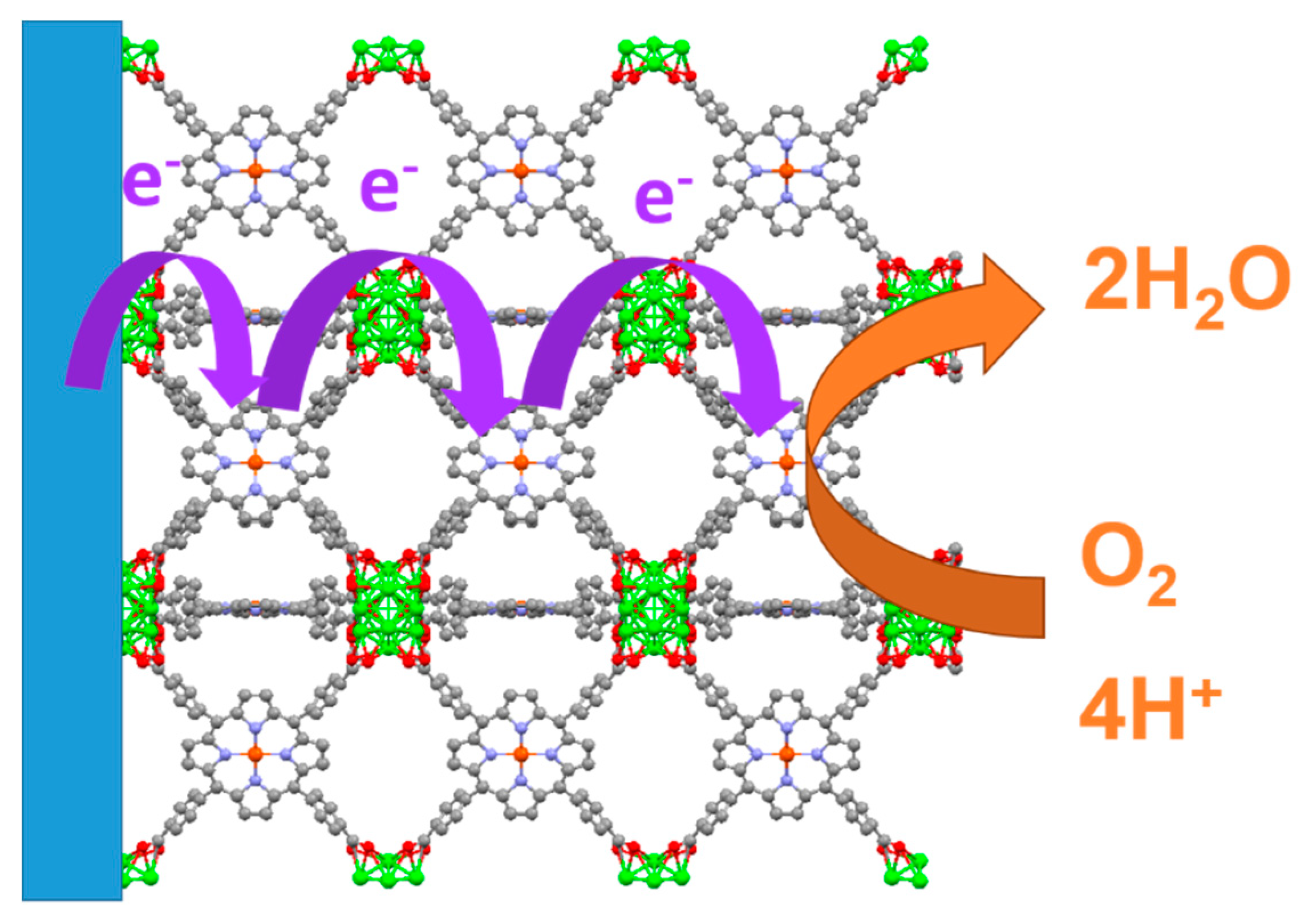
4.3. Oxyben Reduction
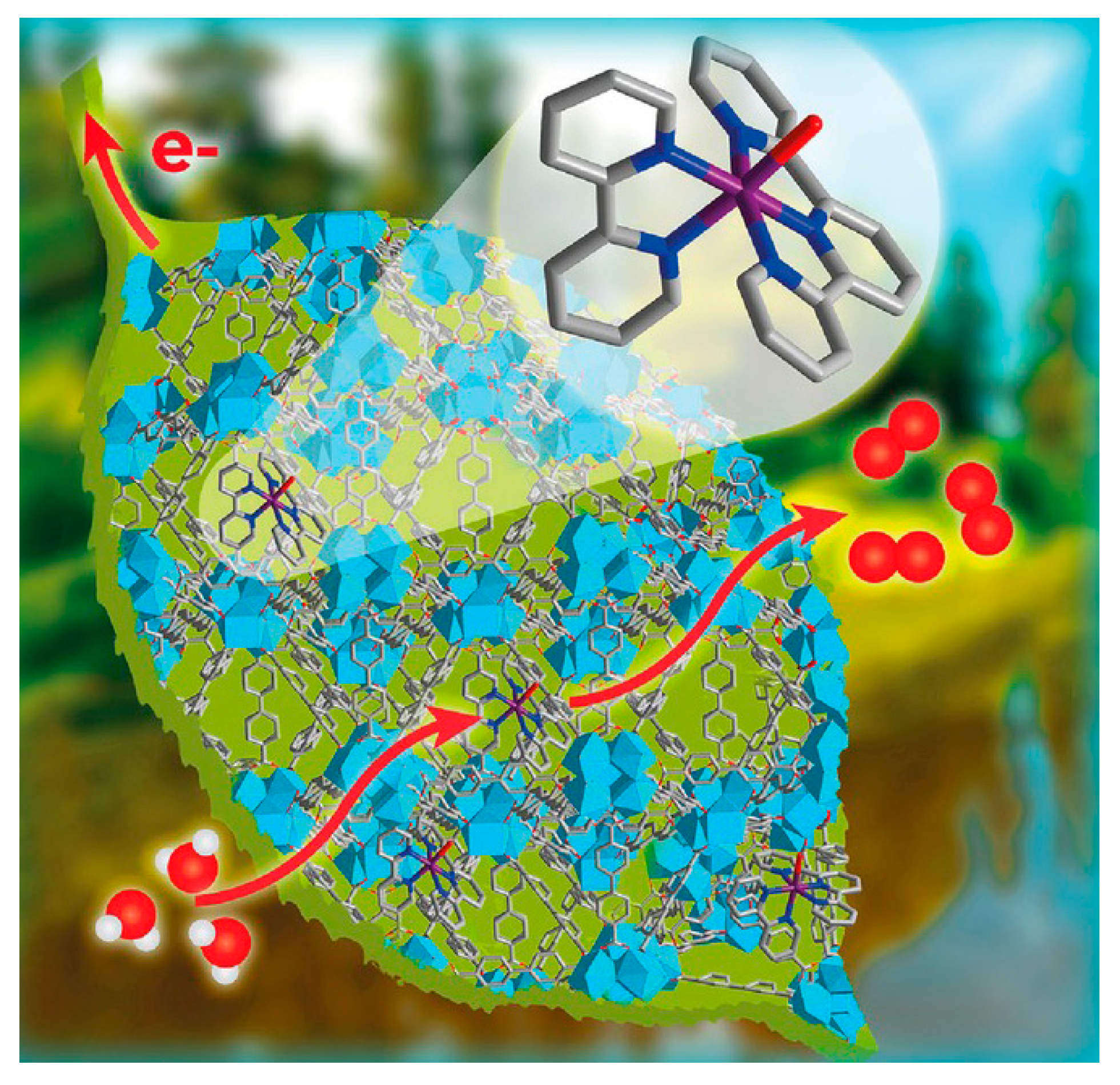
4.4. CO2 Reduction
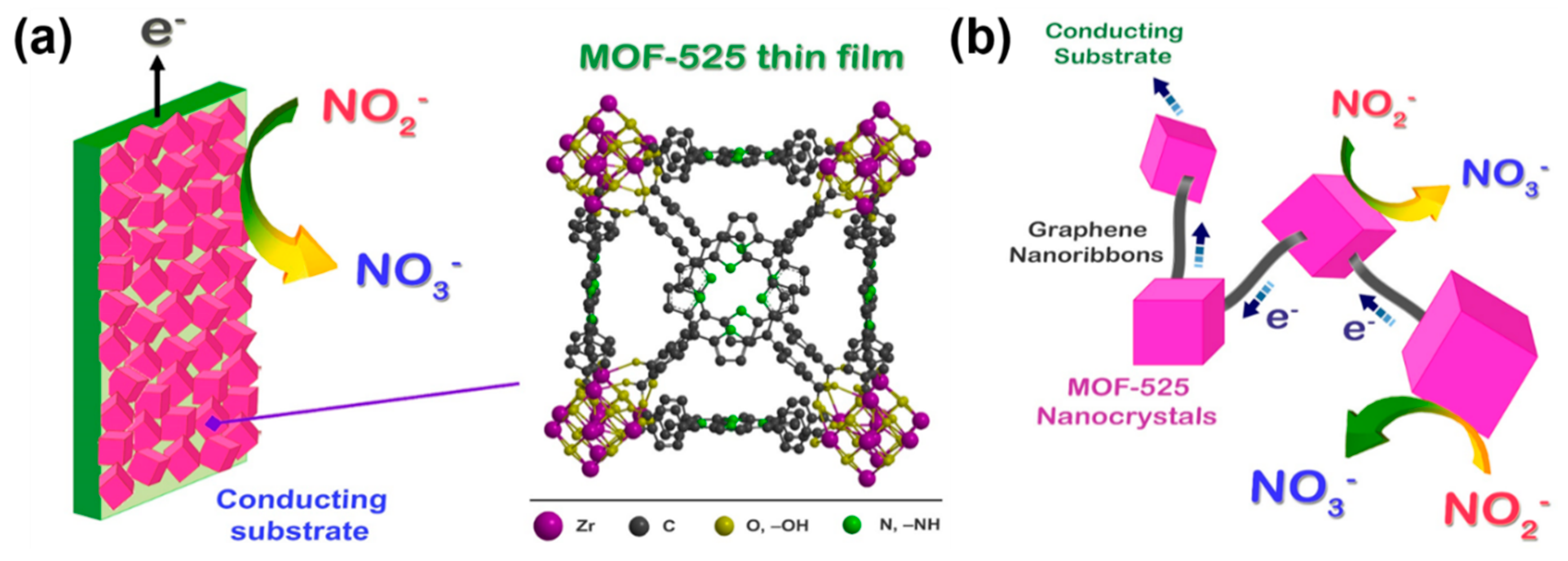
4.5. Electroanalysis
5. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Hwang, D.-W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bard, A.J.; Fox, M.A. Artificial Photosynthesis: Solar Splitting of Water to Hydrogen and Oxygen. Acc. Chem. Res. 1995, 28, 141–145. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent Trends and Perspectives in Electrochemical Water Splitting with an Emphasis on Sulfide, Selenide, and Phosphide Catalysts of Fe, Co, and Ni: A Review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev. 2009, 38, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [Green Version]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Mortimer, R.J. Electrochromic Materials. Annu. Rev. Mater. Res. 2011, 41, 241–268. [Google Scholar] [CrossRef]
- Bertoncello, P.; Ciani, I.; Li, F.; Unwin, P.R. Measurement of Apparent Diffusion Coefficients within Ultrathin Nafion Langmuir−Schaefer Films: Comparison of a Novel Scanning Electrochemical Microscopy Approach with Cyclic Voltammetry. Langmuir 2006, 22, 10380–10388. [Google Scholar] [CrossRef]
- Shi, M.; Anson, F.C. Rapid Oxidation of Ru(NH3)63+ by Os(bpy)33+ within Nafion Coatings on Electrodes. Langmuir 1996, 12, 2068–2075. [Google Scholar] [CrossRef]
- Safranj, A.; Gershuni, S.; Rabani, J. Hydrophobic and ionic exchange of tris(2,2′-bipyridine)ruthenium(II), methylviologen, and sulfonatopropylviologen in Nafion films. Langmuir 1993, 9, 3676–3681. [Google Scholar] [CrossRef]
- Ferey, G. Hybrid porous solids: past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal—Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed]
- Hönicke, I.M.; Senkovska, I.; Bon, V.; Baburin, I.A.; Bönisch, N.; Raschke, S.; Evans, J.D.; Kaskel, S. Balancing Mechanical Stability and Ultrahigh Porosity in Crystalline Framework Materials. Angew. Chem. Int. Ed. 2018, 57, 13780–13783. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.J.; Dinca, M.; Long, J.R. Hydrogen storage in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef]
- Herm, Z.R.; Bloch, E.D.; Long, J.R. Hydrocarbon Separations in Metal—Organic Frameworks. Chem. Mater. 2014, 26, 323–338. [Google Scholar] [CrossRef]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal—Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal—Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Islamoglu, T.; Goswami, S.; Li, Z.; Howarth, A.J.; Farha, O.K.; Hupp, J.T. Postsynthetic Tuning of Metal—Organic Frameworks for Targeted Applications. Acc. Chem. Res. 2017, 50, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. Postsynthetic Methods for the Functionalization of Metal—Organic Frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.B.; Peters, A.W.; Wasielewski, M.R.; Hupp, J.T.; Farha, O.K. Metal—Organic Frameworks as Platform Materials for Solar Fuels Catalysis. ACS Energy Lett. 2018, 3, 598–611. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Katz, M.J.; Isley, W.C., III; Ghosh, P.; Liao, P.; Bury, W.; Wagner, G.W.; Hall, M.G.; DeCoste, J.B.; Peterson, G.W.; et al. Destruction of chemical warfare agents using metal–organic frameworks. Nat. Mater. 2015, 14, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water Stability and Adsorption in Metal—Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal—Organic frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Hendon, C.H.; Tiana, D.; Walsh, A. Conductive metal-organic frameworks and networks: Fact or fantasy? Phys. Chem. Chem. Phys. 2012, 14, 13120–13132. [Google Scholar] [CrossRef]
- Sun, L.; Campbell, M.G.; Dincă, M. Electrically Conductive Porous Metal—Organic Frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef]
- Lin, S.; Usov, P.M.; Morris, A.J. The role of redox hopping in metal—Organic framework electrocatalysis. Chem. Commun. 2018, 54, 6965–6974. [Google Scholar] [CrossRef] [PubMed]
- Blauch, D.N.; Saveant, J.M. Dynamics of electron hopping in assemblies of redox centers. Percolation and diffusion. J. Am. Chem. Soc. 1992, 114, 3323–3332. [Google Scholar] [CrossRef]
- Murray, R.W. Chemically modified electrodes. Acc. Chem. Res. 1980, 13, 135–141. [Google Scholar] [CrossRef]
- Usov, P.M.; Fabian, C.; D’Alessandro, D.M. Rapid determination of the optical and redox properties of a metal—Organic framework via in situ solid state spectroelectrochemistry. Chem. Commun. 2012, 48, 3945–3947. [Google Scholar] [CrossRef] [PubMed]
- Wade, C.R.; Li, M.; Dincă, M. Facile Deposition of Multicolored Electrochromic Metal–Organic Framework Thin Films. Angew. Chem. Int. Ed. 2013, 52, 13377–13381. [Google Scholar] [CrossRef] [PubMed]
- Ahrenholtz, S.R.; Epley, C.C.; Morris, A.J. Solvothermal Preparation of an Electrocatalytic Metalloporphyrin MOF Thin Film and its Redox Hopping Charge-Transfer Mechanism. J. Am. Chem. Soc. 2014, 136, 2464–2472. [Google Scholar] [CrossRef]
- Kung, C.-W.; Wang, T.C.; Mondloch, J.E.; Fairen-Jimenez, D.; Gardner, D.M.; Bury, W.; Klingsporn, J.M.; Barnes, J.C.; Van Duyne, R.; Stoddart, J.F.; et al. Metal—Organic Framework Thin Films Composed of Free-Standing Acicular Nanorods Exhibiting Reversible Electrochromism. Chem. Mater. 2013, 25, 5012–5017. [Google Scholar] [CrossRef]
- Goswami, S.; Ray, D.; Otake, K.-I.; Kung, C.-W.; Garibay, S.J.; Islamoglu, T.; Atilgan, A.; Cui, Y.; Cramer, C.J.; Farha, O.K.; et al. A porous, electrically conductive hexa-zirconium(iv) metal-organic framework. Chem. Sci. 2018, 9, 4477–4482. [Google Scholar] [CrossRef]
- Patwardhan, S.; Schatz, G.C. Theoretical Investigation of Charge Transfer in Metal Organic Frameworks for Electrochemical Device Applications. J. Phys. Chem. C 2015, 119, 24238–24247. [Google Scholar] [CrossRef]
- Palmer, R.H.; Liu, J.; Kung, C.-W.; Hod, I.; Farha, O.K.; Hupp, J.T. Electroactive Ferrocene at or near the Surface of Metal—Organic Framework UiO-66. Langmuir 2018, 34, 4707–4714. [Google Scholar] [CrossRef]
- Lin, S.; Pineda-Galvan, Y.; Maza, W.A.; Epley, C.C.; Zhu, J.; Kessinger, M.C.; Pushkar, Y.; Morris, A.J. Electrochemical Water Oxidation by a Catalyst-Modified Metal—Organic Framework Thin Film. ChemSusChem 2017, 10, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.A.; Bhunia, A.; Fei, H.; Cohen, S.M.; Ott, S. Development of a UiO-Type Thin Film Electrocatalysis Platform with Redox-Active Linkers. J. Am. Chem. Soc. 2018, 140, 2985–2994. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.-W.; Chang, T.-H.; Chou, L.-Y.; Hupp, J.T.; Farha, O.K.; Ho, K.-C. Porphyrin-based metal–organic framework thin films for electrochemical nitrite detection. Electrochem. Commun. 2015, 58, 51–56. [Google Scholar] [CrossRef]
- Hod, I.; Sampson, M.D.; Deria, P.; Kubiak, C.P.; Farha, O.K.; Hupp, J.T. Fe-Porphyrin-Based Metal—Organic Framework Films as High-Surface Concentration, Heterogeneous Catalysts for Electrochemical Reduction of CO2. ACS Catal. 2015, 5, 6302–6309. [Google Scholar] [CrossRef]
- Ling, S.; Slater, B. Unusually Large Band Gap Changes in Breathing Metal—Organic Framework Materials. J. Phys. Chem. C 2015, 119, 16667–16677. [Google Scholar] [CrossRef]
- Hendrickx, K.; Vanpoucke, D.E.P.; Leus, K.; Lejaeghere, K.; Van Yperen-De Deyne, A.; Van Speybroeck, V.; Van Der Voort, P.; Hemelsoet, K. Understanding Intrinsic Light Absorption Properties of UiO-66 Frameworks: A Combined Theoretical and Experimental Study. Inorg. Chem. 2015, 54, 10701–10710. [Google Scholar] [CrossRef] [PubMed]
- Hmadeh, M.; Lu, Z.; Liu, Z.; Gándara, F.; Furukawa, H.; Wan, S.; Augustyn, V.; Chang, R.; Liao, L.; Zhou, F.; et al. New Porous Crystals of Extended Metal-Catecholates. Chem. Mater. 2012, 24, 3511–3513. [Google Scholar] [CrossRef]
- Li, W.-H.; Ding, K.; Tian, H.-R.; Yao, M.-S.; Nath, B.; Deng, W.-H.; Wang, Y.; Xu, G. Conductive Metal–Organic Framework Nanowire Array Electrodes for High-Performance Solid-State Supercapacitors. Adv. Funct. Mater. 2017, 27, 1702067. [Google Scholar] [CrossRef]
- Sheberla, D.; Sun, L.; Blood-Forsythe, M.A.; Er, S.; Wade, C.R.; Brozek, C.K.; Aspuru-Guzik, A.; Dincă, M. High Electrical Conductivity in Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2, a Semiconducting Metal–Organic Graphene Analogue. J. Am. Chem. Soc. 2014, 136, 8859–8862. [Google Scholar] [CrossRef] [PubMed]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.-J.; Shao-Horn, Y.; Dincă, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2016, 16, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Clough, A.J.; Skelton, J.M.; Downes, C.A.; de la Rosa, A.A.; Yoo, J.W.; Walsh, A.; Melot, B.C.; Marinescu, S.C. Metallic Conductivity in a Two-Dimensional Cobalt Dithiolene Metal—Organic Framework. J. Am. Chem. Soc. 2017, 139, 10863–10867. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Lei, T.; Lukatskaya, M.R.; Park, J.; Huang, Z.; Lee, M.; Shaw, L.; Chen, S.; Yakovenko, A.A.; Kulkarni, A.; et al. Robust and conductive two-dimensional metal−organic frameworks with exceptionally high volumetric and areal capacitance. Nat. Energy 2018, 3, 30–36. [Google Scholar] [CrossRef]
- Dou, J.-H.; Sun, L.; Ge, Y.; Li, W.; Hendon, C.H.; Li, J.; Gul, S.; Yano, J.; Stach, E.A.; Dincă, M. Signature of Metallic Behavior in the Metal—Organic Frameworks M3(hexaiminobenzene)2 (M = Ni, Cu). J. Am. Chem. Soc. 2017, 139, 13608–13611. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-F.; Lu, C.-F.; Su, W.-F. Highly Conductive 2D Metal–Organic Framework Thin Film Fabricated by Liquid—Liquid Interfacial Reaction Using One-Pot-Synthesized Benzenehexathiol. Langmuir 2018, 34, 15754–15762. [Google Scholar] [CrossRef] [PubMed]
- Narayan, T.C.; Miyakai, T.; Seki, S.; Dincă, M. High Charge Mobility in a Tetrathiafulvalene-Based Microporous Metal—Organic Framework. J. Am. Chem. Soc. 2012, 134, 12932–12935. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Jacobs, B.; Allendorf, M.D.; Long, J.R. Conductivity, Doping, and Redox Chemistry of a Microporous Dithiolene-Based Metal—Organic Framework. Chem. Mater. 2010, 22, 4120–4122. [Google Scholar] [CrossRef]
- Sun, L.; Miyakai, T.; Seki, S.; Dincă, M. Mn2(2,5-disulfhydrylbenzene-1,4-dicarboxylate): A Microporous Metal–Organic Framework with Infinite (−Mn–S−)∞ Chains and High Intrinsic Charge Mobility. J. Am. Chem. Soc. 2013, 135, 8185–8188. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, H. Control of Charge Transfer in Donor/Acceptor Metal—Organic Frameworks. Acc. Chem. Res. 2013, 46, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, S.; Hosoda, M.; Kajiwara, T.; Miyasaka, H.; Yamashita, M.; Nakanishi, Y.; Kitagawa, Y.; Yamaguchi, K.; Kobayashi, A.; Kitagawa, H. Electroconductive Porous Coordination Polymer Cu[Cu(pdt)2] Composed of Donor and Acceptor Building Units. Inorg. Chem. 2009, 48, 9048–9050. [Google Scholar] [CrossRef]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; El Gabaly, F.; Yoon, H.P.; et al. Tunable Electrical Conductivity in Metal-Organic Framework Thin-Film Devices. Science 2014, 343, 66–69. [Google Scholar] [CrossRef]
- Sengupta, A.; Datta, S.; Su, C.; Herng, T.S.; Ding, J.; Vittal, J.J.; Loh, K.P. Tunable Electrical Conductivity and Magnetic Property of the Two Dimensional Metal Organic Framework [Cu(TPyP)Cu2(O2CCH3)4]. ACS Appl. Mater. Interfaces 2016, 8, 16154–16159. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, H.; Bayer, B.C.; Peterlik, H.; Meyer, J.C.; Lang, W.; Pichler, T. Doping of metal–organic frameworks towards resistive sensing. Sci. Rep. 2017, 7, 2439. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Pirillo, J.; Hijikata, Y.; Zhang, Z.; Awaga, K. Nanopore-induced host–guest charge transfer phenomena in a metal—Organic framework. Chem. Sci. 2018, 9, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.-W.; Otake, K.; Buru, C.T.; Goswami, S.; Cui, Y.; Hupp, J.T.; Spokoyny, A.M.; Farha, O.K. Increased Electrical Conductivity in a Mesoporous Metal–Organic Framework Featuring Metallacarboranes Guests. J. Am. Chem. Soc. 2018, 140, 3871–3875. [Google Scholar] [CrossRef]
- Le Ouay, B.; Boudot, M.; Kitao, T.; Yanagida, T.; Kitagawa, S.; Uemura, T. Nanostructuration of PEDOT in Porous Coordination Polymers for Tunable Porosity and Conductivity. J. Am. Chem. Soc. 2016, 138, 10088–10091. [Google Scholar] [CrossRef]
- Wang, T.C.; Hod, I.; Audu, C.O.; Vermeulen, N.A.; Nguyen, S.T.; Farha, O.K.; Hupp, J.T. Rendering High Surface Area, Mesoporous Metal–Organic Frameworks Electronically Conductive. ACS Appl. Mater. Interfaces 2017, 9, 12584–12591. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.-W.; Platero-Prats, A.E.; Drout, R.J.; Kang, J.; Wang, T.C.; Audu, C.O.; Hersam, M.C.; Chapman, K.W.; Farha, O.K.; Hupp, J.T. Inorganic “Conductive Glass” Approach to Rendering Mesoporous Metal–Organic Frameworks Electronically Conductive and Chemically Responsive. ACS Appl. Mater. Interfaces 2018, 10, 30532–30540. [Google Scholar] [CrossRef]
- Campbell, M.G.; Dincă, M. Metal–Organic Frameworks as Active Materials in Electronic Sensor Devices. Sensors 2017, 17, 1108. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Tang, J.; Salunkhe, R.R.; Jiang, X.; Yu, A.; Wu, K.C.-W.; Yamauchi, Y. Nanoarchitectured Design of Porous Materials and Nanocomposites from Metal-Organic Frameworks. Adv. Mater. 2017, 29, 1604898. [Google Scholar] [CrossRef]
- He, W.; Ifraemov, R.; Raslin, A.; Hod, I. Room-Temperature Electrochemical Conversion of Metal–Organic Frameworks into Porous Amorphous Metal Sulfides with Tailored Composition and Hydrogen Evolution Activity. Adv. Funct. Mater. 2018, 28, 1707244. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yoon, S.J.; Shrestha, N.K.; Lee, S.-H.; Ahn, H.; Han, S.-H. Unusual energy storage and charge retention in Co-based metal–organic-frameworks. Microporous Mesoporous Mater. 2012, 153, 163–165. [Google Scholar] [CrossRef]
- Zhu, G.; Wen, H.; Ma, M.; Wang, W.; Yang, L.; Wang, L.; Shi, X.; Cheng, X.; Sun, X.; Yao, Y. A self-supported hierarchical Co-MOF as a supercapacitor electrode with ultrahigh areal capacitance and excellent rate performance. Chem. Commun. 2018, 54, 10499–10502. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.O.; Jiang, D.; Burrows, A.D.; Halls, J.E.; Marken, F. Conformal transformation of [Co(bdc)(DMF)] (Co-MOF-71, bdc = 1,4-benzenedicarboxylate, DMF = N,N-dimethylformamide) into porous electrochemically active cobalt hydroxide. Electrochem. Commun. 2013, 27, 9–13. [Google Scholar] [CrossRef]
- Babu, K.F.; Kulandainathan, M.A.; Katsounaros, I.; Rassaei, L.; Burrows, A.D.; Raithby, P.R.; Marken, F. Electrocatalytic activity of BasoliteTM F300 metal-organic-framework structures. Electrochem. Commun. 2010, 12, 632–635. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA. 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Colombo, V.; Galli, S.; Choi, H.J.; Han, G.D.; Maspero, A.; Palmisano, G.; Masciocchi, N.; Long, J.R. High thermal and chemical stability in pyrazolate-bridged metal—Organic frameworks with exposed metal sites. Chem. Sci. 2011, 2, 1311–1319. [Google Scholar] [CrossRef]
- Wang, K.; Lv, X.-L.; Feng, D.; Li, J.; Chen, S.; Sun, J.; Song, L.; Xie, Y.; Li, J.-R.; Zhou, H.-C. Pyrazolate-Based Porphyrinic Metal—Organic Framework with Extraordinary Base-Resistance. J. Am. Chem. Soc. 2016, 138, 914–919. [Google Scholar] [CrossRef]
- Lv, X.-L.; Wang, K.; Wang, B.; Su, J.; Zou, X.; Xie, Y.; Li, J.-R.; Zhou, H.-C. A Base-Resistant Metalloporphyrin Metal—Organic Framework for C–H Bond Halogenation. J. Am. Chem. Soc. 2017, 139, 211–217. [Google Scholar] [CrossRef]
- Jamesh, M.I. Recent progress on earth abundant hydrogen evolution reaction and oxygen evolution reaction bifunctional electrocatalyst for overall water splitting in alkaline media. J. Power Sources 2016, 333, 213–236. [Google Scholar] [CrossRef]
- Nohra, B.; El Moll, H.; Rodriguez Albelo, L.M.; Mialane, P.; Marrot, J.; Mellot-Draznieks, C.; O’Keeffe, M.; Ngo Biboum, R.; Lemaire, J.; Keita, B.; et al. Polyoxometalate-Based Metal Organic Frameworks (POMOFs): Structural Trends, Energetics, and High Electrocatalytic Efficiency for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 13363–13374. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-S.; Du, D.-Y.; Guan, W.; Bo, X.-J.; Li, Y.-F.; Guo, L.-P.; Su, Z.-M.; Wang, Y.-Y.; Lan, Y.-Q.; Zhou, H.-C. Ultrastable Polymolybdate-Based Metal–Organic Frameworks as Highly Active Electrocatalysts for Hydrogen Generation from Water. J. Am. Chem. Soc. 2015, 137, 7169–7177. [Google Scholar] [CrossRef] [PubMed]
- Hod, I.; Deria, P.; Bury, W.; Mondloch, J.E.; Kung, C.-W.; So, M.; Sampson, M.D.; Peters, A.W.; Kubiak, C.P.; Farha, O.K.; et al. A porous proton-relaying metal-organic framework material that accelerates electrochemical hydrogen evolution. Nat. Commun. 2015, 6, 8304. [Google Scholar] [CrossRef]
- Noh, H.; Kung, C.-W.; Otake, K.-i.; Peters, A.W.; Li, Z.; Liao, Y.; Gong, X.; Farha, O.K.; Hupp, J.T. Redox-Mediator-Assisted Electrocatalytic Hydrogen Evolution from Water by a Molybdenum Sulfide-Functionalized Metal—Organic Framework. ACS Catal. 2018, 8, 9848–9858. [Google Scholar] [CrossRef]
- Micheroni, D.; Lan, G.; Lin, W. Efficient Electrocatalytic Proton Reduction with Carbon Nanotube-Supported Metal—Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 15591–15595. [Google Scholar] [CrossRef]
- Nivetha, R.; Kollu, P.; Chandar, K.; Pitchaimuthu, S.; Jeong, S.K.; Grace, A.N. Role of MIL-53(Fe)/hydrated–dehydrated MOF catalyst for electrochemical hydrogen evolution reaction (HER) in alkaline medium and photocatalysis. RSC Adv. 2019, 9, 3215–3223. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [Green Version]
- Kanan, M.W.; Nocera, D.G. In Situ Formation of an Oxygen-Evolving Catalyst in Neutral Water Containing Phosphate and Co2+. Science 2008, 321, 1072. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Li, F.-L.; Shao, Q.; Huang, X.; Lang, J.-P. Nanoscale Trimetallic Metal–Organic Frameworks Enable Efficient Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 1888–1892. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Zhao, C. Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat. Commun. 2017, 8, 15341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wu, Y.; Cao, R.; Ren, L.; Chen, M.; Feng, X.; Zhou, J.; Wang, B. Fe/Ni Metal–Organic Frameworks and Their Binder-Free Thin Films for Efficient Oxygen Evolution with Low Overpotential. ACS Appl. Mater. Interfaces 2016, 8, 16736–16743. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-Q.; Liao, P.-Q.; Zhou, D.-D.; He, C.-T.; Wu, J.-X.; Zhang, W.-X.; Zhang, J.-P.; Chen, X.-M. Modular and Stepwise Synthesis of a Hybrid Metal—Organic Framework for Efficient Electrocatalytic Oxygen Evolution. J. Am. Chem. Soc. 2017, 139, 1778–1781. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Ma, D.-D.; Xu, Q.; Wu, X.-T.; Zhu, Q.-L. Semisacrificial Template Growth of Self-Supporting MOF Nanocomposite Electrode for Efficient Electrocatalytic Water Oxidation. Adv. Funct. Mater. 2019, 29, 1807418. [Google Scholar] [CrossRef]
- Xing, J.; Guo, K.; Zou, Z.; Cai, M.; Du, J.; Xu, C. In situ growth of well-ordered NiFe-MOF-74 on Ni foam by Fe2+ induction as an efficient and stable electrocatalyst for water oxidation. Chem. Commun. 2018, 54, 7046–7049. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Li, Y.; Zhang, Y.; Geng, W.; Jia, B.; Tang, J.; Bao, S.; Wang, H.-P.; Fan, Y.; Wei, Z.-W.; et al. Modulating Electronic Structure of Metal-Organic Framework for Efficient Electrocatalytic Oxygen Evolution. Adv. Energy Mater. 2018, 8, 1801564. [Google Scholar] [CrossRef]
- Usov, P.M.; Ahrenholtz, S.R.; Maza, W.A.; Stratakes, B.; Epley, C.C.; Kessinger, M.C.; Zhu, J.; Morris, A.J. Cooperative electrochemical water oxidation by Zr nodes and Ni-porphyrin linkers of a PCN-224 MOF thin film. J. Mater. Chem. A 2016, 4, 16818–16823. [Google Scholar] [CrossRef]
- Johnson, B.A.; Bhunia, A.; Ott, S. Electrocatalytic water oxidation by a molecular catalyst incorporated into a metal-organic framework thin film. Dalton Trans. 2017, 46, 1382–1388. [Google Scholar] [CrossRef]
- Kung, C.-W.; Mondloch, J.E.; Wang, T.C.; Bury, W.; Hoffeditz, W.; Klahr, B.M.; Klet, R.C.; Pellin, M.J.; Farha, O.K.; Hupp, J.T. Metal—Organic Framework Thin Films as Platforms for Atomic Layer Deposition of Cobalt Ions To Enable Electrocatalytic Water Oxidation. ACS Appl. Mater. Interfaces 2015, 7, 28223–28230. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Wang, M.; Han, J.; Wang, H.; Li, Z.; Liu, X. Engineering iridium-based metal organic frameworks towards electrocatalytic water oxidation. Dalton Trans. 2018, 47, 4646–4652. [Google Scholar] [CrossRef]
- Senthil Raja, D.; Chuah, X.-F.; Lu, S.-Y. In Situ Grown Bimetallic MOF-Based Composite as Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting with Ultrastability at High Current Densities. Adv. Energy Mater. 2018, 8, 1801065. [Google Scholar] [CrossRef]
- Bezerra, C.W.B.; Zhang, L.; Lee, K.; Liu, H.; Marques, A.L.B.; Marques, E.P.; Wang, H.; Zhang, J. A review of Fe–N/C and Co–N/C catalysts for the oxygen reduction reaction. Electrochim. Acta 2008, 53, 4937–4951. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.; Loh, K.P. Electrocatalytically Active Graphene–Porphyrin MOF Composite for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 6707–6713. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yin, F.; Chen, B.; Li, G. Synthesis of an ε-MnO2/metal–organic-framework composite and its electrocatalysis towards oxygen reduction reaction in an alkaline electrolyte. J. Mater. Chem. A 2015, 3, 16168–16176. [Google Scholar] [CrossRef]
- Mao, J.; Yang, L.; Yu, P.; Wei, X.; Mao, L. Electrocatalytic four-electron reduction of oxygen with Copper (II)-based metal-organic frameworks. Electrochem. Commun. 2012, 19, 29–31. [Google Scholar] [CrossRef]
- Gonen, S.; Lori, O.; Cohen-Taguri, G.; Elbaz, L. Metal organic frameworks as a catalyst for oxygen reduction: an unexpected outcome of a highly active Mn-MOF-based catalyst incorporated in activated carbon. Nanoscale 2018, 10, 9634–9641. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Yin, F.; Wang, H.; He, X.; Li, G. A metal–organic-framework/carbon composite with enhanced bifunctional electrocatalytic activities towards oxygen reduction/evolution reactions. Int. J. Hydrog. Energy 2017, 42, 17376–17385. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, C.; Gao, X.; Du, C.; Zhuang, Z.; Chen, W. Immobilizing Pd nanoclusters into electronically conductive metal-organic frameworks as bi-functional electrocatalysts for hydrogen evolution and oxygen reduction reactions. Electrochim. Acta 2019, 306, 627–634. [Google Scholar] [CrossRef]
- Lions, M.; Tommasino, J.B.; Chattot, R.; Abeykoon, B.; Guillou, N.; Devic, T.; Demessence, A.; Cardenas, L.; Maillard, F.; Fateeva, A. Insights into the mechanism of electrocatalysis of the oxygen reduction reaction by a porphyrinic metal organic framework. Chem. Commun. 2017, 53, 6496–6499. [Google Scholar] [CrossRef] [PubMed]
- Usov, P.M.; Huffman, B.; Epley, C.C.; Kessinger, M.C.; Zhu, J.; Maza, W.A.; Morris, A.J. Study of Electrocatalytic Properties of Metal—Organic Framework PCN-223 for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2017, 9, 33539–33543. [Google Scholar] [CrossRef] [PubMed]
- Miner, E.M.; Fukushima, T.; Sheberla, D.; Sun, L.; Surendranath, Y.; Dincă, M. Electrochemical oxygen reduction catalysed by Ni3(hexaiminotriphenylene)2. Nat. Commun. 2016, 7, 10942. [Google Scholar] [CrossRef] [PubMed]
- Miner, E.M.; Gul, S.; Ricke, N.D.; Pastor, E.; Yano, J.; Yachandra, V.K.; Van Voorhis, T.; Dincă, M. Mechanistic Evidence for Ligand-Centered Electrocatalytic Oxygen Reduction with the Conductive MOF Ni3(hexaiminotriphenylene)2. ACS Catal. 2017, 7, 7726–7731. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, T.; Tao, H.; Fan, Q.; Han, B. Fundamentals and Challenges of Electrochemical CO2 Reduction Using Two-Dimensional Materials. Chem 2017, 3, 560–587. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic Conversion of Carbon Dioxide to Methane and Methanol on Transition Metal Surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef] [PubMed]
- Janáky, C.; Hursán, D.; Endrődi, B.; Chanmanee, W.; Roy, D.; Liu, D.; de Tacconi, N.R.; Dennis, B.H.; Rajeshwar, K. Electro- and Photoreduction of Carbon Dioxide: The Twain Shall Meet at Copper Oxide/Copper Interfaces. ACS Energy Lett. 2016, 1, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Albo, J.; Vallejo, D.; Beobide, G.; Castillo, O.; Castaño, P.; Irabien, A. Copper-Based Metal—Organic Porous Materials for CO2 Electrocatalytic Reduction to Alcohols. ChemSusChem 2017, 10, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, R.; Senthil Kumar, S.; Anbu Kulandainathan, M. Highly selective electrochemical reduction of carbon dioxide using Cu based metal organic framework as an electrocatalyst. Electrochem. Commun. 2012, 25, 70–73. [Google Scholar] [CrossRef]
- Tan, X.; Yu, C.; Zhao, C.; Huang, H.; Yao, X.; Han, X.; Guo, W.; Cui, S.; Huang, H.; Qiu, J. Restructuring of Cu2O to Cu2O@Cu-Metal–Organic Frameworks for Selective Electrochemical Reduction of CO2. ACS Appl. Mater. Interfaces 2019, 11, 9904–9910. [Google Scholar] [CrossRef]
- Qiu, Y.-L.; Zhong, H.-X.; Zhang, T.-T.; Xu, W.-B.; Su, P.-P.; Li, X.-F.; Zhang, H.-M. Selective Electrochemical Reduction of Carbon Dioxide Using Cu Based Metal Organic Framework for CO2 Capture. ACS Appl. Mater. Interfaces 2018, 10, 2480–2489. [Google Scholar] [CrossRef]
- Perfecto-Irigaray, M.; Albo, J.; Beobide, G.; Castillo, O.; Irabien, A.; Pérez-Yáñez, S. Synthesis of heterometallic metal—Organic frameworks and their performance as electrocatalyst for CO2 reduction. RSC Adv. 2018, 8, 21092–21099. [Google Scholar] [CrossRef]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal—Organic Frameworks for Electrocatalytic Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.-X.; Qian, S.-L.; Bu, F.-Y.; Wu, Y.-C.; Feng, L.-G.; Teng, Y.-L.; Liu, W.-L.; Li, Z.-W. Electrochemical Reduction of CO2 to CO by a Heterogeneous Catalyst of Fe–Porphyrin-Based Metal—Organic Framework. ACS Appl. Energy Mater. 2018, 1, 4662–4669. [Google Scholar] [CrossRef]
- Kung, C.-W.; Audu, C.O.; Peters, A.W.; Noh, H.; Farha, O.K.; Hupp, J.T. Copper Nanoparticles Installed in Metal—Organic Framework Thin Films are Electrocatalytically Competent for CO2 Reduction. ACS Energy Lett. 2017, 2, 2394–2401. [Google Scholar] [CrossRef]
- Dou, S.; Song, J.; Xi, S.; Du, Y.; Wang, J.; Huang, Z.-F.; Xu, Z.J.; Wang, X. Boosting Electrochemical CO2 Reduction on Metal—Organic Frameworks via Ligand Doping. Angew. Chem. Int. Ed. 2019, 58, 4041–4045. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical methods, fundamentals and applications; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Zhang, C.; Wang, M.; Liu, L.; Yang, X.; Xu, X. Electrochemical investigation of a new Cu-MOF and its electrocatalytic activity towards H2O2 oxidation in alkaline solution. Electrochem. Commun. 2013, 33, 131–134. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; He, J.; Zhang, Y.; Yu, J.; Song, Y. Cu-Hemin Metal-Organic-Frameworks/Chitosan-Reduced Graphene Oxide Nanocomposites with Peroxidase-Like Bioactivity for Electrochemical Sensing. Electrochim. Acta 2016, 213, 691–697. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.; Gao, F.; Ni, J.; Zhang, Y.; Lin, Z. Graphene Oxide Directed One-Step Synthesis of Flowerlike Graphene@HKUST-1 for Enzyme-Free Detection of Hydrogen Peroxide in Biological Samples. ACS Appl. Mater. Interfaces 2016, 8, 32477–32487. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, T.; Zhao, J.; Liu, H.; Zheng, B.; Gu, Y.; Yan, X.; Li, Y.; Lu, N.; Zhang, Z.; et al. Boosted Sensor Performance by Surface Modification of Bifunctional rht-Type Metal—Organic Framework with Nanosized Electrochemically Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2017, 9, 2984–2994. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Chen, J.; Pang, H. Copper metal—organic framework nanocrystal for plane effect nonenzymatic electro-catalytic activity of glucose. Nanoscale 2014, 6, 10989–10994. [Google Scholar] [CrossRef]
- Hosseini, H.; Ahmar, H.; Dehghani, A.; Bagheri, A.; Tadjarodi, A.; Fakhari, A.R. A novel electrochemical sensor based on metal-organic framework for electro-catalytic oxidation of L-cysteine. Biosens. Bioelectron. 2013, 42, 426–429. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Ma, J.-G.; Li, H.; Chen, D.-M.; Gu, W.; Yang, G.-M.; Cheng, P. Metal—organic framework biosensor with high stability and selectivity in a bio-mimic environment. Chem. Commun. 2015, 51, 9161–9164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nsabimana, A.; Zhu, L.; Bo, X.; Han, C.; Li, M.; Guo, L. Metal organic frameworks/macroporous carbon composites with enhanced stability properties and good electrocatalytic ability for ascorbic acid and hemoglobin. Talanta 2014, 129, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Wang, Q.; Gao, F.; Gao, F.; Yang, Y.; Guo, H. Highly Dispersible and Stable Copper Terephthalate Metal–Organic Framework—Graphene Oxide Nanocomposite for an Electrochemical Sensing Application. ACS Appl. Mater. Interfaces 2014, 6, 11573–11580. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Ganesan, V.; Sonkar, P.K.; Gupta, R.; Rastogi, P.K. Electrochemical investigation of gold nanoparticles incorporated zinc based metal-organic framework for selective recognition of nitrite and nitrobenzene. Electrochim. Acta 2016, 200, 276–282. [Google Scholar] [CrossRef]
- Chen, H.; Yang, T.; Liu, F.; Li, W. Electrodeposition of gold nanoparticles on Cu-based metal-organic framework for the electrochemical detection of nitrite. Sens. Actuators B Chem. 2019, 286, 401–407. [Google Scholar] [CrossRef]
- Kung, C.-W.; Li, Y.-S.; Lee, M.-H.; Wang, S.-Y.; Chiang, W.-H.; Ho, K.-C. In situ growth of porphyrinic metal-organic framework nanocrystals on graphene nanoribbons for the electrocatalytic oxidation of nitrite. J. Mater. Chem. A 2016, 4, 10673–10682. [Google Scholar] [CrossRef]
- Huang, T.Y.; Kung, C.W.; Liao, Y.T.; Kao, S.Y.; Cheng, M.; Chang, T.H.; Henzie, J.; Alamri Hatem, R.; Alothman Zeid, A.; Yamauchi, Y.; et al. Enhanced Charge Collection in MOF-525–PEDOT Nanotube Composites Enable Highly Sensitive Biosensing. Adv. Sci. 2017, 4, 1700261. [Google Scholar] [CrossRef]
- Cao, M.; Yin, X.; Bo, X.; Guo, L. High-performance electrocatalyst based on metal-organic framework/macroporous carbon composite for efficient detection of luteolin. J. Electroanal. Chem. 2018, 824, 153–160. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Huang, W.; Zhang, T.; Hu, X.; Perman, J.A.; Ma, S. A metal–organic framework and conducting polymer based electrochemical sensor for high performance cadmium ion detection. J. Mater. Chem. A 2017, 5, 8385–8393. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Chen, H.; Hu, X.; Ma, S. Fabrication of Highly Sensitive and Stable Hydroxylamine Electrochemical Sensor Based on Gold Nanoparticles and Metal—Metalloporphyrin Framework Modified Electrode. ACS Appl. Mater. Interfaces 2016, 8, 18173–18181. [Google Scholar] [CrossRef]
- Yang, J.; Yang, L.; Ye, H.; Zhao, F.; Zeng, B. Highly dispersed AuPd alloy nanoparticles immobilized on UiO-66-NH2 metal-organic framework for the detection of nitrite. Electrochim. Acta 2016, 219, 647–654. [Google Scholar] [CrossRef]
- Gong, C.; Shen, Y.; Chen, J.; Song, Y.; Chen, S.; Song, Y.; Wang, L. Microperoxidase-11@PCN-333 (Al)/three-dimensional macroporous carbon electrode for sensing hydrogen peroxide. Sens. Actuators B Chem. 2017, 239, 890–897. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-H.; Wang, Y.-S.; Chen, Y.-C.; Kung, C.-W. Metal–Organic Frameworks Toward Electrocatalytic Applications. Appl. Sci. 2019, 9, 2427. https://doi.org/10.3390/app9122427
Li J-H, Wang Y-S, Chen Y-C, Kung C-W. Metal–Organic Frameworks Toward Electrocatalytic Applications. Applied Sciences. 2019; 9(12):2427. https://doi.org/10.3390/app9122427
Chicago/Turabian StyleLi, Jun-Hong, Yi-Sen Wang, Yu-Chuan Chen, and Chung-Wei Kung. 2019. "Metal–Organic Frameworks Toward Electrocatalytic Applications" Applied Sciences 9, no. 12: 2427. https://doi.org/10.3390/app9122427
APA StyleLi, J.-H., Wang, Y.-S., Chen, Y.-C., & Kung, C.-W. (2019). Metal–Organic Frameworks Toward Electrocatalytic Applications. Applied Sciences, 9(12), 2427. https://doi.org/10.3390/app9122427





