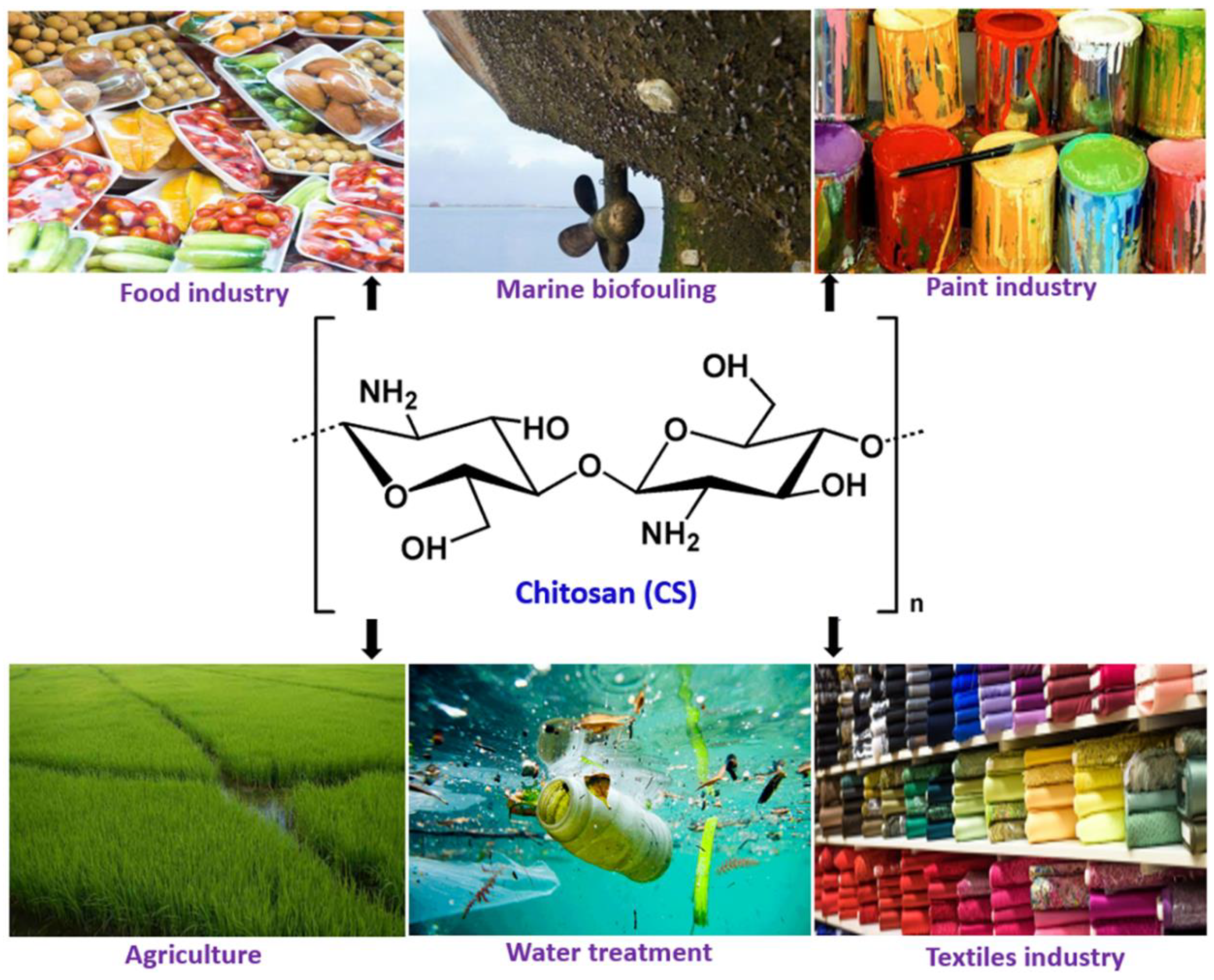
Chitosan Nanocomposite Coatings for Food, Paints, and Water Treatment Applications
Abstract
1. Introduction
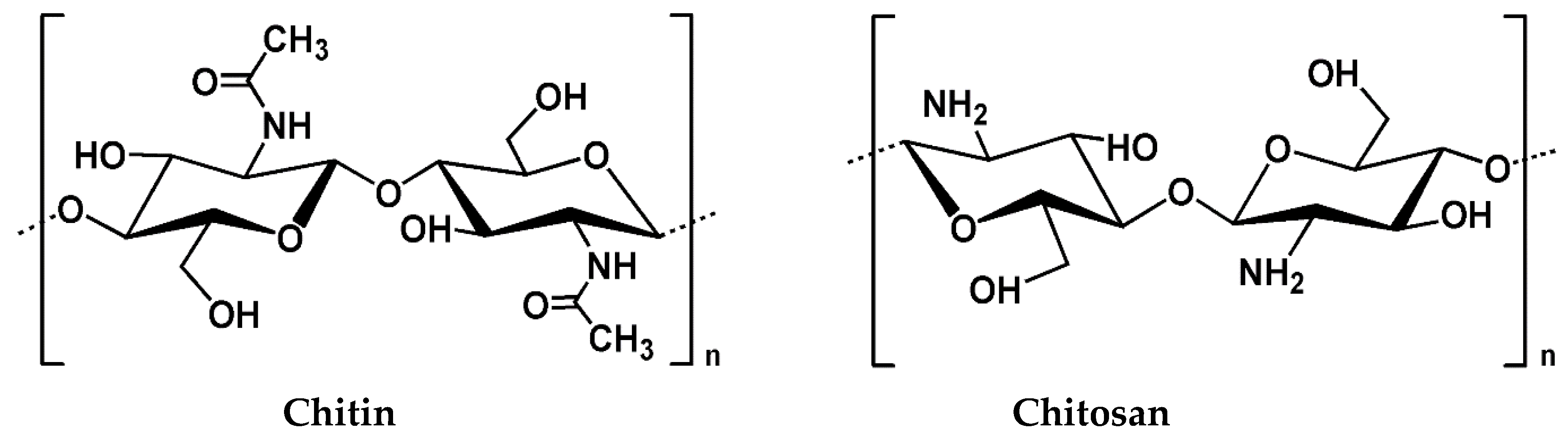
2. Chitosan and its Properties
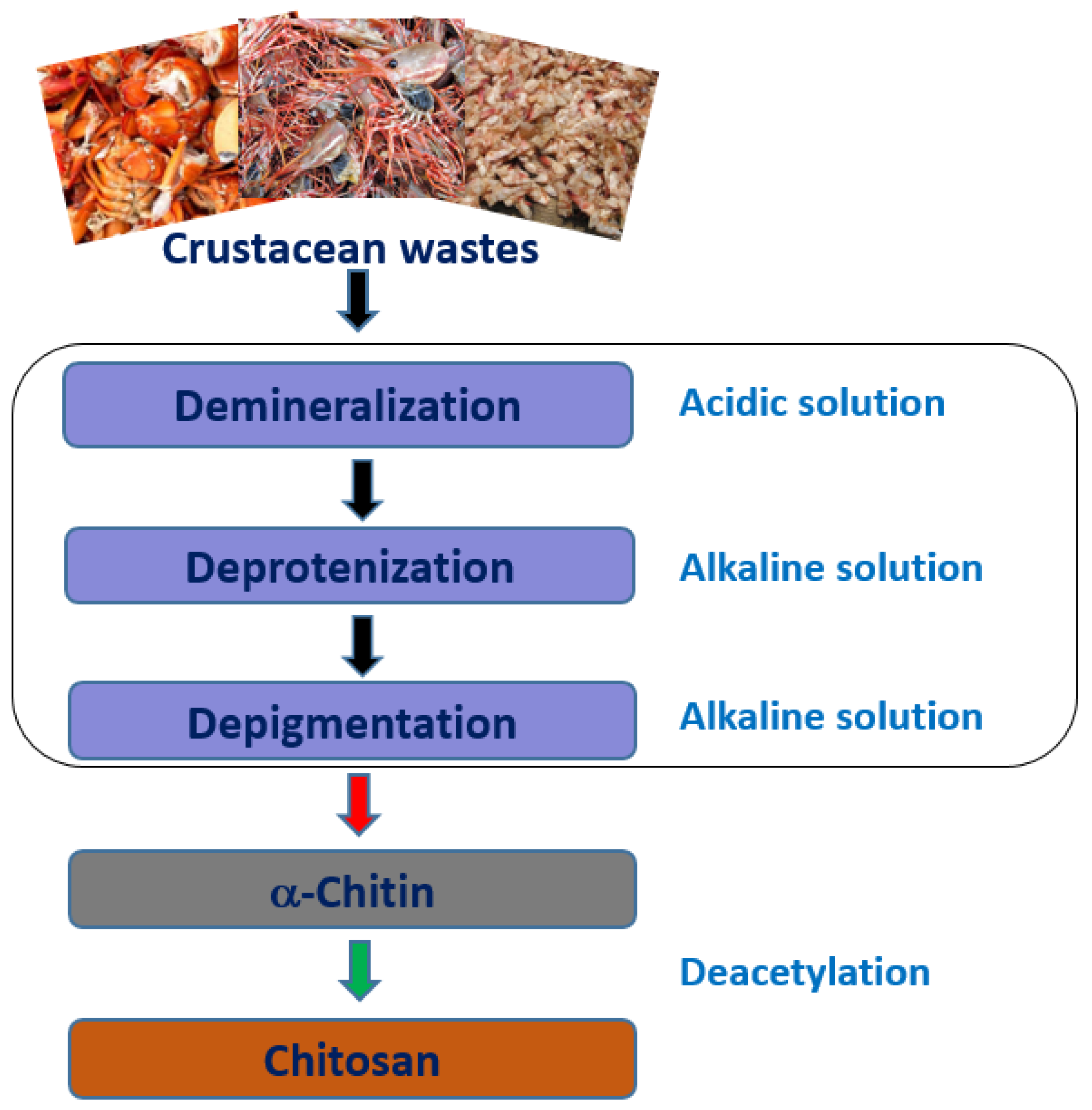
2.1. Source and Extraction
2.2. Physico-Chemical Properties of Chitosan
2.2.1. Degree of Deacetylation (DD)
2.2.2. Molecular Weight (MW)
2.2.3. Solubility
2.3. Antimicrobial Properties
2.4. Self-Healing Properties
3. Chitosan-Based Nanocomposites
3.1. Chitosan-Metal/Metal Oxide
3.2. Chitosan-Carbon Materials
3.3. Chitosan-Polymer Mixture or Copolymer
3.4. Chitosan-Clay Composites
| Chitosan Molecular Weight/Viscosity | Type of Nanomaterials in Composite | Name of Nanomaterial/Polymer/Clay | Preparation Method of Chitosan Nanocomposite | Form of Chitosan Nanocomposites | Specific Application | Key/Enhanced Properties | Application Field | Reference |
|---|---|---|---|---|---|---|---|---|
| 100 kDa | Metal | Ag nanoparticles | In situ reduction on chitosan | Thin film coating on bandage | Antibacterial activity against E. coli and S. aureus | Inactivation bacterial metabolism | Antimicrobial | [100] |
| Medium molecular weight | Metal | Ag nanoparticles | In situ reduction on chitosan | Ag nanoparticles anchored on chitosan particles | Sensing of ammonia in solution | Sensitive in optical absorption intensity and wavelength | Environment | [101] |
| Medium molecular weight | Metal oxide | ZnO nanoparticles | Blending | Thin film coating | Antifouling prevention | Anti-diatom activity and antibacterial activity against the marine bacterium | Anti-biofouling | [85,86] |
| Low viscosity | Metal oxide | SiO2 nanoparticles | In situ Stöber method grown on chitosan | Slurry packed in liquid chromatography (LC) column | Adsorption of rare-earth elements | High adsorption efficiency, selectivity, and reusability | Environmental | [87] |
| 190–310 kDa | Carbon | Graphene oxide | Cross-linking | Thin film | Antimicrobial against E. coli and B. subtilis | Improved mechanical and antimicrobial properties | Antimicrobial | [88] |
| 300 kDa | Carbon | Graphene oxide | Cross-linking | Hydrogel | Removal of dyes and metal ions from water | Tunable surface charge; efficient removal of pollutants | Environmental | [89] |
| N/A | Polymer | low density poly-ethylene (LDPE) film | Grafting | Coating | Significant changes in surface wettability | Improved anti-thrombogenic properties | Antifouling | [92] |
| N/A | Clay | Halloysite clay nanotubes | Electrostatical adsorption | Coating | Anticorrosive protective | Improved passive barrier protective and self-healing | Environmental | [96] |
| 50–190 kDa | Clay | Bentonite and sepiolite | Blend | Thin film | Winemaking application | Enhanced immobilization of protease but negatively affected catalytic properties | Antimicrobial | [97] |
| Medium molecular weight | Clay | Bentonite | Gelation and lyophilization | Bead | Carbon dioxide adsorption | High adsorption capacity under moderate condition | Environmental | [98] |
4. Applications of Chitosan-Based Nanocomposites
4.1. Water Purification
| Chitosan-Based Nanocomposite | Dye/Metal | pH | Removal Process | References |
|---|---|---|---|---|
| CS/AgNPs | Methyl orange | 3–11 | Photocatalytic decolourization | [107] |
| CS/AuNPs | 4-nitrophenol | No data | Catalytic reduction | [108] |
| CS/TiO2 | Rhodamine-B, Congo red | 3–11 | Photocatalytic degradation under visible light irradiation | [109] |
| CS/Polyaniline/CdS | Reactive Blue-19 | 6 | Adsorption | [110] |
| CS/PVA/ZnO | Acid Black-1 | No data | Adsorption | [111] |
| CS/SiO2/CNT | Direct Blue-71, Reactive Blue-19 | 0–12 | Adsorption | [112] |
| CS/lignin/titania | Brilliant Black | No data | Adsorption | [113] |
| CS/Bio-silica | Acid Red 88 | 1–12 | Adsorption | [114] |
| CS/Palladium | 4-Nitrophenol | No data | Catalytic hydrogenation | [115] |
| CS/SnO2 | Methyl orange, Rhodamine-B | No data | Photocatalytic degradation | [116] |
| CS/Ag3PO4/CdS | Methyl orange | 3–8 | Photocatalytic decolourization | [117] |
| CS/zirconium tungstate | Reactive Blue-21, Reactive Red-141, Rhodamine-6G and binary mixture; RB+RR, RR+RH, RB + RH | No data | Photocatalytic degradation | [118] |
| CS/SnO2 intercalated polyaniline (PANI) | Methylene blue Reactive yellow-15 | No data | Photocatalytic degradation under direct sunlight irradiation | [119] |
| CS/TiO2 fibers supported zero-valent metal nanoparticles (like Cu0, Co0, Ag0 and Ni0) | Methyl orange, Congo red (CR), Methylene blue, Acridine orange, 4-nitrohphenol, 2-nitrophenol, 3-nitrophenol, 2,6-dinitrophenol | No data | Catalytic reduction | [120] |
| CS coated cotton cloth supported zero-valent metal nanoparticles | Methyl orange, Methylene blue,4-nitrohphenol, Rhodamine-B | No data | Catalytic reduction | [121] |
| CS/MoO3/TiO2 | Methyl orange | No data | Photocatalytic degradation under solar light | [122] |
4.2. Antifouling Paints and Coatings
| Application | Carrier/Additive | Type of the Study | Effective Against | References |
|---|---|---|---|---|
| Films | No | Laboratory | Bryozoan | [130] |
| Films | No | Field | Micro-fouling | [131] |
| Ultra-thin nanocoatings | No | Laboratory | Bacteria | [138] |
| Films | Polyelectrolyte brushes | Laboratory and field | Pathogens, micro-fouling and macro-fouling | [134] |
| Paints | Silicon-polyurethane | Field | Micro-fouling and macro-fouling | [129] |
| ZnO nanocomposites | ZnO nanoparticles | Laboratory | Micro-fouling | [86] |
| ZnO nanocomposites | ZnO nanoparticles | Laboratory | Pathogenic bacteria and fungi | [85] |
| Silver nanocomposite films | Silver nanoparticles | Laboratory | Pathogenic bacteria | [132] |
| Cellulose membranes | No | Laboratory | Pathogenic bacteria | [136] |
| PAN-chitosan membranes | No | Laboratory | Bacteria | [139] |
| Chitosan membranes | No | Laboratory | Pathogenic bacteria | [135] |
| Forward osmosis membranes | Graphene oxide nanosheets | Laboratory | Proteins | [137] |
| PES membranes | Fe3O4 nanoparticles | Laboratory | Proteins | [140] |
| PES membranes | Silver nanoparticles | Laboratory | Proteins, bacteria | [141] |
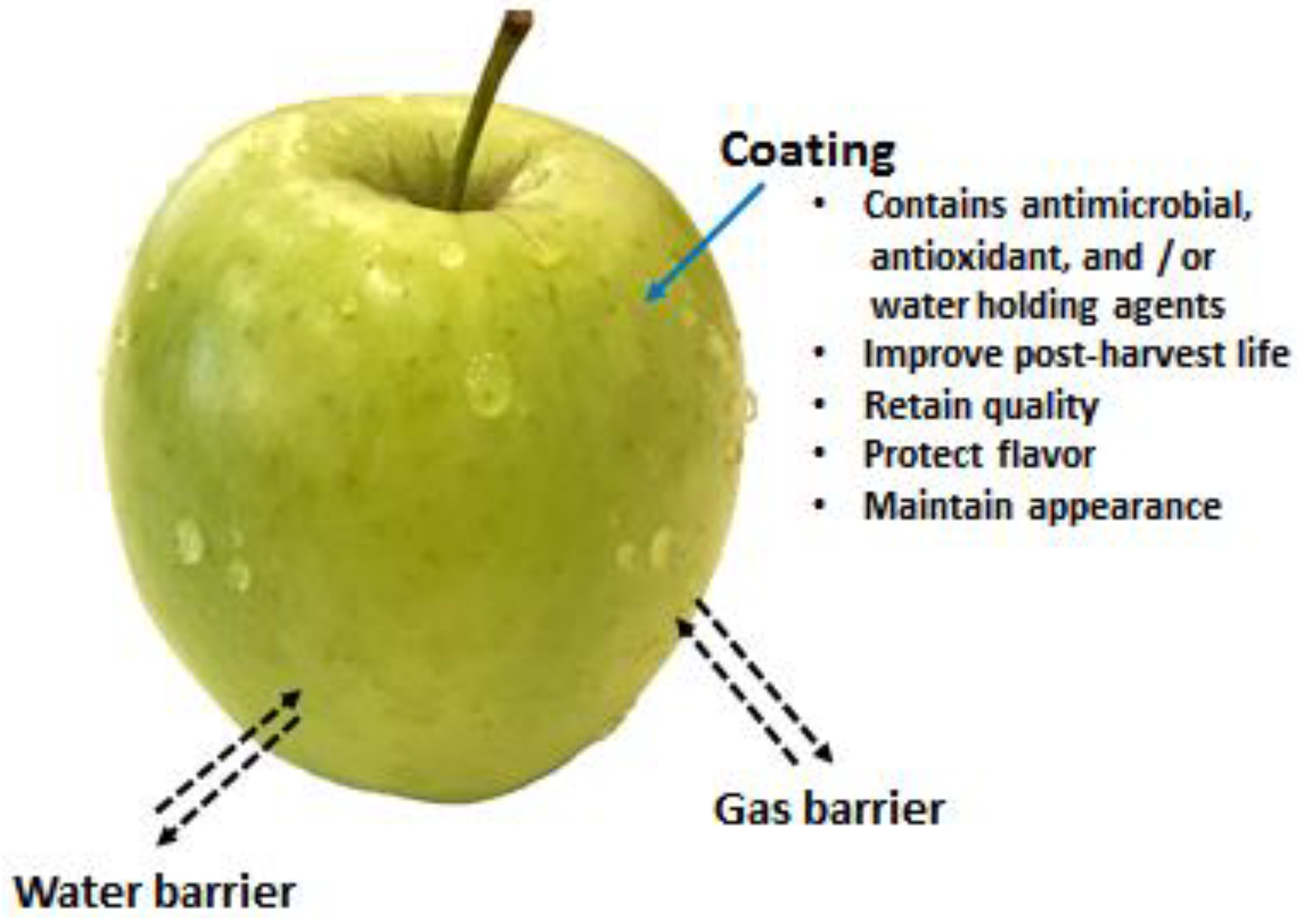
4.3. Shelf-Life Extension of Fruits and Vegetables
4.3.1. Packaging Films
4.3.2. Coatings of Fruits and Vegetables
- Offer barrier properties against moisture and oxygen
- Help to deliver antimicrobial activity to inhibit or delay the microbial growth
- Deliver antioxidant effects that help to reduce the oxidation process, loss of colour, vitamins, etc.
- Help to maintain the loss of volatile components and stop acquiring foreign odours
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Tsigos, I.; Martinou, A.; Kafetzopoulos, D.; Bouriotis, V. Chitin deacetylases: New, versatile tools in biotechnology. Trends Biotechnol. 2000, 18, 305–312. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Crit. Rev. Biotechnol. 2012, 33, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol–chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Kumari, S.; Rath, P.K. Extraction and Characterization of Chitin and Chitosan from (Labeo rohit) Fish Scales. Procedia Mater. Sci. 2014, 6, 482–489. [Google Scholar] [CrossRef]
- Doiphode, N.; Joshi, C.; Ghormade, V.; Deshpande, M.V. Biotechnological Applications of Dimorphic Yeasts. In Yeast Biotechnology: Diversity and Applications; Springer: Dordrecht, The Netherlands, 2009; pp. 635–650. [Google Scholar]
- Amorim, R.V.S.; Ledingham, W.M.; Kennedy, J.F.; Campos-Takaki, G.M. Chitosan from Syncephalastrum racemosum Using Sugar Cane Substrates as Inexpensive Carbon Sources. Food Biotechnol. 2006, 20, 43–53. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Khodaiyan, F.; Razavi, S.H.; Mousavi, M. Improvement of chitosan production from Persian Gulf shrimp waste by response surface methodology. Food Hydrocoll. 2016, 59, 50–58. [Google Scholar] [CrossRef]
- Teli, M.D.; Sheikh, J. Extraction of chitosan from shrimp shells waste and application in antibacterial finishing of bamboo rayon. Int. J. Biol. Macromol. 2012, 50, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, F.; Yousefzadi, M.; Toiserkani, H.; Najafipour, S. Bioconversion of shrimp waste Penaeus merguiensis using lactic acid fermentation: An alternative procedure for chemical extraction of chitin and chitosan. Int. J. Biol. Macromol. 2017, 104, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Ghorbel-Bellaaj, O.; Nasri, R.; Chaabouni, M.; Rinaudo, M.; Nasri, M. Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochem. 2012, 47, 2032–2039. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll. 2013, 31, 166–171. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Abdelmalek, B.E.; Sila, A.; Haddar, A.; Bougatef, A.; Ayadi, M.A. β-Chitin and chitosan from squid gladius: Biological activities of chitosan and its application as clarifying agent for apple juice. Int. J. Biol. Macromol. 2017, 104, 953–962. [Google Scholar] [CrossRef]
- Marei, N.H.; El-Samie, E.A.; Salah, T.; Saad, G.R.; Elwahy, A.H.M. Isolation and characterization of chitosan from different local insects in Egypt. Int. J. Biol. Macromol. 2016, 82, 871–877. [Google Scholar] [CrossRef]
- Nemtsev, S.V.; Zueva, O.Y.; Khismatullin, M.R.; Albulov, A.I.; Varlamov, V.P. Isolation of Chitin and Chitosan from Honeybees. Appl. Biochem. Microbiol. 2004, 40, 39–43. [Google Scholar] [CrossRef]
- Abdel-Gawad, K.M.; Hifney, A.F.; Fawzy, M.A.; Gomaa, M. Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll. 2017, 63, 593–601. [Google Scholar] [CrossRef]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Baron, R.D.; Pérez, L.L.; Salcedo, J.M.; Córdoba, L.P.; Sobral, P.J.d.A. Production and characterization of films based on blends of chitosan from blue crab (Callinectes sapidus) waste and pectin from Orange (Citrus sinensis Osbeck) peel. Int. J. Biol. Macromol. 2017, 98, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Barber, A.R.; Corbin, K.; Zhang, W. Lobster processing by-products as valuable bioresource of marine functional ingredients, nutraceuticals, and pharmaceuticals. Bioresour. Bioprocess. 2017, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef] [PubMed]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, L.; Zhong, W. A new linear potentiometric titration method for the determination of deacetylation degree of chitosan. Carbohydr. Polym. 2003, 54, 457–463. [Google Scholar] [CrossRef]
- Dos Santos, Z.M.; Caroni, A.L.P.F.; Pereira, M.R.; da Silva, D.R.; Fonseca, J.L.C. Determination of deacetylation degree of chitosan: A comparison between conductometric titration and CHN elemental analysis. Carbohydr. Res. 2009, 344, 2591–2595. [Google Scholar] [CrossRef]
- Kasaai, M.R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Carbohydr. Polym. 2010, 79, 801–810. [Google Scholar] [CrossRef]
- Desbrières, J.; Martinez, C.; Rinaudo, M. Hydrophobic derivatives of chitosan: Characterization and rheological behaviour. Int. J. Biol. Macromol. 1996, 19, 21–28. [Google Scholar] [CrossRef]
- Kasaai, M. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohydr. Polym. 2008, 71, 497–508. [Google Scholar] [CrossRef]
- Wu, T.; Zivanovic, S. Determination of the degree of acetylation (DA) of chitin and chitosan by an improved first derivative UV method. Carbohydr. Polym. 2008, 73, 248–253. [Google Scholar] [CrossRef]
- Wu, C.; Kao, C.Y.; Tseng, S.-Y.; Chen, K.C.; Chen, S.-F. Determination of the degree of deacetylation of chitosan by capillary zone electrophoresis. Carbohydr. Polym. 2014, 111, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef]
- Li, X.; Xia, W. Effects of concentration, degree of deacetylation and molecular weight on emulsifying properties of chitosan. Int. J. Biol. Macromol. 2011, 48, 768–772. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhong, Y.; Zhao, Y. Effect of deacetylation degree on properties of Chitosan films using electrostatic spraying technique. Food Control 2019, 97, 25–31. [Google Scholar] [CrossRef]
- Paul, T.; Halder, S.K.; Das, A.; Ghosh, K.; Mandal, A.; Payra, P.; Barman, P.; Das Mohapatra, P.K.; Pati, B.R.; Mondal, K.C. Production of chitin and bioactive materials from Black tiger shrimp (Penaeus monodon) shell waste by the treatment of bacterial protease cocktail. 3 Biotech 2015, 5, 483–493. [Google Scholar] [CrossRef]
- Peniche, C.; Peniche, H.; Pérez, J. Chitosan based self-assembled nanoparticles in drug delivery. Polymers 2018, 10, 235. [Google Scholar]
- Jongsri, P.; Wangsomboondee, T.; Rojsitthisak, P.; Seraypheap, K. Effect of molecular weights of chitosan coating on postharvest quality and physicochemical characteristics of mango fruit. LWT 2016, 73, 28–36. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhuang, C.; Gu, W.; Zhao, Y. Effect of molecular weight on the properties of chitosan films prepared using electrostatic spraying technique. Carbohydr. Polym. 2019, 212, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Min, B.J.; Kim, Y.-T.; Kimmel, R.M.; Cooksey, K.; Park, S.I. Antimicrobial activity against foodborne pathogens of chitosan biopolymer films of different molecular weights. LWT -Food Sci. Technol. 2011, 44, 565–569. [Google Scholar] [CrossRef]
- Zheng, L.-Y.; Zhu, J.-F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Shelma, R.; Sharma, C.P. Acyl modified chitosan derivatives for oral delivery of insulin and curcumin. J. Mater. Sci. Mater. Med. 2010, 21, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Long, Z.; Xiao, H.; Dong, C. Preparation of N, N, N-trimethyl chitosan via a novel approach using dimethyl carbonate. Carbohydr. Polym. 2017, 169, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, R.S.; Divyashree, K.N.; Viswanath, P.; Srinivas, P.; Raj, B. Preparation of N-vanillyl chitosan and 4-hydroxybenzyl chitosan and their physico-mechanical, optical, barrier, and antimicrobial properties. Carbohydr. Polym. 2012, 87, 110–116. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Kim, D.-G.; Jang, M.-K.; Nah, J.-W. Preparation and spectroscopic characterization of methoxy poly(ethylene glycol)-grafted water-soluble chitosan. Carbohydr. Res. 2008, 343, 282–289. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abd El-Ghany, N.A. Preparation and antimicrobial activity of some carboxymethyl chitosan acyl thiourea derivatives. Int. J. Biol. Macromol. 2012, 50, 1280–1285. [Google Scholar] [CrossRef]
- Botelho da Silva, S.; Krolicka, M.; van den Broek, L.A.M.; Frissen, A.E.; Boeriu, C.G. Water-soluble chitosan derivatives and pH-responsive hydrogels by selective C-6 oxidation mediated by TEMPO-laccase redox system. Carbohydr. Polym. 2018, 186, 299–309. [Google Scholar] [CrossRef]
- Fu, Y.; Xiao, C.; Liu, J. Facile fabrication of quaternary water soluble chitosan-sodium alginate gel and its affinity characteristic toward multivalent metal ion. Environ. Technol. Innov. 2019, 13, 340–345. [Google Scholar] [CrossRef]
- Alfaro, L.; Chotiko, A.; Chouljenko, A.; Janes, M.; King, J.M.; Sathivel, S. Development of water-soluble chitosan powder and its antimicrobial effect against inoculated Listeria innocua NRRL B-33016 on shrimp. Food Control 2018, 85, 453–458. [Google Scholar] [CrossRef]
- Chouljenko, A.; Chotiko, A.; Reyes, V.; Alfaro, L.; Liu, C.; Dzandu, B.; Sathivel, S. Application of water-soluble chitosan to shrimp for quality retention. LWT 2016, 74, 571–579. [Google Scholar] [CrossRef]
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 285–287. [Google Scholar] [CrossRef]
- Sarwar, A.; Katas, H.; Zin, N.M. Antibacterial effects of chitosan–tripolyphosphate nanoparticles: Impact of particle size molecular weight. J. Nanoparticle Res. 2014, 16, 2517. [Google Scholar] [CrossRef]
- Xing, K.; Chen, X.G.; Kong, M.; Liu, C.S.; Cha, D.S.; Park, H.J. Effect of oleoyl-chitosan nanoparticles as a novel antibacterial dispersion system on viability, membrane permeability and cell morphology of Escherichia coli and Staphylococcus aureus. Carbohydr. Polym. 2009, 76, 17–22. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef]
- Tamara, F.; Lin, C.; Mi, F.-L.; Ho, Y.-C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Li, X.; Feng, X.; Yang, S.; Fu, G.; Wang, T.; Su, Z. Chitosan kills Escherichia coli through damage to be of cell membrane mechanism. Carbohydr. Polym. 2010, 79, 493–499. [Google Scholar] [CrossRef]
- Ataei, S.; Khorasani, S.N.; Neisiany, R.E. Biofriendly vegetable oil healing agents used for developing self-healing coatings: A review. Prog. Org. Coat. 2019, 129, 77–95. [Google Scholar] [CrossRef]
- Scheiner, M.; Dickens, T.J.; Okoli, O. Progress towards self-healing polymers for composite structural applications. Polymer 2016, 83, 260–282. [Google Scholar] [CrossRef]
- Garcia, S.J. Effect of polymer architecture on the intrinsic self-healing character of polymers. Eur. Polym. J. 2014, 53, 118–125. [Google Scholar] [CrossRef]
- Mauldin, T.C.; Kessler, M.R. Self-healing polymers and composites. Int. Mater. Rev. 2010, 55, 317–346. [Google Scholar] [CrossRef]
- Szabó, T.; Molnár-Nagy, L.; Bognár, J.; Nyikos, L.; Telegdi, J. Self-healing microcapsules and slow release microspheres in paints. Prog. Org. Coat. 2011, 72, 52–57. [Google Scholar] [CrossRef]
- Hefni, H.H.H.; Azzam, E.M.; Badr, E.A.; Hussein, M.; Tawfik, S.M. Synthesis, characterization and anticorrosion potentials of chitosan-g-PEG assembled on silver nanoparticles. Int. J. Biol. Macromol. 2016, 83, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Chitosan-based self-healing protective coatings doped with cerium nitrate for corrosion protection of aluminum alloy 2024. Progr. Org. Coat. 2012, 75, 8–13. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, D.; Wan, Y. 2-Mercaptobenzothiazole doped chitosan/11-alkanethiolate acid composite coating: Dual function for copper protection. Appl. Surface Sci. 2011, 257, 10529–10534. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Pancrecious, J.K.; Rajan, T.P.D.; Pai, B.C. Chapter 17—Smart Coatings. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Mohapatra, S., Nguyen, T.A., Nguyen-Tri, P., Eds.; Woodhead Publishing: Duxford, UK, 2019; pp. 341–372. [Google Scholar]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Functionalized chitosan-based coatings for active corrosion protection. Surface Coat. Technol. 2013, 226, 51–59. [Google Scholar] [CrossRef]
- Mohandas, A.; Deepthi, S.; Biswas, R.; Jayakumar, R. Chitosan based metallic nanocomposite scaffolds as antimicrobial wound dressings. Bioact. Mater. 2018, 3, 267–277. [Google Scholar] [CrossRef]
- Ding, F.; Li, H.; Du, Y.; Shi, X. Recent advances in chitosan-based self-healing materials. Res. Che. Intermed. 2018, 44, 4827–4840. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Freire, C.S.R.; Fernandes, S.C.M.; Kallip, S.; Lisenkov, A.; Gandini, A.; Ferreira, M.G.S. Self-healing protective coatings with “green” chitosan based pre-layer reservoir of corrosion inhibitor. J. Mater. Chem. 2011, 21, 4805. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S. Synthesis and characterization of a novel amphiphilic chitosan–polylactide graft copolymer. Carbohydr. Polym. 2005, 59, 165–171. [Google Scholar] [CrossRef]
- Wong, K.; Sun, G.; Zhang, X.; Dai, H.; Liu, Y.; He, C.; Leong, K.W. PEI-g-chitosan, a Novel Gene Delivery System with Transfection Efficiency Comparable to Polyethylenimine in vitro and after Liver Administration in vivo. Bioconjugate Chem. 2006, 17, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhattacharya, W.; Singh, M.; Halder, D.; Mitra, A. Plant latex capped colloidal silver nanoparticles: A potent anti-biofilm and fungicidal formulation. J. Mol. Liq. 2017, 230, 705–713. [Google Scholar] [CrossRef]
- Kumari, R.; Brahma, G.; Rajak, S.; Singh, M.; Kumar, S. Antimicrobial activity of green silver nanoparticles produced using aqueous leaf extract of Hydrocotyle rotundifolia. Orient. Pharm. Exp. Med. 2016, 16, 195–201. [Google Scholar] [CrossRef]
- Swargiary, M.; Kumar, S. One pot phytosynthesis of gold nanoparticles using aqueous extract of elephant apple- an eco-friendly approach. Orient. Pharm. Exp. Med. 2017, 17, 285–289. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I. Electrodeposition of hydroxyapatite–silver–chitosan nanocomposite coatings. Surface Coat. Technol. 2008, 202, 3815–3821. [Google Scholar] [CrossRef]
- Mishra, S.K.; Ferreira, J.M.F.; Kannan, S. Mechanically stable antimicrobial chitosan–PVA–silver nanocomposite coatings deposited on titanium implants. Carbohydr. Polym. 2015, 121, 37–48. [Google Scholar] [CrossRef]
- Pounraj, S.; Somu, P.; Paul, S. Chitosan and graphene oxide hybrid nanocomposite film doped with silver nanoparticles efficiently prevents biofouling. Appl. Surface Sci. 2018, 452, 487–497. [Google Scholar] [CrossRef]
- Lin, B.; Luo, Y.; Teng, Z.; Zhang, B.; Zhou, B.; Wang, Q. Development of silver/titanium dioxide/chitosan adipate nanocomposite as an antibacterial coating for fruit storage. LWT-Food Sci. Technol. 2015, 63, 1206–1213. [Google Scholar] [CrossRef]
- Ortiz-Duarte, G.; Pérez-Cabrera, L.E.; Artés-Hernández, F.; Martínez-Hernández, G.B. Ag-chitosan nanocomposites in edible coatings affect the quality of fresh-cut melon. Postharvest Biol. Technol. 2019, 147, 174–184. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Burgess, J.G. Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 2017, 168, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Roosen, J.; Spooren, J.; Binnemans, K. Adsorption performance of functionalized chitosan–silica hybrid materials toward rare earths. J. Mater. Chem. A 2014, 2, 19415–19426. [Google Scholar] [CrossRef]
- Grande, C.D.; Mangadlao, J.; Fan, J.; De Leon, A.; Delgado-Ospina, J.; Rojas, J.G.; Rodrigues, D.F.; Advincula, R. Chitosan Cross-Linked Graphene Oxide Nanocomposite Films with Antimicrobial Activity for Application in Food Industry. Macromol. Symp. 2017, 374, 1600114. [Google Scholar] [CrossRef]
- Yan, H.; Yang, H.; Li, A.; Cheng, R. pH-tunable surface charge of chitosan/graphene oxide composite adsorbent for efficient removal of multiple pollutants from water. Chem. Eng. J. 2016, 284, 1397–1405. [Google Scholar] [CrossRef]
- Dong, X.; Wei, C.; Liang, J.; Liu, T.; Kong, D.; Lv, F. Thermosensitive hydrogel loaded with chitosan-carbon nanotubes for near infrared light triggered drug delivery. Colloids Surfaces B Biointerfaces 2017, 154, 253–262. [Google Scholar] [CrossRef]
- Papadimitriou, L.; Kaliva, M.; Vamvakaki, M.; Chatzinikolaidou, M. Immunomodulatory Potential of Chitosan-graft-poly(ε-caprolactone) Copolymers toward the Polarization of Bone-Marrow-Derived Macrophages. ACS Biomater. Sci. Eng. 2017, 3, 1341–1349. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Ramkumar, M.C.; Arun Kumar, A.; Padmanabhan, P.V.A.; Pichumani, M.; Bendavid, A.; Cools, P.; De Geyter, N.; Morent, R.; Kumar, V.; et al. Evaluation of surface properties of low density polyethylene (LDPE) films tailored by atmospheric pressure non-thermal plasma (APNTP) assisted co-polymerization and immobilization of chitosan for improvement of antifouling properties. Mater. Sci. Eng. C 2019, 94, 150–160. [Google Scholar] [CrossRef]
- Trivedi, P.; Saloranta-Simell, T.; Maver, U.; Gradišnik, L.; Prabhakar, N.; Smått, J.-H.; Mohan, T.; Gericke, M.; Heinze, T.; Fardim, P. Chitosan–Cellulose Multifunctional Hydrogel Beads: Design, Characterization and Evaluation of Cytocompatibility with Breast Adenocarcinoma and Osteoblast Cells. Bioengineering 2018, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, Y.; Yang, X.; Xiong, Y.; Li, Y.; Chen, B.; Lai, W.-F.; Rogach, A.L. Water-Soluble Biocompatible Copolymer Hypromellose Grafted Chitosan Able to Load Exogenous Agents and Copper Nanoclusters with Aggregation-Induced Emission. Adv. Funct. Mater. 2018, 28, 1802848. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Huang, L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: A pilot study in vitro. Biotechnol. Biotechnol. Equip. 2017, 31, 766–773. [Google Scholar] [CrossRef]
- Njoku, D.I.; Cui, M.; Xiao, H.; Shang, B.; Li, Y. Understanding the anticorrosive protective mechanisms of modified epoxy coatings with improved barrier, active and self-healing functionalities: EIS and spectroscopic techniques. Sci. Rep. 2017, 7, 15597. [Google Scholar] [CrossRef]
- Benucci, I.; Liburdi, K.; Cacciotti, I.; Lombardelli, C.; Zappino, M.; Nanni, F.; Esti, M. Chitosan/clay nanocomposite films as supports for enzyme immobilization: An innovative green approach for winemaking applications. Food Hydrocoll. 2018, 74, 124–131. [Google Scholar] [CrossRef]
- Azharul Islam, M.; Tan, Y.L.; Atikul Islam, M.; Romić, M.; Hameed, B.H. Chitosan–bleaching earth clay composite as an efficient adsorbent for carbon dioxide adsorption: Process optimization. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 554, 9–15. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.-C.; Chen, M.; Shi, Q.; Sun, R.; Wang, X. Chitosan/rectorite nanocomposite with injectable functionality for skin hemostasis. J. Mater. Chem. B 2018, 6, 6544–6549. [Google Scholar] [CrossRef]
- Susilowati, E.; MaryaniAshadi, *!!! REPLACE !!!*. Preparation of silver-chitosan nanocomposites and coating on bandage for antibacterial wound dressing application. AIP Conf. Proc. 2016, 1710, 030015. [Google Scholar]
- El-Sherbiny, I.M.; Hefnawy, A.; Salih, E. New core–shell hyperbranched chitosan-based nanoparticles as optical sensor for ammonia detection. Int. J. Biol. Macromol. 2016, 86, 782–788. [Google Scholar] [CrossRef]
- Ogugbue, C.J.; Sawidis, T. Bioremediation and Detoxification of Synthetic Wastewater Containing Triarylmethane Dyes by Aeromonas hydrophila Isolated from Industrial Effluent. Biotechnol. Res. Int. 2011, 2011, 1–11. [Google Scholar] [CrossRef]
- Brüschweiler, B.J. Toxicity of non-regulated aromatic amines from azo dyes in textiles: Knowns and unknowns. Toxicol. Lett. 2013, 221, S54. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]
- Shen, C.; Shen, Y.; Wen, Y.; Wang, H.; Liu, W. Fast and highly efficient removal of dyes under alkaline conditions using magnetic chitosan-Fe(III) hydrogel. Water Res. 2011, 45, 5200–5210. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Khan, S.B.; Kamal, T.; Anwar, Y.; Alamry, K.A.; Asiri, A.M. Anti-bacterial chitosan/zinc phthalocyanine fibers supported metallic and bimetallic nanoparticles for the removal of organic pollutants. Carbohydr. Polym. 2017, 173, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Nithya, A.; JeevaKumari, H.L.; Rokesh, K.; Ruckmani, K.; Jeganathan, K.; Jothivenkatachalam, K. A versatile effect of chitosan-silver nanocomposite for surface plasmonic photocatalytic and antibacterial activity. J. Photochem. Photobiol. B Biol. 2015, 153, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, O.; Bonardd, S.; Saldías, C.; Radic, D.; Leiva, Á. Biobased Chitosan Nanocomposite Films Containing Gold Nanoparticles: Obtainment, Characterization, and Catalytic Activity Assessment. ACS Appl. Mater. Interfaces 2017, 9, 16561–16570. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.T.; Nithya, A.; Jothivenkatachalam, K. Photocatalytic and antimicrobial activities of chitosan-TiO2 nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Rasoulifard, M.H.; Seyed Dorraji, M.S.; Amani-Ghadim, A.R.; Keshavarz-babaeinezhad, N. Visible-light photocatalytic activity of chitosan/polyaniline/CdS nanocomposite: Kinetic studies and artificial neural network modeling. Appl. Catal. A Gen. 2016, 514, 60–70. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnakumar, B.; Sobral, A.J.F.N.; Koh, J. Bio-based (chitosan/PVA/ZnO) nanocomposites film: Thermally stable and photoluminescence material for removal of organic dye. Carbohydr. Polym. 2019, 205, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M. Synthesis and characterization of magnetic nanocomposite of chitosan/SiO2/carbon nanotubes and its application for dyes removal. J. Clean. Prod. 2017, 145, 105–113. [Google Scholar] [CrossRef]
- Masilompane, T.M.; Chaukura, N.; Mishra, S.B.; Mishra, A.K. Chitosan-lignin-titania nanocomposites for the removal of brilliant black dye from aqueous solution. Int. J. Biol. Macromol. 2018, 120, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Darvishi Cheshmeh Soltani, R.; Khataee, A.R.; Safari, M.; Joo, S.W. Preparation of bio-silica/chitosan nanocomposite for adsorption of a textile dye in aqueous solutions. Int. Biodeterior. Biodegrad. 2013, 85, 383–391. [Google Scholar] [CrossRef]
- Dhanavel, S.; Manivannan, N.; Mathivanan, N.; Gupta, V.K.; Narayanan, V.; Stephen, A. Preparation and characterization of cross-linked chitosan/palladium nanocomposites for catalytic and antibacterial activity. J. Mol. Liq. 2018, 257, 32–41. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saravanan, R.; Agarwal, S.; Gracia, F.; Khan, M.M.; Qin, J.; Mangalaraja, R.V. Degradation of azo dyes under different wavelengths of UV light with chitosan-SnO2 nanocomposites. J. Mol. Liq. 2017, 232, 423–430. [Google Scholar] [CrossRef]
- Cao, Q.; Xiao, L.; Zeng, L.; Cao, C.; Wang, J. Ag3PO4/chitosan/CdS nanocomposites exhibiting high photocatalytic activities under visible-light illumination. Powder Technol. 2017, 321, 1–8. [Google Scholar] [CrossRef]
- Vanamudan, A.; Sadhu, M.; Pamidimukkala, P.S. Nanostructured zirconium tungstate and its bionanocomposite with chitosan: Wet peroxide photocatalytic degradation of dyes. J. Taiwan Inst. Chem. Eng. 2018, 85, 74–82. [Google Scholar] [CrossRef]
- Karpuraranjith, M.; Thambidurai, S. Biotemplate-SnO2 particles intercalated PANI matrix: Enhanced photo catalytic activity for degradation of MB and RY-15 dye. Polym. Degrad. Stab. 2016, 133, 108–118. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Alamry, K.A.; Asiri, A.M. Chitosan-titanium oxide fibers supported zero-valent nanoparticles: Highly efficient and easily retrievable catalyst for the removal of organic pollutants. Sci. Rep. 2018, 8, 6260. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Alamry, K.A.; Asiri, A.M.; Sobahi, T.R.A. Chitosan coated cotton cloth supported zero-valent nanoparticles: Simple but economically viable, efficient and easily retrievable catalysts. Sci. Rep. 2017, 7, 16957. [Google Scholar] [CrossRef]
- Magesan, P.; Sanuja, S.; Umapathy, M.J. Novel hybrid chitosan blended MoO3–TiO2 nanocomposite film: Evaluation of its solar light photocatalytic and antibacterial activities. RSC Adv. 2015, 5, 42506–42515. [Google Scholar] [CrossRef]
- Zhou, J.; Lü, Q.-F.; Luo, J.-J. Efficient removal of organic dyes from aqueous solution by rapid adsorption onto polypyrrole–based composites. J. Clean. Prod. 2017, 167, 739–748. [Google Scholar] [CrossRef]
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: Some basic aspects. Mar. Ecol. Progr. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef]
- Qian, P.Y.; Lau, S.C.K.; Dahms, H.U.; Dobretsov, S.; Harder, T. Marine Biofilms as Mediators of Colonization by Marine Macroorganisms: Implications for Antifouling and Aquaculture. Mar. Biotechnol. 2007, 9, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R.; Briand, J.-F. Marine biofilms on artificial surfaces: Structure and dynamics. Environ. Microbiol. 2013, 15, 2879–2893. [Google Scholar] [CrossRef] [PubMed]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Progr. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2010, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, É.; Bonnet, C.; Lemarchand, K. Biofouling Growth in Cold Estuarine Waters and Evaluation of Some Chitosan and Copper Anti-Fouling Paints. Int. J. Mol. Sci. 2009, 10, 3209–3223. [Google Scholar] [CrossRef] [PubMed]
- Al-Naamani, L.S. Antifouling properties of chitosan coatings on plastic substrates. J. Agric. Mar. Sci. [JAMS] 2019, 23, 92. [Google Scholar] [CrossRef][Green Version]
- Dobretsov, S.; Abed, R.M.M.; Muthukrishnan, T.; Sathe, P.; Al-Naamani, L.; Queste, B.Y.; Piontkovski, S. Living on the edge: Biofilms developing in oscillating environmental conditions. Biofouling 2018, 34, 1064–1077. [Google Scholar] [CrossRef]
- Thomas, V.; Yallapu, M.M.; Sreedhar, B.; Bajpai, S.K. Fabrication, Characterization of Chitosan/Nanosilver Film and Its Potential Antibacterial Application. J. Biomater. Sci. Polym. Ed. 2009, 20, 2129–2144. [Google Scholar] [CrossRef]
- Reighard, K.P.; Hill, D.B.; Dixon, G.A.; Worley, B.V.; Schoenfisch, M.H. Disruption and eradication of P. aeruginosabiofilms using nitric oxide-releasing chitosan oligosaccharides. Biofouling 2015, 31, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Elshaarawy, R.F.M.; Mustafa, F.H.A.; van Geelen, L.; Abou-Taleb, A.E.A.; Tadros, H.R.Z.; Kalscheuer, R.; Janiak, C. Mining marine shell wastes for polyelectrolyte chitosan anti-biofoulants: Fabrication of high-performance economic and ecofriendly anti-biofouling coatings. Carbohydr. Polym. 2017, 172, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Imai, M.; Suzuki, I.; Sawai, J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochem. Eng. J. 2008, 40, 485–491. [Google Scholar] [CrossRef]
- Nigmatullin, R.; Konovalova, V.; Pobigay, G. Development of antimicrobial membranes via the surface tethering of chitosan. J. Appl. Polym. Sci. 2009, 111, 1697–1705. [Google Scholar] [CrossRef]
- Salehi, H.; Rastgar, M.; Shakeri, A. Anti-fouling and high water permeable forward osmosis membrane fabricated via layer by layer assembly of chitosan/graphene oxide. Appl. Surface Sci. 2017, 413, 99–108. [Google Scholar] [CrossRef]
- Bulwan, M.; Wójcik, K.; Zapotoczny, S.; Nowakowska, M. Chitosan-Based Ultrathin Films as Antifouling, Anticoagulant and Antibacterial Protective Coatings. J. Biomater. Sci. Polym. Ed. 2012, 23, 1963–1980. [Google Scholar] [CrossRef]
- Shanthana Lakshmi, D.; Jaiswar, S.; saxena, M.; Tasselli, F.; Raval, H.D. Preparation and performance of biofouling resistant PAN/chitosan hollow fiber membranes. 3 Biotech 2017, 7, 224. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H.; Beygzadeh, M. Novel high flux antifouling nanofiltration membranes for dye removal containing carboxymethyl chitosan coated Fe3O4 nanoparticles. Desalination 2014, 349, 145–154. [Google Scholar] [CrossRef]
- Wang, R.; Song, X.; Xiang, T.; Liu, Q.; Su, B.; Zhao, W.; Zhao, C. Mussel-inspired chitosan-polyurethane coatings for improving the antifouling and antibacterial properties of polyethersulfone membranes. Carbohydr. Polym. 2017, 168, 310–319. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Gong, T.; Li, C.; Bian, B.; Wu, Y.; Dawuda, M.M.; Liao, W. Advances in application of small molecule compounds for extending the shelf life of perishable horticultural products: A review. Sci. Hortic. 2018, 230, 25–34. [Google Scholar] [CrossRef]
- Swaminathan, M.S. Food Losses and Food Waste. In Combating Hunger and Achieving Food Security; Cambridge University Press: Cambridge, UK, 2015; pp. 37–46. [Google Scholar]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Preparation of sulfur nanoparticle-incorporated antimicrobial chitosan films. Food Hydrocoll. 2018, 82, 116–123. [Google Scholar] [CrossRef]
- Xu, D.A.N.; Qin, H.-R.; Ren, D.A.N.; Yu, Y.-L. Influence of Coating Time on the Preservation Performance of Chitosan/Montmorillonite Composite Coating on Tangerine Fruits. In The 21st IAPRI World Conference on Packaging; DEStech Publications, Inc.: Zhuhai, China, 2018. [Google Scholar]
- Cosme Silva, G.M.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Naskar, A.; Khan, H.; Sarkar, R.; Kumar, S.; Halder, D.; Jana, S. Anti-biofilm activity and food packaging application of room temperature solution process based polyethylene glycol capped Ag-ZnO-graphene nanocomposite. Mater. Sci. Eng. C 2018, 91, 743–753. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Basumatary, K.; Daimary, P.; Das, S.K.; Thapa, M.; Singh, M.; Mukherjee, A.; Kumar, S. Lagerstroemia speciosa fruit-mediated synthesis of silver nanoparticles and its application as filler in agar based nanocomposite films for antimicrobial food packaging. Food Packag. Shelf Life 2018, 17, 99–106. [Google Scholar] [CrossRef]
- Kumar, S.; Mitra, A.; Halder, D. Centella asiatica leaf mediated synthesis of silver nanocolloid and its application as filler in gelatin based antimicrobial nanocomposite film. LWT 2017, 75, 293–300. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dutta, J.; Dobretsov, S. Nanocomposite Zinc Oxide-Chitosan Coatings on Polyethylene Films for Extending Storage Life of Okra (Abelmoschus esculentus). Nanomaterials 2018, 8, 479. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Mujeeb Rahman, P.; Abdul Mujeeb, V.M.; Muraleedharan, K.; Thomas, S.K. Chitosan/nano ZnO composite films: Enhanced mechanical, antimicrobial and dielectric properties. Arab. J. Chem. 2018, 11, 120–127. [Google Scholar] [CrossRef]
- Saral Sarojini, K.; Indumathi, M.P.; Rajarajeswari, G.R. Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar]
- Chutichude, B.; Chutichude, P. Effects of Chitosan Coating to Some Postharvest Characteristics of Hylocercus undatus (Haw) Brit. and Rose Fruit. Int. J. Agric. Res. 2011, 6, 82–92. [Google Scholar] [CrossRef][Green Version]
- Lin, B.; Du, Y.; Liang, X.; Wang, X.; Wang, X.; Yang, J. Effect of chitosan coating on respiratory behavior and quality of stored litchi under ambient temperature. J. Food Eng. 2011, 102, 94–99. [Google Scholar] [CrossRef]
- Kaya, M.; Česonienė, L.; Daubaras, R.; Leskauskaitė, D.; Zabulionė, D. Chitosan coating of red kiwifruit (Actinidia melanandra) for extending of the shelf life. Int. J. Biol. Macromol. 2016, 85, 355–360. [Google Scholar] [CrossRef]
- Batista Silva, W.; Cosme Silva, G.M.; Santana, D.B.; Salvador, A.R.; Medeiros, D.B.; Belghith, I.; da Silva, N.M.; Cordeiro, M.H.M.; Misobutsi, G.P. Chitosan delays ripening and ROS production in guava (Psidium guajava L.) fruit. Food Chem. 2018, 242, 232–238. [Google Scholar] [CrossRef]
- Drevinskas, T.; Naujokaitytė, G.; Maruška, A.; Kaya, M.; Sargin, I.; Daubaras, R.; Česonienė, L. Effect of molecular weight of chitosan on the shelf life and other quality parameters of three different cultivars of Actinidia kolomikta (kiwifruit). Carbohydr. Polym. 2017, 173, 269–275. [Google Scholar] [CrossRef]
- Castelo Branco Melo, N.F.; de MendonçaSoares, B.L.; Marques Diniz, K.; Ferreira Leal, C.; Canto, D.; Flores, M.A.P.; Henrique da Costa Tavares-Filho, J.; Galembeck, A.; Montenegro Stamford, T.L.; Montenegro Stamford-Arnaud, T.; et al. Effects of fungal chitosan nanoparticles as eco-friendly edible coatings on the quality of postharvest table grapes. Postharvest Biol. Technol. 2018, 139, 56–66. [Google Scholar] [CrossRef]
- Candir, E.; Ozdemir, A.E.; Aksoy, M.C. Effects of chitosan coating and modified atmosphere packaging on postharvest quality and bioactive compounds of pomegranate fruit cv. ‘Hicaznar’. Sci. Hortic. 2018, 235, 235–243. [Google Scholar] [CrossRef]
- Maqbool, M.; Ali, A.; Alderson, P.G.; Zahid, N.; Siddiqui, Y. Effect of a Novel Edible Composite Coating Based on Gum Arabic and Chitosan on Biochemical and Physiological Responses of Banana Fruits during Cold Storage. J. Agric. Food Chem. 2011, 59, 5474–5482. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Gökmen, V. Extending the shelf-life of pomegranate arils with chitosan-ascorbic acid coating. LWT-Food Sci. Technol. 2017, 76, 172–180. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, H. Effect of preharvest chitosan- g -salicylic acid treatment on postharvest table grape quality, shelf life, and resistance to Botrytis cinerea -induced spoilage. Sci. Hortic. 2017, 224, 367–373. [Google Scholar] [CrossRef]
- Eshetu, A.; Ibrahim, A.M.; Forsido, S.F.; Kuyu, C.G. Effect of beeswax and chitosan treatments on quality and shelf life of selected mango (Mangifera indica L.) cultivars. Heliyon 2019, 5, e01116. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Taher, M.A. Influence of edible coatings chitosan/PVP blending with salicylic acid on biochemical fruit skin browning incidence and shelf life of guava fruits cv. ‘Banati’. Sci. Hortic. 2018, 235, 424–436. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Magwaza, L.S. Evaluating the efficacy of moringa leaf extract, chitosan and carboxymethyl cellulose as edible coatings for enhancing quality and extending postharvest life of avocado (Persea americana Mill.) fruit. Food Packag. Shelf Life 2017, 11, 40–48. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H.D. Active chitosan/PVA with ascorbic acid and berry quality of ‘Superior seedless’ grapes. Sci. Hortic. 2017, 224, 286–292. [Google Scholar] [CrossRef]
- Treviño-Garza, M.Z.; García, S.; Heredia, N.; Alanís-Guzmán, M.G.; Arévalo-Niño, K. Layer-by-layer edible coatings based on mucilages, pullulan and chitosan and its effect on quality and preservation of fresh-cut pineapple (Ananas comosus). Postharvest Biol. Technol. 2017, 128, 63–75. [Google Scholar] [CrossRef]
- Xu, D.; Qin, H.; Ren, D. Prolonged preservation of tangerine fruits using chitosan/montmorillonite composite coating. Postharvest Biol. Technol. 2018, 143, 50–57. [Google Scholar] [CrossRef]
- Shi, S.; Wang, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125–131. [Google Scholar] [CrossRef]
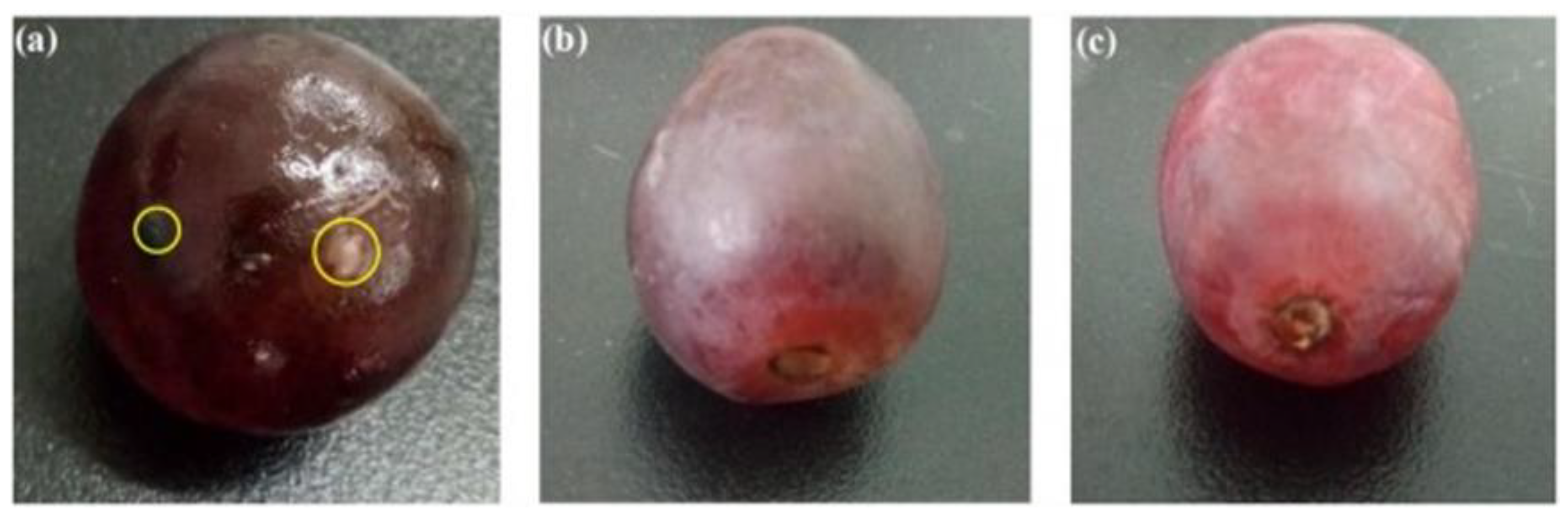
| CS/CS-Composite Film | CS Concentration in FFS (%, w/v) | Nano-Filler Concentration (% w/w of Solid Matter) | Food Systems/Applications | Antimicrobial Activity (Microorganism Tested) | Effect of Films | Thickness, Mechanical and Permeability Properties | References | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Thickness (μm) | TS (MPa) | EAB (%) | WVP or WVTR | |||||||
| CS/TiO2 | 2.5 | TiO2-10 | Red grapes | E. coli, S. aureus, C. albicans, A. niger | Red grapes were preserved for 22 days with composite film compared to 15 days with bare chitosan film before mildew occurred | 70 | 46.33 ± 1.88 | 25.77 ± 2.91 | No data | [147] |
| CS/TiO2 | 2.0 | TiO2-1.0 | Tomato fruit | No data | Composite film delayed the ripening process of tomato fruits and loss in quality | No data | 16.43 ± 0.46 | 53.06 ± 2.15 | 19.14 ± 1.13 (gm−2d−1) | [159] |
| CS/nano ZnO | 2.0 | Zinc acetate-100 | No data | E. coli, S. aureus | Composite films showed good antimicrobial activity against both Gram-negative and Gram-positive bacteria | No data | 41.73 ± 0.48 | 32.44 ± 0.52 | 22.53 ± 0.31 (g/h m2) | [160] |
| CS/Gelatin/AgNPs | 1.8 | AgNPs-0.1 | Red grapes | No data | Shelf-life of red grapes were extended by 14 days compared to control | 87.60 ± 2.19 | 21.19 ± 1.01 | 27.23 ± 0.76 | No data | [155] |
| CS/Bacterial cellulose nanocrystals (BCNC)/AgNPs | 1.0 | AgNPs-1.0 | No data | S. aureus, B. cereus, E. Coli, Ps. Aeruginosa, C. albicans | Addition of BCNC and/or AgNPs in chitosan film improved its physical, mechanical, and antimicrobial properties | 140 | 42.89 ± 1.76 | 22.50 ± 2.07 | 2.16 ± 0.06 (×10−10 g/smPa) | [151] |
| CS/Polyurethane (PU)/nano ZnO | CS/PU ratio 0.25:0.75 | ZnONPs–1.0, 3.0 and 5.0 | Carrot pieces | E. coli, S. aureus | Composite films efficiently enhanced the shelf-life of carrot pieces by 9 days compared to control | 100 | 8.1 | 2.156 | 163.0 (g/m2/day) | [161] |
| CS/Sulfur nanoparticles (SNP) | 2.67 | SNP-2.0 | No data | E. coli, L. monocytogenes | Composite films showed high antibacterial activity against foodborne pathogens and could be potentially used in antimicrobial food packaging | 55.6 ± 4.7 | 39.4 ± 4.4 | 8.3 ± 3.1 | 0.96 ± 0.17 (×10−9 g.m/m2.Pa.s) | [148] |
| Chitosan Based Coating Formulations | Chitosan % (w/v) | Fruits and Vegetables Type | Effects of Coating | References |
|---|---|---|---|---|
| CS only | 1.0–3.0 | Rose or Pitaya fruit (Hylocercus undatus) | Coating containing 3% chitosan played the beneficial role for slowing down fruit withering, and maintained the fresh appearance and extended the maximal storability at ambient temperature | [162] |
| CS only | 1.0 | Litchi fruit | Coating effectively reduced the respiration rate as well as transpiration rate of litchi fruits during storage | [163] |
| CS only | 1.0 | Red kiwifruit (Actinidia melanandra) | Chitosan coating was found to enhance the shelf-life of kiwifruit | [164] |
| CS only | 1.0–3.0 | Mango fruit (Mangifera indica) | Coating delayed the climacteric peak, water loss, firmness, and ultimately prolonged the quality attributes of fruit | [150] |
| CS only | 1.0–3.0 | Guava fruit (Psidium guajava) | Coating effectively maintained the quality of guava fruits by increasing antioxidant value, and delaying ripening of fruits during storage at room temperature | [165] |
| CS only (Low, med, and high molecular weight) | 1.0 | Kiwifruit (Actinidia kolomikta) | High-molecular-weight chitosan showed more pronounced improvements on the shelf-life of kiwifruit | [166] |
| CS-nanoparticles | 1.0 | Table grapes | Edible coatings of chitosan nanoparticle efficiently delayed the ripening process and led to reduced weight loss, soluble solids, and sugar contents | [167] |
| CS coating with modified atmosphere packaging (MAP) | 1.0 | Pomegranate fruit | Chitosan coating with MAP treatment led to maintenance of visual quality and initial red aril colour intensity of fruits for up to 6 months under cold storage at 6 °C. | [168] |
| CS/Gum Arabic | 1.0 | Banana fruit | Coating delayed ripening by decreasing the rate of respiration of fruits and enhanced shelf-life for up to 33 days | [169] |
| CS/Ascorbic acid | 1.0–2.0 | Pomegranate arils | Shelf-life of arils could be extended to 21 days at 5 °C from 10 days for uncoated arils (control) | [170] |
| CS/g-Salicylic acid | 1.0 | Table grapes | Composite coatings on grapes improved the post-harvest life by decreasing the rate of respiration, decay incidence, and weight loss, and by maintaining/improving levels of total soluble solids, titratable acidity, and sensory attributes during storage | [171] |
| CS/beeswax | 1.5–2.0 | Mango fruit (M. indica) | Coating on fruits ultimately maintained firmness and improved shelf-life up to 3 weeks compared to control (uncoated) | [172] |
| CS/ PVP/ Salicylic acid (SA) | 1.0 | Guava fruit (P. guajava) | Fruits coated with CS/PVP-SA showed minimum loss in water and browning of skin that helped in maintaining fruit colour and firmness | [173] |
| CS/Alginate with Pomegranate peel extract (PPE) | 1.0 | Guava fruit (P. guajava) | Coatings proved efficient in maintaining the quality of guava for up to 20 days at low temperature storage | [174] |
| CS/CMC/ Moringa leaf extract | 0.5–1.0 | Avocado fruit (Persea americana) | Coating on fruits efficiently reduced the respiration rate, moisture loss, and firmness that ultimately helps in improving fruit quality and shelf-life | [175] |
| CS/PVA blended with ascorbic acid (AA) | N/A | ‘Superior seedless’ grapes | Coating treatment significantly reduced water loss, shattering of berry, and delayed the colour change and titratable acidity | [176] |
| CS/Pullulan, CS/Linseed, CS/Nopal cactus, CS/Aloe mucilage | 1.5 | Fresh-cut pineapple (Ananas comosus) | Layer-by-layer edible coatings were beneficial to maintain the quality and extension of the shelf-life of cut pineapple | [177] |
| CS/MMT | 1.5 | Tangerine fruits | Coated fruit showed reduced decay rate, loss in weight, and improvements in total soluble solids and titratable acidity | [178] |
| CS/nano-silica | 2.0 | Longan fruit | Coating shown to improve the quality of longan fruits for the period of extended storage | [179] |
| CS-AgNPs | 1.0 | Fresh-cut melon | Coatings decreased the rate of respiration and rate of production of ethylene compared to uncoated samples and thus had better sensory quality up to 13 days at 5 °C | [84] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Chitosan Nanocomposite Coatings for Food, Paints, and Water Treatment Applications. Appl. Sci. 2019, 9, 2409. https://doi.org/10.3390/app9122409
Kumar S, Ye F, Dobretsov S, Dutta J. Chitosan Nanocomposite Coatings for Food, Paints, and Water Treatment Applications. Applied Sciences. 2019; 9(12):2409. https://doi.org/10.3390/app9122409
Chicago/Turabian StyleKumar, Santosh, Fei Ye, Sergey Dobretsov, and Joydeep Dutta. 2019. "Chitosan Nanocomposite Coatings for Food, Paints, and Water Treatment Applications" Applied Sciences 9, no. 12: 2409. https://doi.org/10.3390/app9122409
APA StyleKumar, S., Ye, F., Dobretsov, S., & Dutta, J. (2019). Chitosan Nanocomposite Coatings for Food, Paints, and Water Treatment Applications. Applied Sciences, 9(12), 2409. https://doi.org/10.3390/app9122409







