Ultra-Low Interfacial Tension Foam System for Enhanced Oil Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Liquid
2.2. Additives
2.3. Foaming Process
2.4. Foaming Capacity and Foam Stability
2.5. Oil-Liquid IFT of Liquid Phase
2.6. Core-Flooding Experiment
3. Results and Discussion
3.1. Foaming Properties and Oil-Liquid IFT of Surfactants
3.2. Foaming Properties and Oil-Liquid IFT of Binary Surfactants
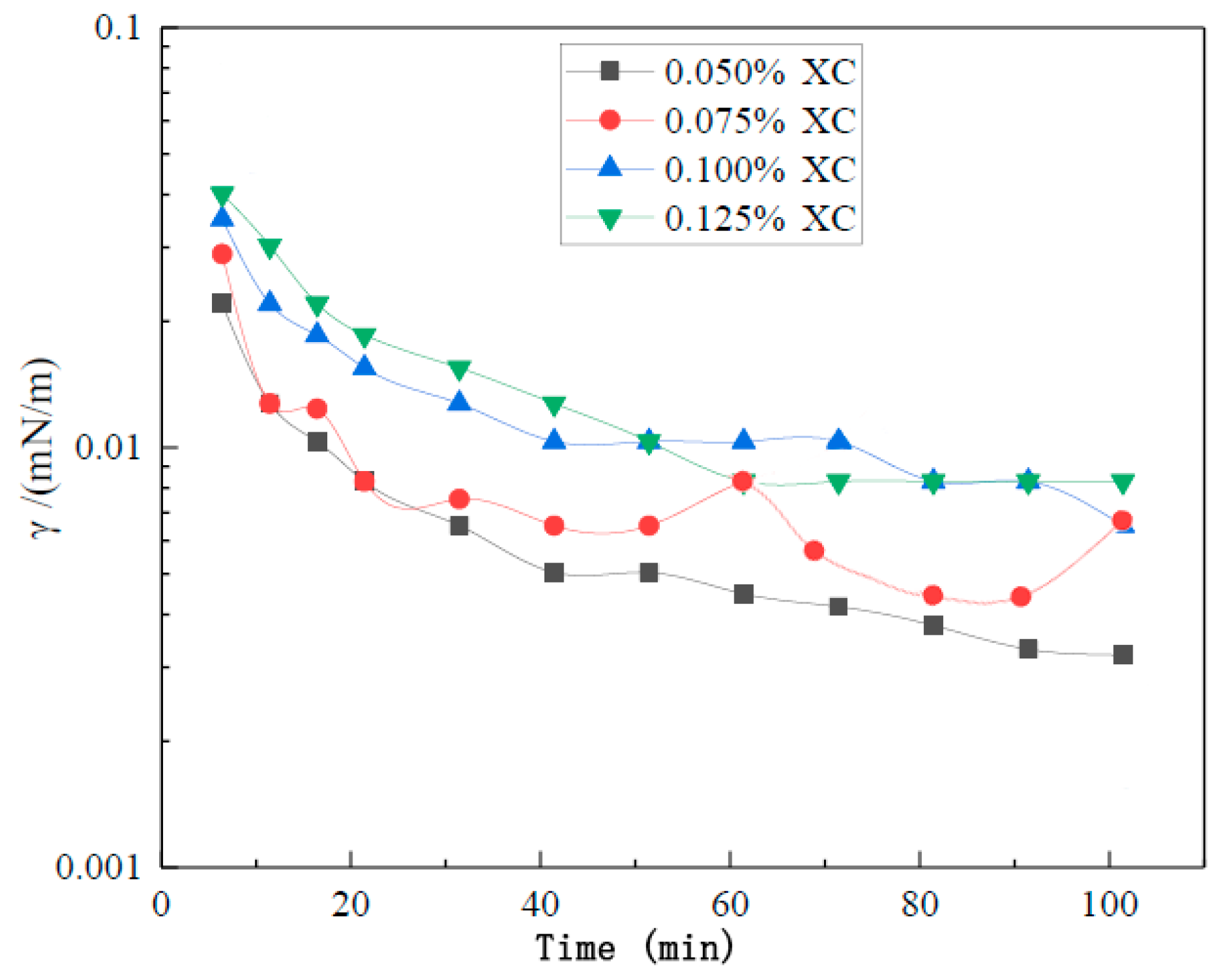
3.3. Foam Stabilizer Selection
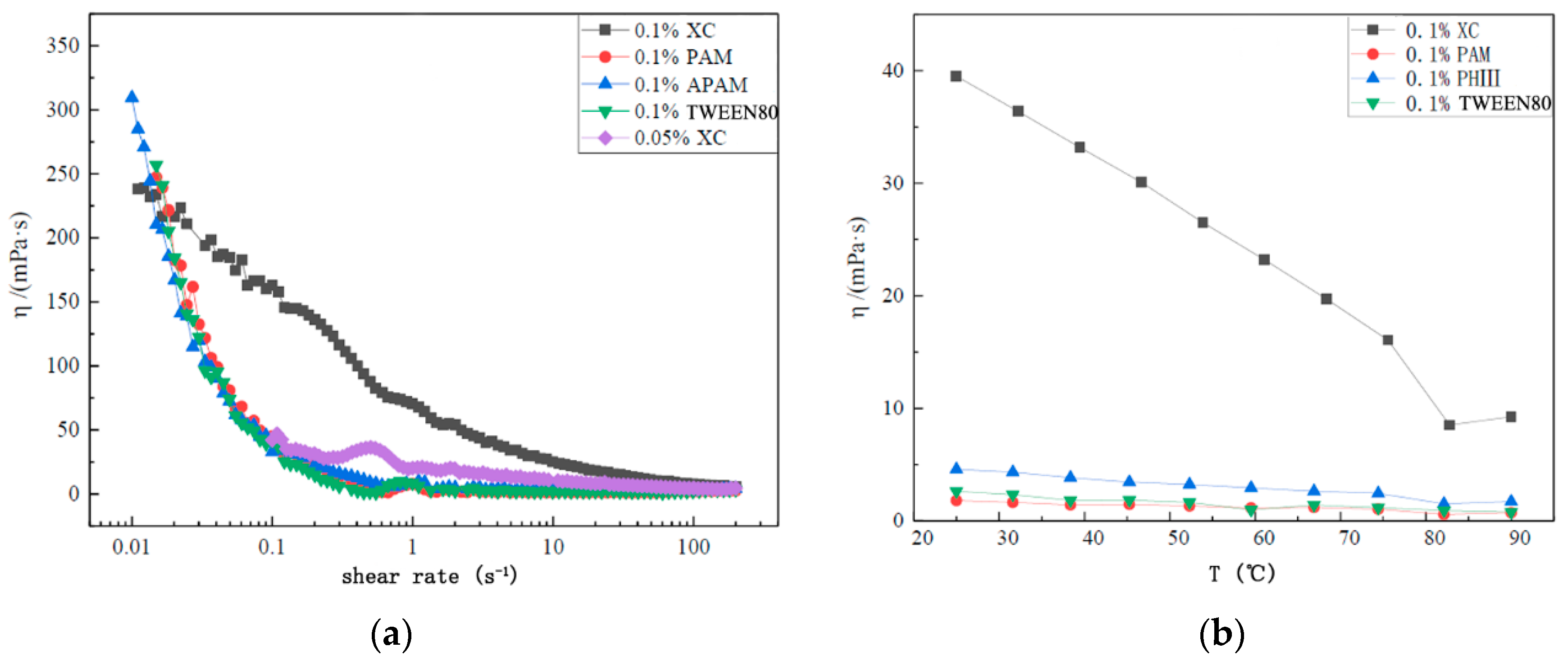
3.4. Optimization of Foam System Performance
3.5. Macroscopic Flow Characteristics and EOR Effect
4. Conclusions and Proposals
4.1. Conclusions
- The foam system should have both a high foaming capacity and a low oil-liquid IFT obtained by using different surfactant and polymer structures and adjusting the composition of chemical additives.
- The compounding of DHPB and SDS (mass ratio at 1:1.5) exhibited the best foaming properties (FCI: 117,600 mL·min) and the lowest oil-liquid IFT (0.0076 mN/m). The compounding of surfactants with the same carbon number showed better results.
- Increasing polymer concentration lowered the oil-liquid IFT reduction rate, and led to an increase in the oil-liquid IFT. The viscosity of the liquid phase depends on the polymer concentration, which expected to affect the diffusion of surfactants and gas.
- Ultra-low IFT foam system could function as both surfactant-polymer system and foam system in flooding process, which means it mainly functions in oil-liquid IFT reduction in high permeability conditions and blocks the high permeability channels in low permeability conditions. This feature meant the ultra-low IFT foam system could be more applicable in the EOR process.
4.2. Proposals
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.; Elraies, K.A.; Tan, I.M.; Hashmet, M.R. Experimental investigation of associative polymer performance for CO2, foam enhanced oil recovery. J. Pet. Sci. Eng. 2017, 157, 971–979. [Google Scholar] [CrossRef]
- Talebian, S.H.; Masoudi, R.; Tan, I.M.; Zitha, P.L.J. Foam assisted CO2-EOR: A review of concept, challenges, and future prospects. J. Pet. Sci. Eng. 2014, 120, 202–215. [Google Scholar] [CrossRef]
- Awan, A.R.; Teigland, R.; Kleppe, J. A Survey of North Sea Enhanced-Oil-Recovery Projects Initiated During the Years 1975 to 2005. SPE Reserv. Eval. Eng. 2008, 11, 497–512. [Google Scholar] [CrossRef]
- Jones, S.A.; Van Der Bent, V.; Farajzadeh, R.; Rossen, W.R.; Vincent-Bonnieu, S. Surfactant screening for foam EOR: Correlation between bulk and core-flood experiments. Colloids Surf. A Physicochem. Eng. Asp. 2016, 500, 166–176. [Google Scholar] [CrossRef]
- Guo, H.; Faber, M.J.; Buijse, M.A.; Zitha, P.L. A Novel Alkaline-Surfactant-Foam EOR Process. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 19–21 July 2011. [Google Scholar]
- Ocampo, A.; Restrepo, A.; Cifuentes, H.; Hester, J.; Orozco, N.; Gil, C.; Castro, E.; Lopera, S.; Gonzalez, C. Successful Foam EOR Pilot in a Mature Volatile Oil Reservoir Under Miscible Gas Injection. J. Pet. Technol. 2013, 65, 117–119. [Google Scholar]
- Druetta, P.; Raffa, P.; Picchioni, F. Plenty of Room at the Bottom: Nanotechnology as Solution to an Old Issue in Enhanced Oil Recovery. Appl. Sci. 2018, 8, 2596. [Google Scholar] [CrossRef]
- Talebian, S.H.; Masoudi, R.; Tan, I.M.; Zitha, P.L. Foam assisted CO2-EOR; Concepts, Challenges and Applications. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2–4 July 2013. [Google Scholar]
- Ahmed, S.; Elraies, K.A.; Tan, I.M.; Mumtaz, M. A Review on CO2 Foam for Mobility Control: Enhanced Oil Recovery. In ICIPEG 2016; Springer: Singapore, 2017. [Google Scholar]
- Srisuriyachai, F.; Pancharoen, M.; Laochamroonvoraponse, R.; Vathanapanich, Y. Reduction of Surfactant Adsorption: A Study of Dynamic Surfactant Adsorption/Desorption in Shaly-sandstone Reservoir. In Proceedings of the IOR 2019—20th European Symposium on Improved Oil Recovery, Pau, France, 8–11 May 2019. [Google Scholar]
- Gu, Q.; Liu, H.; Zhang, Y. Lattice Boltzmann Simulation of Immiscible Two-Phase Displacement in Two-Dimensional Berea Sandstone. Appl. Sci. 2018, 8, 1497. [Google Scholar] [CrossRef]
- Ologundudu, T.; Odiyo, J.; Ekosse, G.I. Fluoride Sorption Efficiency of Vermiculite Functionalised with Cationic Surfactant: Isotherm and Kinetics. Appl. Sci. 2016, 6, 277. [Google Scholar] [CrossRef]
- Sahner, R.A.; Trivedi, K.; Puliafito, A. Generalized Correlations for Predicting Solubility, Swelling and Viscosity Behavior of CO2-Crude Oil Systems. J. Petrol. Technol. 1965, 17, 102–106. [Google Scholar]
- Kang, P.S.; Lim, J.S.; Huh, C. Artificial Neural Network Model to Estimate the Viscosity of Polymer Solutions for Enhanced Oil Recovery. Appl. Sci. 2016, 6, 188. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, M.C.; Dudley, K.L.; Lawrence, R.W. Conductive Carbon Nanofiber–Polymer Foam Structures. Adv. Mater. 2010, 17, 1999–2003. [Google Scholar] [CrossRef]
- Holland, P.M.; Rubingh, D.N. Nonideal Multlcomponent Mixed Mlcelle Model. J. Phys. Chem. 1983, 87, 1984–1990. [Google Scholar] [CrossRef]
- Hsu, Y.Y.; Gresser, J.D.; Trantolo, D.J.; Lyons, C.M.; Gangadharam, P.R.; Wise, D.L. Effect of polymer foam morphology and density on kinetics of in vitro controlled release of isoniazid from compressed foam matrices. J. Biomed. Mater. Res. Part A 1997, 35, 107–116. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Andrianov, A.; Zitha, P.L.J. Investigation of Immiscible and Miscible Foam for Enhancing Oil Recovery. Ind. Eng. Chem. Res. 2010, 49, 1910–1919. [Google Scholar] [CrossRef]
- Tsujii, K.; Okahashi, K.; Takeuchi, T. Addition-compound formation between anionic and zwitter-ionic surfactants in water. J. Phys. Chem. 1982, 86, 1437–1441. [Google Scholar] [CrossRef]
- Suh, K.W.; Krueger, D.C.; Zehner, B.E. Method for the Preparation of Styrene Polymer Foam. U.S. Patent NO. 4,438,224, 1986. [Google Scholar]
- Zhou, S. Advances in Energy Science and Equipment Engineering II. Eur. J. Nutr. 2015, 45, 187–195. [Google Scholar]
- Tang, S.; Tian, L.; Lu, J.; Wang, Z.; Xie, Y.; Yang, X.; Lei, X. A Novel Low Tension Foam Flooding for Improving Post-Chemical-Flood in Shuanghe Oilfield. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar]
- Rosen, M.J.; Hua, X.Y. Surface concentrations and molecular interactions in binary mixtures of surfactants. J. Colloid Interface Sci. 1982, 86, 164–172. [Google Scholar] [CrossRef]
- Xiangchun, Z.; Junhui, Z.; Zhixue, S. Performance evaluation and study of flooding control of foam gel in SZ36-1 oilfield. Petrochem. Ind. Appl. 2012, 41, 57–59. [Google Scholar]
- Willett, J.L.; Shogren, R.L. Processing and properties of extruded starch/polymer foams. Polymer 2002, 43, 5935–5947. [Google Scholar] [CrossRef]
- Osterloh, W.T.; Jante, M.J. Effects of Gas and Liquid Velocity on Steady-State Foam Flow at High Temperature. In Proceedings of the SPE/DOE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 22–24 April 1992; p. 24179. [Google Scholar]
- Martín, M.; García, J.M.; Montes, F.J.; Galán, M.A. On the effect of the orifice configuration on the coalescence of growing bubbles. Chem. Eng. Process. Process Intensif. 2008, 47, 1799–1809. [Google Scholar] [CrossRef]
- Kamp, A.M.; Chesters, A.K.; Colin, C.; Fabre, J. Bubble coalescence in turbulent flows: A mechanistic model for turbulence-induced coalescence applied to microgravity bubbly pipe flow. Int. J. Multiph. Flow 2001, 27, 1363–1396. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Wu, Y.; Li, X. Enhanced oil recovery by air-foam flooding system in tight oil reservoirs: Study on the profile-controlling mechanisms. J. Pet. Sci. Eng. 2017, 150, 208–221. [Google Scholar] [CrossRef]
- Chen, B.; Reynolds, A.C. Ensemble-based optimization of the water-alternating-gas-injection process. SPE J. 2016, 2, 11–20. [Google Scholar] [CrossRef]
- Yu, J.; Mo, D.; Liu, N.; Lee, R. The application of nanoparticle-stabilized CO2 foam for oil recovery. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 8–10 April 2013. [Google Scholar]

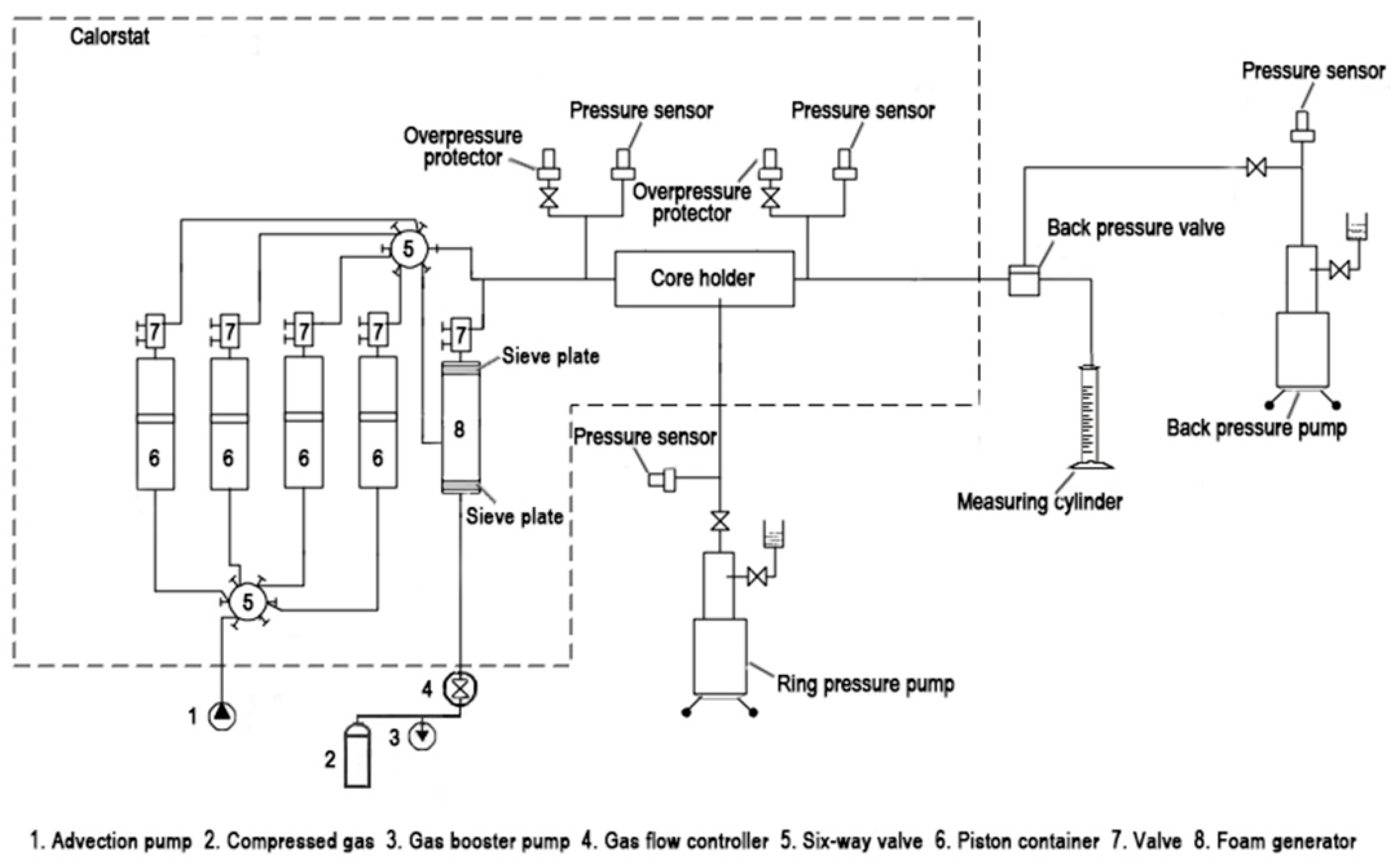

| Ion | Na++K+ | Ca2+ | Mg2+ | HCO3− | SO42− | Cl− | CO32− | Salinity |
|---|---|---|---|---|---|---|---|---|
| concentration (mg/L) | 4459 | 356 | 116 | 966 | 539 | 2989 | 437 | 9862 |
| Inorganic Salt | Purity | Manufacturer |
|---|---|---|
| NaCl | AR | Macklin |
| KCl | AR | Macklin |
| CaCl2·2H2O | AR | Macklin |
| MgCl2·6H2O | AR | Macklin |
| Na2CO3 | AR | Macklin |
| Na2SO4 | AR | Macklin |
| NaHCO3 | AR | Macklin |
| Chemical | Purity | Type | Critical Micelle Concentration (CMC) at 25 °C |
| Tetradecyl hydroxypropyl phosphate betaine (THPB) | 40% | Amphoteric | 1200 mg/L |
| Dodecyl hydroxypropyl phosphate betaine (DHPB) | 45% | Amphoteric | 1360 mg/L |
| 3-sulfopropyltetradecyl dimethyl betaine (3-SDB) | 98% | Amphoteric | 1000 mg/L |
| Cetyltrimethylammonium bromide (CTAB) | 99% | Cationic | 343 mg/L |
| Fatty amine polyoxyethylene ether (AC, C14) | 99% | Nonionic | 97 mg/L |
| α-Sodium olefin sulfonate (AOS, C12) | 92% | Anionic | 1932 mg/L |
| Sodium sulfate (SO) | 96% | Anionic | 365 mg/L |
| Sodium lauryl sulfate (AES) | 70% | Anionic | 2177 mg/L |
| Sodium dodecyl sulfate (SDS) | 96% | Anionic | 2307 mg/L |
| sodium dodecyl benzene sulfonate (SDBS) | 99% | Anionic | 418 mg/L |
| Chemical | Purity | Type | Viscosity at 45 °C, 1 g/L |
| Anionic polyacrylamide (APAM, 5 million molecular weight) | 99% | Polymer | 3331 mPa·s |
| Xanthan gum (XC) | USP | Polymer | 9472 mPa·s |
| Nonionic polyacrylamide (PAM, 5 million molecular weight) | 99% | Polymer | 3922 mPa·s |
| Polysorbate 80 (Tween 80) | 40% | Polymer | 493 mPa·s |
| Parameter | Symbol | Definition | Unit |
|---|---|---|---|
| Foaming volume | V0 | Volume of foam after foaming | mL |
| Residual-liquid volume | Vt | Volume of liquid remaining after foaming | mL |
| Foam half-life | t1/2 | Time required for the foam’s volume to be reduced by half of its initial volume | min |
| Drainage half-life | tD | Time required for the foam to lose half of its liquid | s |
| Foam composite index | FCI | Function of foaming volume and foam half-life | mL·min |
| Number | Water Permeability (mD) | Porosity (%) | Pore Volume (mL) |
|---|---|---|---|
| 1 | 109 | 23.5 | 6.920 |
| 2 | 111 | 23.2 | 6.832 |
| 3 | 110 | 23.7 | 6.979 |
| 4 | 1.2 | 25.7 | 7.568 |
| 5 | 1 | 25.9 | 7.627 |
| 6 | 1 | 26.1 | 7.686 |
| Number | Group | Compounding | Core |
|---|---|---|---|
| 1 | Normal foam system | 1.5 g/L SDS + 2 g/L PAM | NO.1 |
| 2 | ASP | 1 g/L SDBS + 6 g/L Na2CO3 + 1 g/L PAM | NO.2 |
| 3 | Target foam system | 1.2 g/L DHPB + 1.8 g/L SDS + 0.075 g/L XC | NO.3 |
| 4 | Normal foam system | 1.5 g/L SDS + 2 g/L PAM | NO.4 |
| 5 | ASP | 1 g/L SDBS + 6 g/L Na2CO3 + 1 g/L PAM | NO.5 |
| 6 | Target foam system | 1.2 g/L DHPB + 1.8 g/L SDS + 0.075 g/L XC | NO.6 |
| Surfactant | Concentration (g/L) | V0 (mL) | Vt (mL) | t1/2 (min) | tD (s) | FCI (mL·min) | IFT (mN/m) |
|---|---|---|---|---|---|---|---|
| THPB | 0.05 | 210 | 65 | 151 | 175 | 23,782.5 | 0.4068 |
| 0.1 | 265 | 35 | 158 | 180 | 31,402.5 | 0.5533 | |
| 0.15 | 235 | 40 | 168 | 54 | 29,610 | 0.6883 | |
| DHPB | 0.05 | 190 | 50 | 110 | 92 | 15,675 | 0.3245 |
| 0.1 | 215 | 25 | 129 | 103 | 20,801 | 0.2834 | |
| 0.15 | 235 | 15 | 131 | 115 | 23,088 | 0.2214 | |
| 3-SDB | 0.05 | 155 | 65 | 110 | 57 | 12,787.5 | 0.8971 |
| 0.1 | 200 | 50 | 127 | 52 | 19,050 | 0.7324 | |
| 0.15 | 185 | 50 | 176 | 67 | 24,420 | 0.6021 | |
| CTAB | 0.05 | 190 | 45 | 60 | 35 | 8550 | 0.2914 |
| 0.1 | 240 | 40 | 57 | 28 | 10,260 | 0.2429 | |
| 0.15 | 180 | 55 | 60 | 31 | 8100 | 0.3755 | |
| AN | 0.05 | 140 | 60 | 120 | 33 | 12,600 | 0.8395 |
| 0.1 | 130 | 65 | 96 | 30 | 9360 | 0.8524 | |
| 0.15 | 145 | 50 | 80 | 23 | 8700 | 0.9455 | |
| AOS | 0.05 | 255 | 35 | 83 | 135 | 15,873.75 | 1.2123 |
| 0.1 | 280 | 20 | 90 | 160 | 18,900 | 1.1084 | |
| 0.15 | 285 | 15 | 73 | 104 | 15,603.75 | 1.3633 | |
| SO | 0.05 | No bubble | 0.0025 | ||||
| 0.1 | 35 | 95 | 140 | 84 | 3675 | 0.0016 | |
| 0.15 | 60 | 80 | 600 | 90 | 27,000 | 0.0038 | |
| AES | 0.05 | 110 | 60 | 92 | 100 | 7590 | 2.3112 |
| 0.1 | 265 | 25 | 78 | 129 | 15,502.5 | 2.2219 | |
| 0.15 | 260 | 30 | 90 | 133 | 17,550 | 2.3794 | |
| SDS | 0.05 | 250 | 25 | 90 | 77 | 16,875 | 0.795 |
| 0.1 | 260 | 25 | 108 | 70 | 21,060 | 0.792 | |
| 0.15 | 275 | 20 | 99 | 73 | 20,419 | 1.0666 | |
| SDBS | 0.05 | 175 | 25 | 18 | 42 | 2362.5 | 0.7434 |
| 0.1 | 220 | 20 | 19 | 43 | 3135 | 0.6241 | |
| 0.15 | 220 | 20 | 22 | 52 | 3630 | 0.6432 | |
| Compound System | Mass Ratio | V0 (mL) | Vt (mL) | t1/2 (min) | tD (s) | FCI (mL·min) | IFT (mN/m) |
|---|---|---|---|---|---|---|---|
| THPB:AOS | 1.5:1 | 245 | 15 | 122 | 76 | 22,417.5 | 0.7145 |
| 1:1 | 260 | 15 | 103 | 71 | 20,085 | 0.7982 | |
| 1:1.5 | 270 | 15 | 92 | 78 | 18,630 | 0.9054 | |
| THPB:AES | 1.5:1 | 360 | 10 | 101 | 145 | 27,270 | 0.0154 |
| 1:1 | 450 | 10 | 114 | 161 | 38,475 | 0.0134 | |
| 1:1.5 | 530 | 5 | 300 | 192 | 119,250 | 0.0107 | |
| THPB:SDS | 1.5:1 | 310 | 15 | 76 | 78 | 17,670 | 0.0692 |
| 1:1 | 330 | 15 | 91 | 78 | 22,522.5 | 0.0631 | |
| 1:1.5 | 370 | 10 | 102 | 81 | 28,305 | 0.0545 | |
| DHPB:AOS | 1.5:1 | 95 | 35 | 47 | 43 | 3348.75 | 0.5789 |
| 1:1 | 115 | 30 | 46 | 47 | 3967.5 | 0.6541 | |
| 1:1.5 | 145 | 30 | 57 | 47 | 6198.75 | 0.7763 | |
| DHPB:AES | 1.5:1 | 80 | 45 | 18 | 36 | 1080 | 0.7316 |
| 1:1 | 90 | 45 | 18 | 39 | 1215 | 0.7145 | |
| 1:1.5 | 115 | 40 | 21 | 37 | 1811.25 | 0.7682 | |
| DHPB:SDS | 1.5:1 | 470 | 5 | 234 | 240 | 82,485 | 0.0089 |
| 1:1 | 515 | 5 | 242 | 256 | 93,472.5 | 0.0084 | |
| 1:1.5 | 560 | 5 | 280 | 334 | 117,600 | 0.0076 |
| Temperature (°C) | Concentration of XC (g/L) | V0 (mL) | Vt (mL) | t1/2 (min) | tD (s) | FCI (mL·min) |
|---|---|---|---|---|---|---|
| 25 | 0.05 | 535 | 5 | 278 | 631 | 111,547.5 |
| 0.075 | 510 | 5 | 263 | 672 | 100,597.5 | |
| 0.1 | 460 | 0 | 242 | 714 | 83,490 | |
| 0.125 | 435 | 0 | 233 | 747 | 76,016.25 | |
| 45 | 0.05 | 540 | 5 | 292 | 609 | 118,260 |
| 0.075 | 525 | 0 | 281 | 681 | 110,643.75 | |
| 0.1 | 490 | 0 | 264 | 698 | 97,020 | |
| 0.125 | 450 | 0 | 247 | 704 | 83,362.5 | |
| 65 | 0.05 | 495 | 15 | 231 | 519 | 85,758.75 |
| 0.075 | 505 | 15 | 234 | 545 | 88,627.5 | |
| 0.1 | 455 | 10 | 209 | 571 | 71,321.25 | |
| 0.125 | 435 | 5 | 213 | 574 | 69,491.25 | |
| 85 | 0.05 | 395 | 25 | 168 | 292 | 49,770 |
| 0.075 | 420 | 25 | 184 | 341 | 57,960 | |
| 0.1 | 415 | 15 | 181 | 392 | 56,336.25 | |
| 0.125 | 405 | 15 | 190 | 403 | 57,712.5 |
| Number | Oil Saturation (%) | E0 (%) | Ec (%) |
|---|---|---|---|
| 1 | 78.23 | 60.61 | 8.33 |
| 2 | 79.18 | 57.30 | 10.67 |
| 3 | 79.23 | 61.78 | 11.32 |
| 4 | 69.26 | 48.44 | 8.69 |
| 5 | 68.18 | 45.29 | 6.59 |
| 6 | 66.71 | 43.11 | 11.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Liu, S.; Luo, D.; Peng, B. Ultra-Low Interfacial Tension Foam System for Enhanced Oil Recovery. Appl. Sci. 2019, 9, 2155. https://doi.org/10.3390/app9102155
Liu Q, Liu S, Luo D, Peng B. Ultra-Low Interfacial Tension Foam System for Enhanced Oil Recovery. Applied Sciences. 2019; 9(10):2155. https://doi.org/10.3390/app9102155
Chicago/Turabian StyleLiu, Qi, Shuangxing Liu, Dan Luo, and Bo Peng. 2019. "Ultra-Low Interfacial Tension Foam System for Enhanced Oil Recovery" Applied Sciences 9, no. 10: 2155. https://doi.org/10.3390/app9102155
APA StyleLiu, Q., Liu, S., Luo, D., & Peng, B. (2019). Ultra-Low Interfacial Tension Foam System for Enhanced Oil Recovery. Applied Sciences, 9(10), 2155. https://doi.org/10.3390/app9102155







