Duration of the Symptoms and Brain Aging in Women with Fibromyalgia: A Cross-Sectional Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Questionnaires and Procedure
2.3. EEG Recording and Data Processing
2.4. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics of the Whole Sample and Correlation between EEG Frequency Bands and Duration of Symptoms
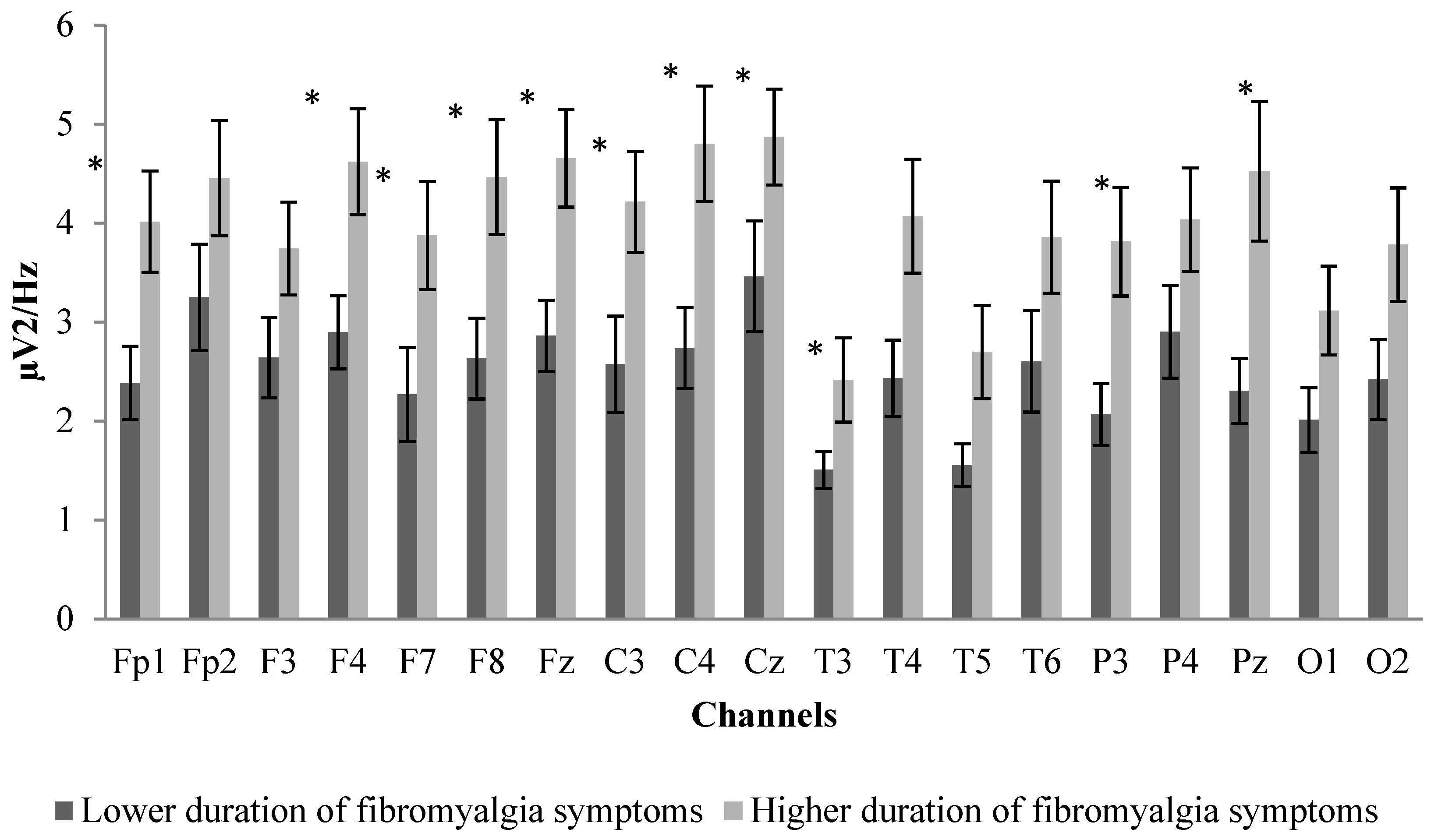
3.2. Between-Group Differences in Sociodemographic Characteristics, Age, Impact of Fibromyalgia, and EEG Power Spectrum
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| EEG | Electroencephalogram |
| FIQ | Fibromyalgia Impact Questionnaire |
| MRI | Magnetic resonance imaging |
| SPSS | Statistical Package for Social Sciences |
References
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Huijnen, I.P.J.; Verbunt, J.A.; Meeus, M.; Smeets, R. Energy Expenditure during Functional Daily Life Performances in Patients with Fibromyalgia. Pain Pract. 2015, 15, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, C.S.; Clark, S.R.; Bennett, R.M. Fibromyalgia and quality of life: A comparative analysis. J. Rheumatol. 1993, 20, 475–479. [Google Scholar] [PubMed]
- Stern, J.; Jeanmonod, D.; Sarnthein, J. Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. Neuroimage 2006, 31, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Roldan, A.M.; Cifre, I.; Sitges, C.; Montoya, P. Altered Dynamic of EEG Oscillations in Fibromyalgia Patients at Rest. Pain Med. 2016, 17, 1058–1068. [Google Scholar] [CrossRef]
- Desmeules, J.A.; Cedraschi, C.; Rapiti, E.; Baumgartner, E.; Finckh, A.; Cohen, P.; Dayer, P.; Vischer, T.L. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003, 48, 1420–1429. [Google Scholar] [CrossRef]
- Williams, D.A.; Gracely, R.H. Functional magnetic resonance imaging findings in fibromyalgia. Arthritis Res. Ther. 2006, 8. [Google Scholar] [CrossRef][Green Version]
- Staud, R.; Craggs, J.G.; Perlstein, W.M.; Robinson, M.E.; Price, D.D. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur. J. Pain 2008, 12, 1078–1089. [Google Scholar] [CrossRef]
- Gracely, R.H.; Petzke, F.; Wolf, J.M.; Clauw, D.J. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002, 46, 1333–1343. [Google Scholar] [CrossRef]
- Burgmer, M.; Pogatzki-Zahn, E.; Gaubitz, M.; Wessoleck, E.; Heuft, G.; Pfleiderer, B. Altered brain activity during pain processing in fibromyalgia. Neuroimage 2009, 44, 502–508. [Google Scholar] [CrossRef]
- Hargrove, J.B.; Bennett, R.M.; Simons, D.G.; Smith, S.J.; Nagpal, S.; Deering, D.E. Quantitative Electroencephalographic Abnormalities in Fibromyalgia Patients. Clin. EEG Neurosci. 2010, 41, 132–139. [Google Scholar] [CrossRef]
- Villafaina, S.; Collado-Mateo, D.; Fuentes-García, J.P.; Cano-Plasencia, R.; Gusi, N. Impact of Fibromyalgia on Alpha-2 EEG Power Spectrum in the Resting Condition: A Descriptive Correlational Study. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Buzsáki, G. Rhythms of the Brain; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Schuff, N.; Woerner, N.; Boreta, L.; Kornfield, T.; Shaw, L.M.; Trojanowski, J.Q.; Thompson, P.M.; Jack, C.R.; Weiner, M.W.; the Alzheimer’s; et al. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain 2009, 132, 1067–1077. [Google Scholar] [CrossRef]
- Prichep, L.S.; John, E.R.; Ferris, S.H.; Reisberg, B.; Almas, M.; Alper, K.; Cancro, R. Quantitative EEG correlates of cognitive deterioration in the elderly. Neurobiol. Aging 1994, 15, 85–90. [Google Scholar] [CrossRef]
- Grunwald, M.; Busse, F.; Hensel, A.; Kruggel, T.; Riedel-Heller, S.; Wolf, M.; Arendt, T.; Gertz, H.J. Correlation between cortical theta activity and hippocampal volumes in health, mild cognitive impairment, and mild dementia. J. Clin. Neurophysiol. 2001, 18, 178–184. [Google Scholar] [CrossRef]
- Grunwald, M.; Hensel, A.; Wolf, H.; Weiss, T.; Gertz, H.J. Does the hippocampal atrophy correlate with the cortical theta power in elderly subjects with a range of cognitive impairment? J. Clin. Neurophysiol. 2007, 24, 22–26. [Google Scholar] [CrossRef]
- Mutso, A.A.; Radzicki, D.; Baliki, M.N.; Huang, L.; Banisadr, G.; Centeno, M.V.; Radulovic, J.; Martina, M.; Miller, R.J.; Apkarian, A.V. Abnormalities in Hippocampal Functioning with Persistent Pain. J. Neurosci. 2012, 32, 5747–5756. [Google Scholar] [CrossRef]
- Gormsen, L.; Rosenberg, R.; Flemming, W.; Troels, S. Depression, anxiety, health-related quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur. J. Pain 2010, 14, e121–e127.e8. [Google Scholar] [CrossRef]
- Dunn, K.M.; Croft, P.R. The importance of symptom duration in determining prognosis. Pain 2006, 121, 126–132. [Google Scholar] [CrossRef]
- Baliki, M.N.; Geha, P.Y.; Apkarian, A.V.; Chialvo, D.R. Beyond feeling: Chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 1398–1403. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Sosa, Y.; Sonty, S.; Levy, R.M.; Harden, R.N.; Parrish, T.B.; Gitelman, D.R. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 10410–10415. [Google Scholar] [CrossRef]
- Bennett, R. The Fibromyalgia Impact Questionnaire (FIQ): A review of its development, current version, operaflng characteristics and uses. Clin. Exp. Rheumatol. 2005, 23, S154–S162. [Google Scholar] [PubMed]
- Ruffini, G.; Dunne, S.; Farres, E.; Cester, I.; Watts, P.C.P.; Silva, S.R.P.; Grau, C.; Fuentemilla, L.; Marco-Pallares, J.; Vandecasteele, B.; et al. ENOBIO dry electrophysiology electrode; first human trial plus wireless electrode system. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 6690–6694. [Google Scholar]
- Collado-Mateo, D.; Adsuar, J.C.; Olivares, P.R.; Cano-Plasencia, R.; Gusi, N. Using a dry electrode EEG device during balance tasks in healthy young-adult males: Test-retest reliability analysis. Somatosens. Mot. Res. 2015, 32, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.P.; Makeig, S.; Westerfield, M.; Townsend, J.; Courchesne, E.; Sejnowski, T.J. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin. Neurophysiol. 2000, 111, 1745–1758. [Google Scholar] [CrossRef]
- Amris, K.; Luta, G.; Christensen, R.; Danneskiold-Samsoe, B.; Bliddal, H.; Waehrens, E.E. Predictors of improvement in observed functional ability in patients with fibromyalgia as an outcome of rehabilitation. J. Rehabil. Med. 2016, 48, 65–71. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Fine, P.G. Long-term consequences of chronic pain: Mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med. 2011, 12, 996–1004. [Google Scholar] [CrossRef]
- Fallon, N.; Chiu, Y.; Nurmikko, T.; Stancak, A. Altered theta oscillations in resting EEG of fibromyalgia syndrome patients. Eur. J. Pain 2017, 22, 49–57. [Google Scholar] [CrossRef]
- Choe, M.K.; Lim, M.; Kim, J.S.; Lee, D.S.; Chung, C.K. Disrupted Resting State Network of Fibromyalgia in Theta frequency. Sci. Rep. 2018, 8, 2064. [Google Scholar] [CrossRef]
- Fernandez, A.; Arrazola, J.; Maestu, F.; Amo, C.; Gil-Gregorio, P.; Wienbruch, C.; Ortiz, T. Correlations of hippocampal atrophy and focal low-frequency magnetic activity in Alzheimer disease: Volumetric MR imaging-magnetoencephalographic study. Am. J. Neuroradiol. 2003, 24, 481–487. [Google Scholar]
- Moretti, D.V.; Miniussi, C.; Frisoni, G.B.; Geroldi, C.; Zanetti, O.; Binetti, G.; Rossini, P.M. Hippocampal atrophy and EEG markers in subjects with mild cognitive impairment. Clin. Neurophysiol. 2007, 118, 2716–2729. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.V. Theta and alpha EEG frequency interplay in subjects with mild cognitive impairment: Evidence from EEG, MRI, and SPECT brain modifications. Front. Aging Neurosci. 2015, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- McCrae, C.S.; O’Shea, A.M.; Boissoneault, J.; Vatthauer, K.E.; Robinson, M.E.; Staud, R.; Perlstein, W.M.; Craggs, J.G. Fibromyalgia patients have reduced hippocampal volume compared with healthy controls. J. Pain Res. 2015, 8, 47–52. [Google Scholar] [CrossRef]
- Rodriguez-Raecke, R.; Niemeier, A.; Ihle, K.; Ruether, W.; May, A. Structural Brain Changes in Chronic Pain Reflect Probably Neither Damage Nor Atrophy. PLoS ONE 2013, 8, e54475. [Google Scholar] [CrossRef] [PubMed]
- Emad, Y.; Ragab, Y.; Zeinhom, F.; El-Khouly, G.; Abou-Zeid, A.; Rasker, J.J. Hippocampus dysfunction may explain symptoms of fibromyalgia syndrome. A study with single-voxel magnetic resonance spectroscopy. J. Rheumatol. 2008, 35, 1371–1377. [Google Scholar]
- Schmidt-Wilcke, T.; Leinisch, E.; Straube, A.; Kampfe, N.; Draganski, B.; Diener, H.C.; Bogdahn, U.; May, A. Gray matter decrease in patients with chronic tension type headache. Neurology 2005, 65, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Kuchinad, A.; Schweinhardt, P.; Seminowicz, D.A.; Wood, P.B.; Chizh, B.A.; Bushnell, M.C. Accelerated brain gray matter loss in fibromyalgia patients: Premature aging of the brain? J. Neurosci. 2007, 27, 4004–4007. [Google Scholar] [CrossRef]
- Lutz, J.; Jaeger, L.; de Quervain, D.; Krauseneck, T.; Padberg, F.; Wichnalek, M.; Beyer, A.; Stahl, R.; Zirngibl, B.; Morhard, D.; et al. White and Gray Matter Abnormalities in the Brain of Patients with Fibromyalgia A Diffusion-Tensor and Volumetric Imaging Study. Arthritis Rheum. 2008, 58, 3960–3969. [Google Scholar] [CrossRef]
- Wartolowska, K.; Hough, M.G.; Jenkinson, M.; Andersson, J.; Wordsworth, B.P.; Tracey, I. Structural changes of the brain in rheumatoid arthritis. Arthritis Rheum. 2012, 64, 371–379. [Google Scholar] [CrossRef]
- Jin, C.; Yuan, K.; Zhao, L.; Zhao, L.; Yu, D.; von Deneen, K.M.; Zhang, M.; Qin, W.; Sun, W.; Tian, J. Structural and functional abnormalities in migraine patients without aura. NMR Biomed. 2013, 26, 58–64. [Google Scholar] [CrossRef]
- Seminowicz, D.A.; Wideman, T.H.; Naso, L.; Hatami-Khoroushahi, Z.; Fallatah, S.; Ware, M.A.; Jarzem, P.; Bushnell, M.C.; Shir, Y.; Ouellet, J.A.; et al. Effective Treatment of Chronic Low Back Pain in Humans Reverses Abnormal Brain Anatomy and Function. J. Neurosci. 2011, 31, 7540–7550. [Google Scholar] [CrossRef] [PubMed]

| Variable | Participants (N = 29) Mean (SD) Frequency (%) |
|---|---|
| Age (years) | 55.89 (9.50) |
| ≤45 (frequency and %) | 4 (13.80%) |
| 45–50 (frequency and %) | 8 (27.59%) |
| 51–60 (frequency and %) | 8 (27.59%) |
| ≥61 (frequency and %) | 9 (31.02%) |
| Duration of symptoms | 20.69 (13.60) |
| ≤10 years | 7 (24.14%) |
| 11–20 years | 9 (31.02%) |
| 21–30 years | 7 (24.14%) |
| ≥31 years | 6 (20.70%) |
| FIQ-100 | 48.45 (15.21) |
| Stiffness | 5.21 (2.72) |
| Sadness | 3.55 (2.87) |
| Pain | 6.00 (2.28) |
| Anxiety | 5.48 (2.63) |
| Education level (n of patients) | |
| Able to write and read a | 1 (3.40%) |
| Primary education | 14 (48.30%) |
| Secondary education | 12 (41.40%) |
| Higher education | 2 (6.90%) |
| Medication (n of patients) | |
| Antidepressants | 11 (37.9%) |
| Analgesics/relaxants | 10 (34.5%) |
| Complementary therapies | |
| Massages | 5 (17.2%) |
| Physiotherapy | 8 (27.6%) |
| Comorbidities | |
| Type 2 diabetes | 2 (6.9%) |
| Hyperlipidemia | 7 (24.1%) |
| Hypertension | 7 (24.1%) |
| Years Suffering from Fibromyalgia Symptoms | Age of the Participants | |||||||
|---|---|---|---|---|---|---|---|---|
| EEG Scalp Locations a | Spearman Rho Correlation | p-Value | Kendall’s Tau-b | p-Value | Spearman Rho Correlation | p-Value | Kendall’s Tau-b | p-Value |
| Fp1 | 0.264 | 0.046 * | 0.401 | 0.031* | 0.070 | 0.598 | 0.115 | 0.552 |
| Fp2 | 0.179 | 0.176 | 0.238 | 0.213 | −0.101 | 0.452 | −0.155 | 0.421 |
| Fz | 0.276 | 0.037 * | 0.408 | 0.028 * | 0.013 | 0.925 | 0.068 | 0.725 |
| F3 | 0.154 | 0.244 | 0.249 | 0.192 | −0.085 | 0.522 | −0.049 | 0.802 |
| F4 | 0.247 | 0.063 | 0.348 | 0.065 | 0.013 | 0.925 | 0.022 | 0.908 |
| F7 | 0.177 | 0.182 | 0.341 | 0.071 | 0.189 | 0.158 | 0.244 | 0.201 |
| F8 | 0.229 | 0.084 | 0.352 | 0.061 | 0.050 | 0.707 | 0.071 | 0.715 |
| Cz | 0.291 | 0.028 * | 0.430 | 0.020 * | 0.093 | 0.486 | 0.154 | 0.424 |
| C4 | 0.219 | 0.098 | 0.347 | 0.065 | 0.131 | 0.328 | 0.205 | 0.286 |
| C3 | 0.222 | 0.094 | 0.358 | 0.057 | 0.008 | 0.955 | 0.022 | 0.911 |
| Pz | 0.269 | 0.042 * | 0.393 | 0.035 * | 0.076 | 0.572 | 0.101 | 0.603 |
| P3 | 0.287 | 0.031 * | 0.422 | 0.023 * | 0.113 | 0.397 | 0.167 | 0.387 |
| P4 | 0.097 | 0.464 | 0.157 | 0.415 | 0.219 | 0.102 | 0.323 | 0.088 |
| T3 | 0.164 | 0.215 | 0.256 | 0.180 | 0.045 | 0.735 | 0.062 | 0.749 |
| T4 | 0.155 | 0.387 | 0.158 | 0.414 | 0.171 | 0.200 | 0.221 | 0.250 |
| T5 | 0.182 | 0.170 | 0.156 | 0.223 | 0.159 | 0.236 | 0.233 | 0.233 |
| T6 | 0.159 | 0.229 | 0.241 | 0.208 | 0.126 | 0.347 | 0.197 | 0.305 |
| O1 | 0.204 | 0.123 | 0.318 | 0.093 | –0.045 | 0.735 | –0.056 | 0.772 |
| O2 | 0.242 | 0.068 | 0.352 | 0.061 | 0.309 | 0.117 | 0.213 | 0.122 |
| Variable | Shorter Duration of Fibromyalgia Symptoms (N = 15) Mean (SD) | Longer Duration of Fibromyalgia Symptoms (N = 14) Mean (SD) |
|---|---|---|
| Age (years) | 52.07 (9.10) | 59.36 (8.69) |
| Year since initial diagnosis | 10.27 (4.92) | 31.86 (10.61) |
| FIQ-100 | 51.65 (17.74) | 44.75 (12.87) |
| Stiffness | 6.13 (2.53) | 4.21 (2.64) |
| Sadness | 4.00 (2.75) | 3.07 (3.02) |
| Pain | 6.00 (2.39) | 6.00 (2.25) |
| Anxiety | 5.53 (2.56) | 5.43 (2.79) |
| Education level (n of patients) | ||
| Able to write and read a | 0 (0%) | 2 (7.1%) |
| Primary education | 7 (46.70%) | 7 (50.00%) |
| Secondary education | 7 (46.70%) | 5 (35.7%) |
| Higher education | 1 (6.70%) | 1 (7.1%) |
| Medication (n of patients) | ||
| Antidepressants | 7 (46.7%) | 4 (28.6%) |
| Analgesics/relaxants | 7 (46.7%) | 3 (21.4%) |
| Complementary physical therapies | ||
| Massages | 2 (13.3%) | 3 (21.4%) |
| Physiotherapy | 5 (33.3%) | 3 (21.4%) |
| Comorbidities | ||
| Type 2 diabetes | 0 (0%) | 2 (14.3%) |
| Hyperlipidemia | 2 (13.3%) | 5 (35.7%) |
| Hypertension | 2 (13.3%) | 5 (35.7%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villafaina, S.; Collado-Mateo, D.; Fuentes-García, J.P.; Domínguez-Muñoz, F.J.; Gusi, N. Duration of the Symptoms and Brain Aging in Women with Fibromyalgia: A Cross-Sectional Study. Appl. Sci. 2019, 9, 2106. https://doi.org/10.3390/app9102106
Villafaina S, Collado-Mateo D, Fuentes-García JP, Domínguez-Muñoz FJ, Gusi N. Duration of the Symptoms and Brain Aging in Women with Fibromyalgia: A Cross-Sectional Study. Applied Sciences. 2019; 9(10):2106. https://doi.org/10.3390/app9102106
Chicago/Turabian StyleVillafaina, Santos, Daniel Collado-Mateo, Juan P. Fuentes-García, Francisco J. Domínguez-Muñoz, and Narcís Gusi. 2019. "Duration of the Symptoms and Brain Aging in Women with Fibromyalgia: A Cross-Sectional Study" Applied Sciences 9, no. 10: 2106. https://doi.org/10.3390/app9102106
APA StyleVillafaina, S., Collado-Mateo, D., Fuentes-García, J. P., Domínguez-Muñoz, F. J., & Gusi, N. (2019). Duration of the Symptoms and Brain Aging in Women with Fibromyalgia: A Cross-Sectional Study. Applied Sciences, 9(10), 2106. https://doi.org/10.3390/app9102106









