Progress in the Utilization Efficiency Improvement of Hot Carriers in Plasmon-Mediated Heterostructure Photocatalysis
Abstract
1. Introduction
2. Basic Mechanisms in Plasmon-Mediated Heterostructure
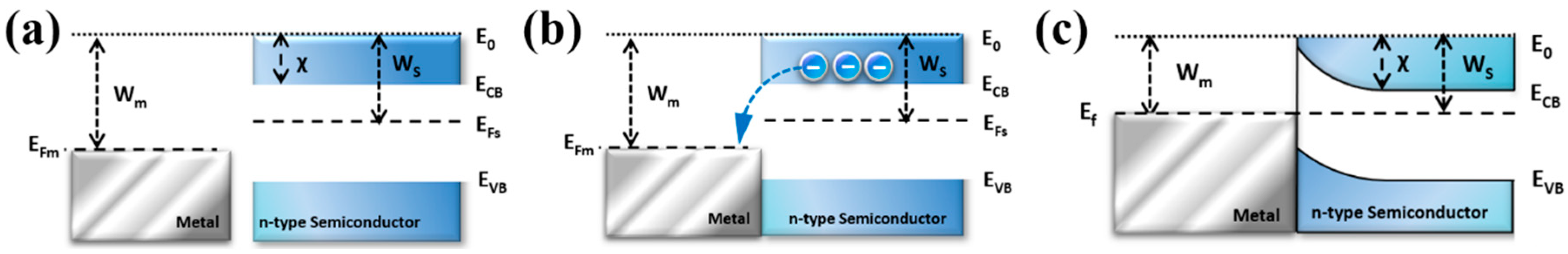
2.1. Formation of Plasmon-Mediated Heterostructure Based on Schottky Contact
2.2. Process of Photocatalysis
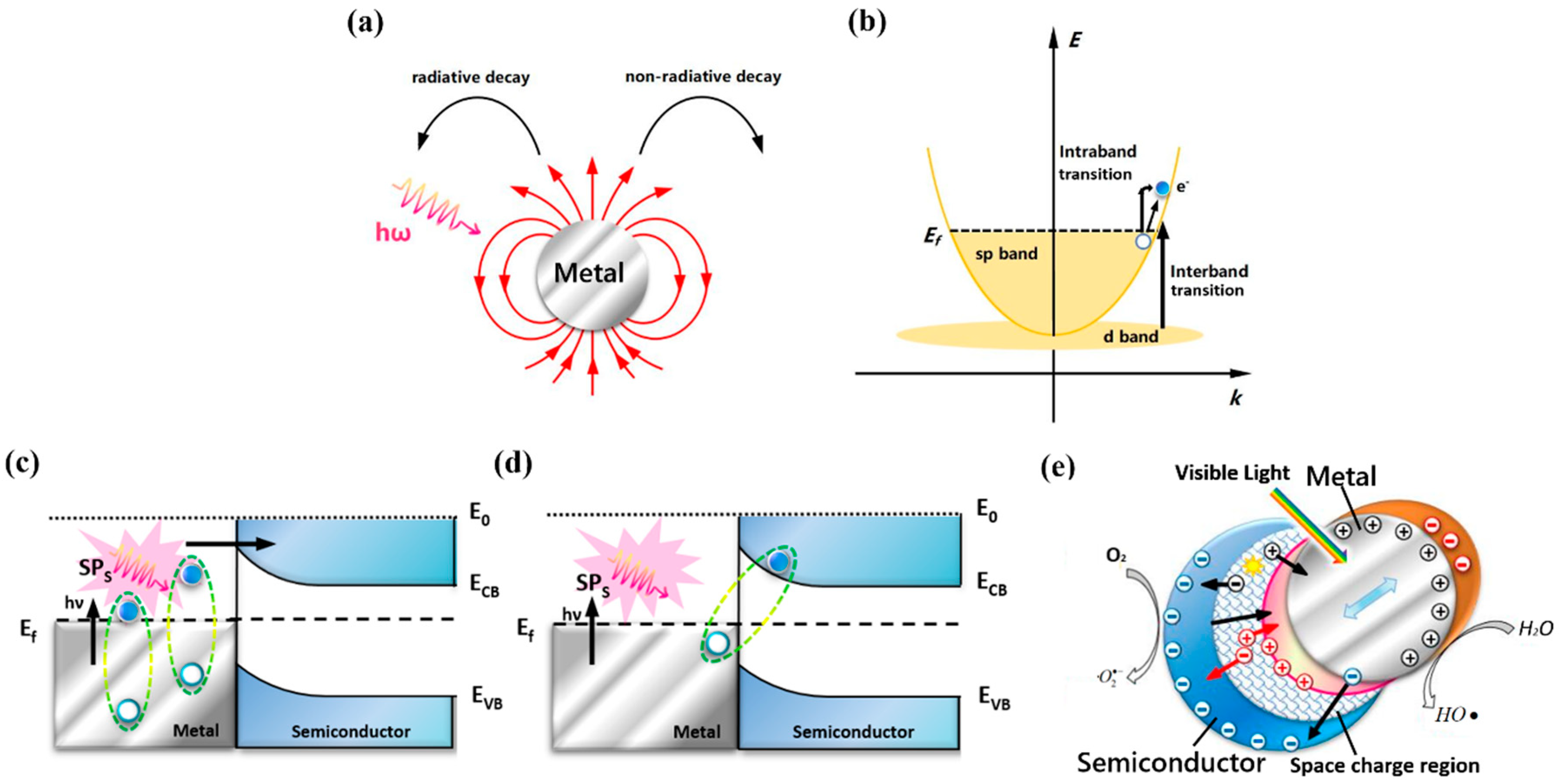
2.2.1. The Generation of HCs
2.2.2. Carriers Separation
2.2.3. Surface Reactions
3. Structural Design of Plasmon-Mediated Photocatalysts
3.1. Generating Efficiency Improvement of HCs
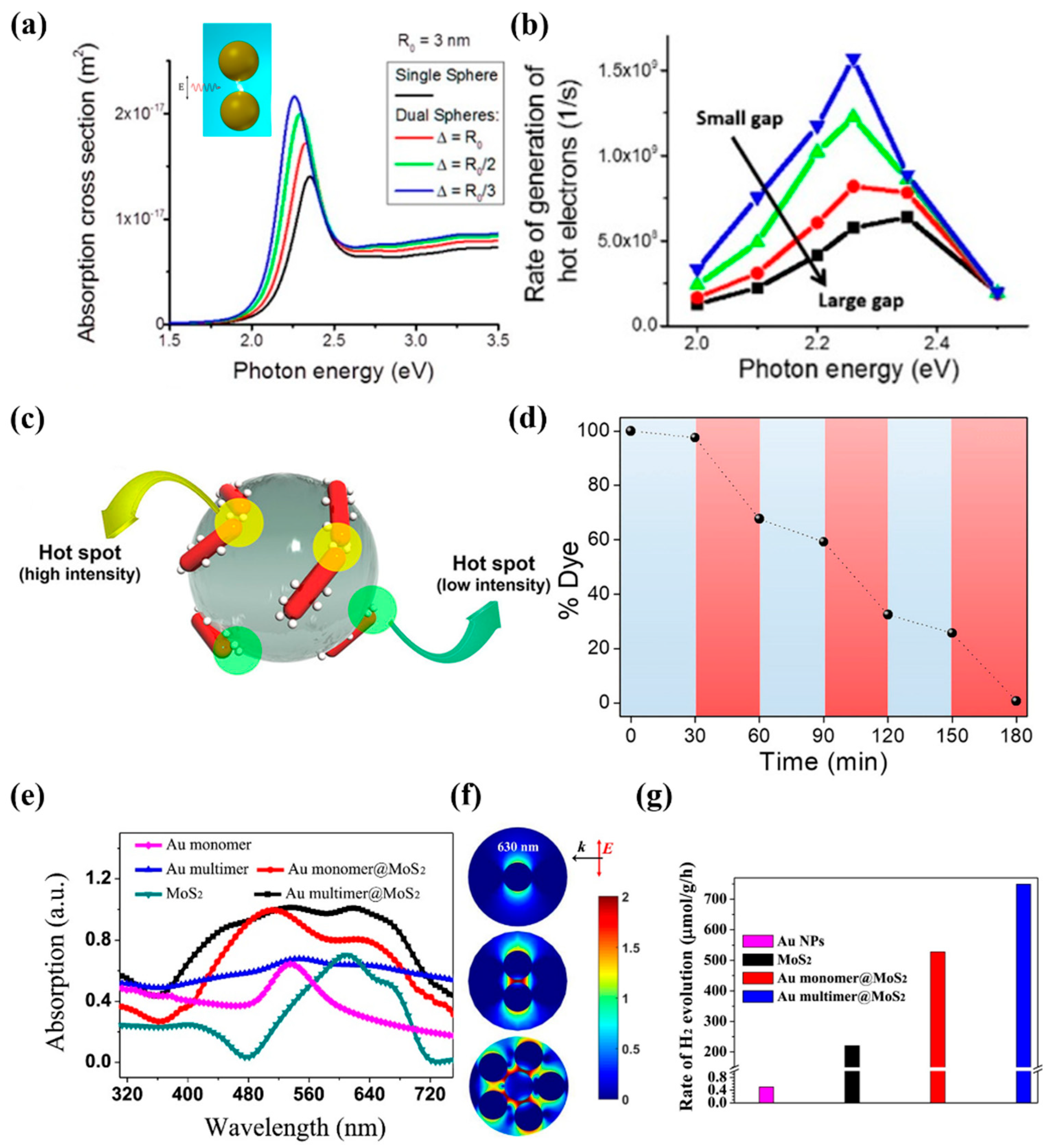
3.1.1. Design of Structures with “Hot Spots”
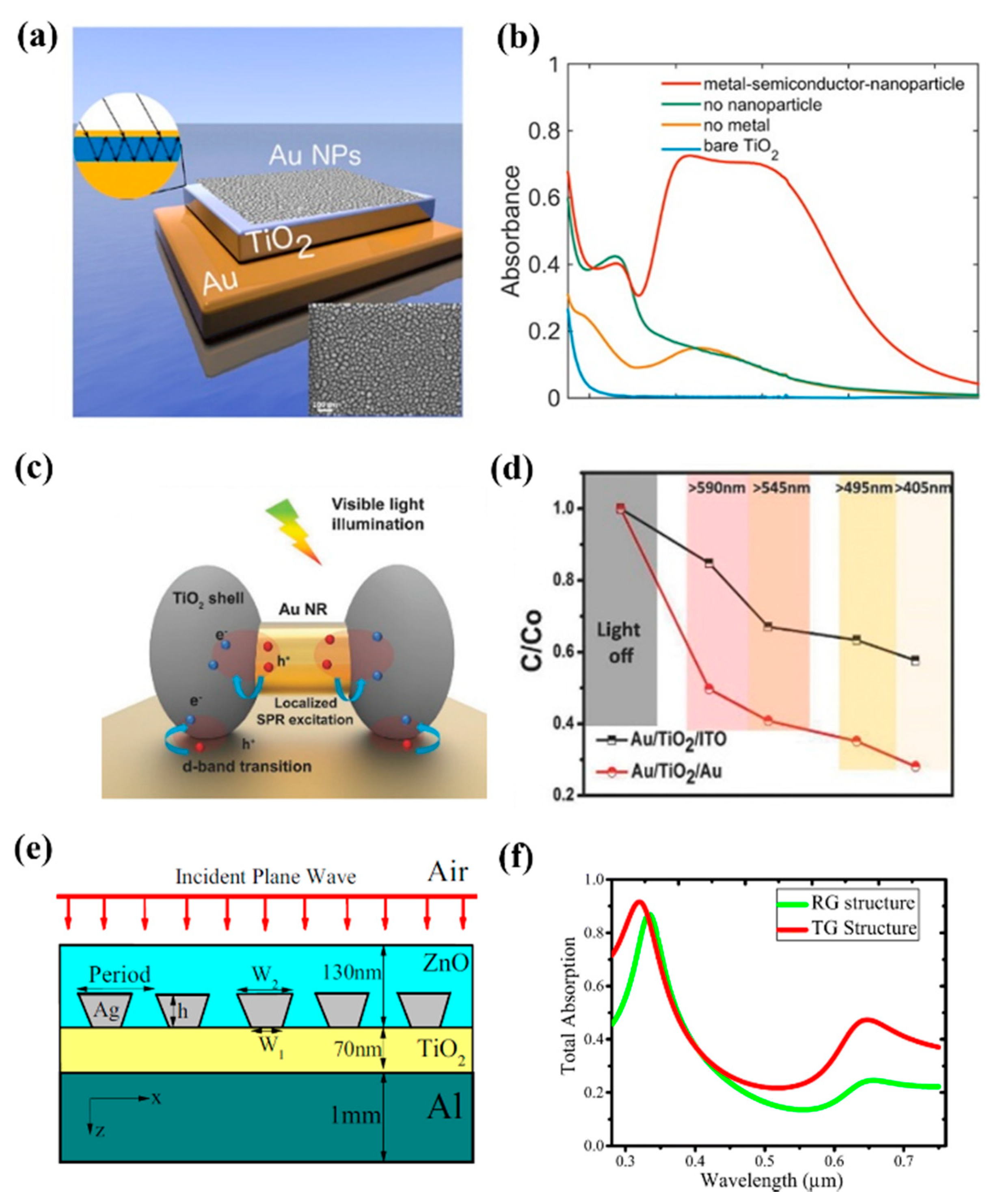
3.1.2. Plasmonic Cavity for Efficient Photons Trapping
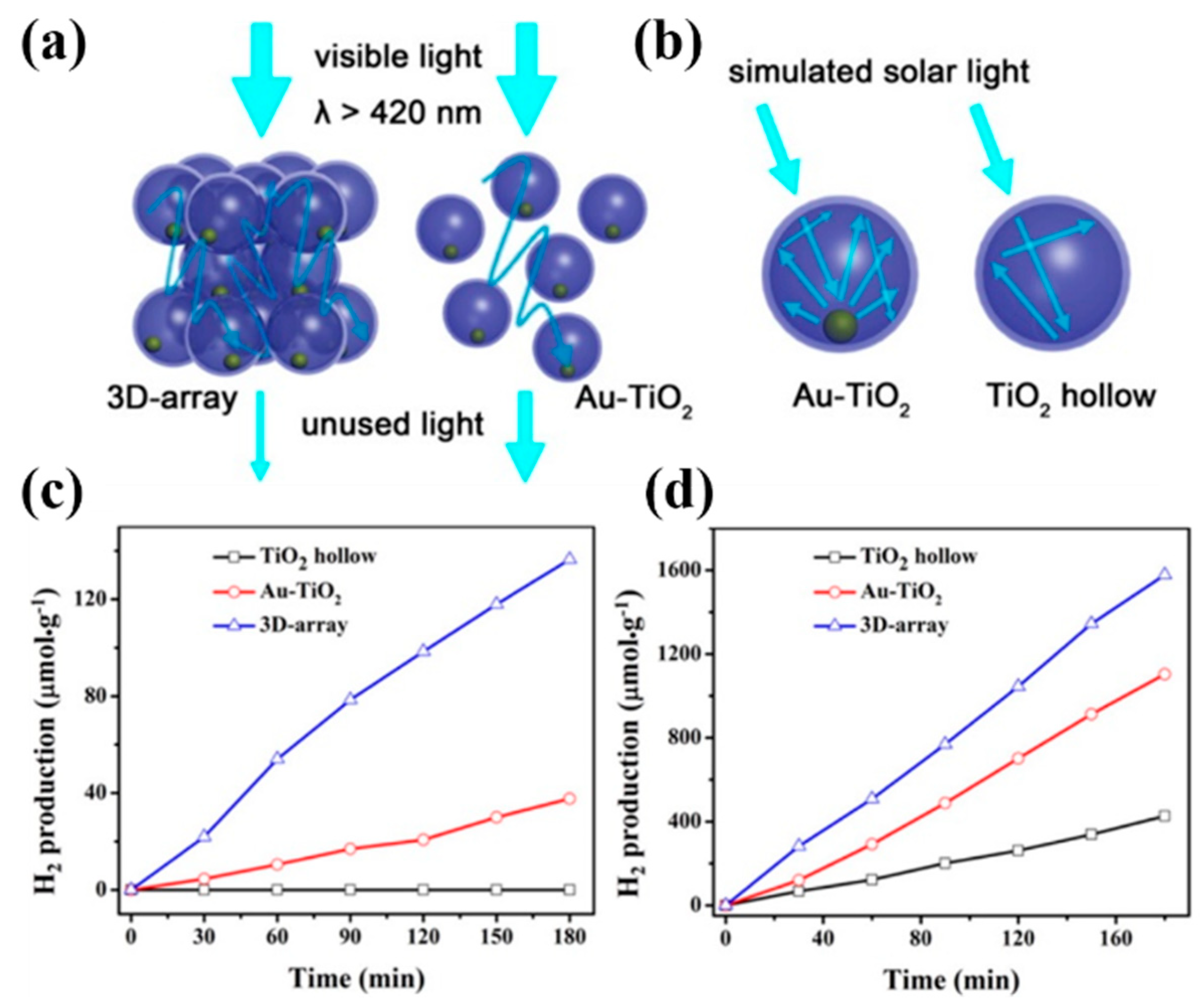
3.1.3. Hollow Heterostructure Designing
3.2. Extraction of HCs
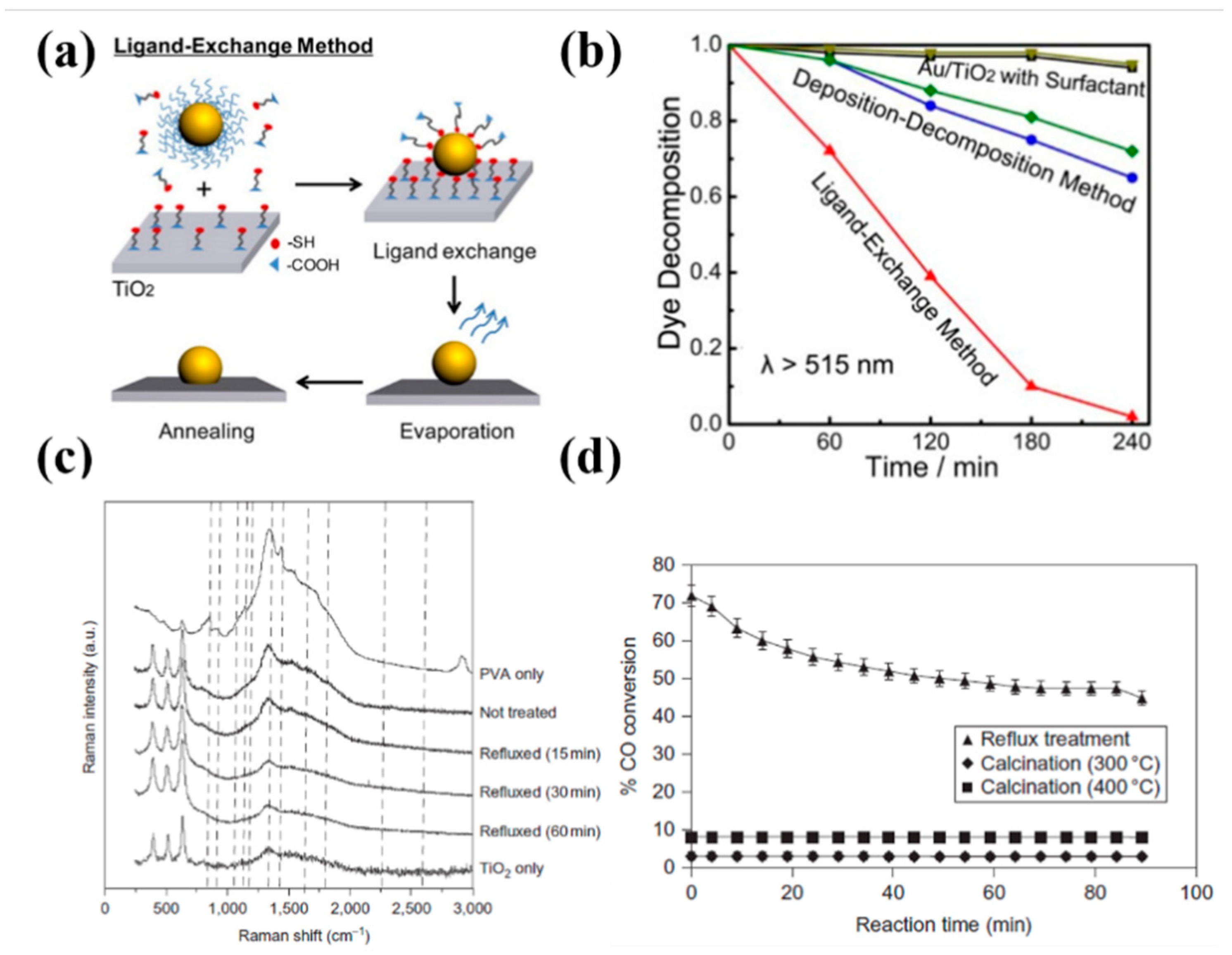
3.2.1. Surface and Interfacial Quality Engineering
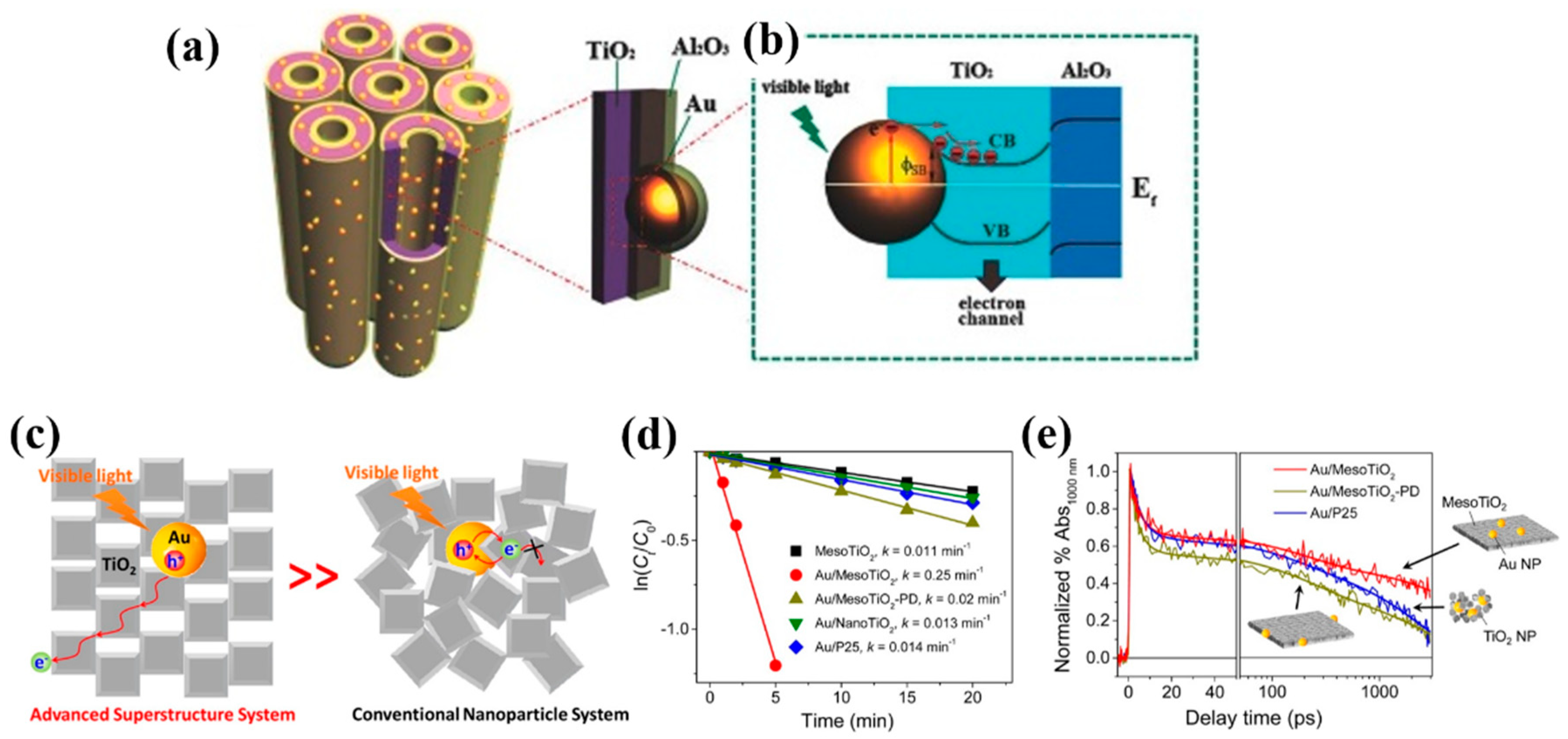
3.2.2. Efficient Separation of Interfacial HCs
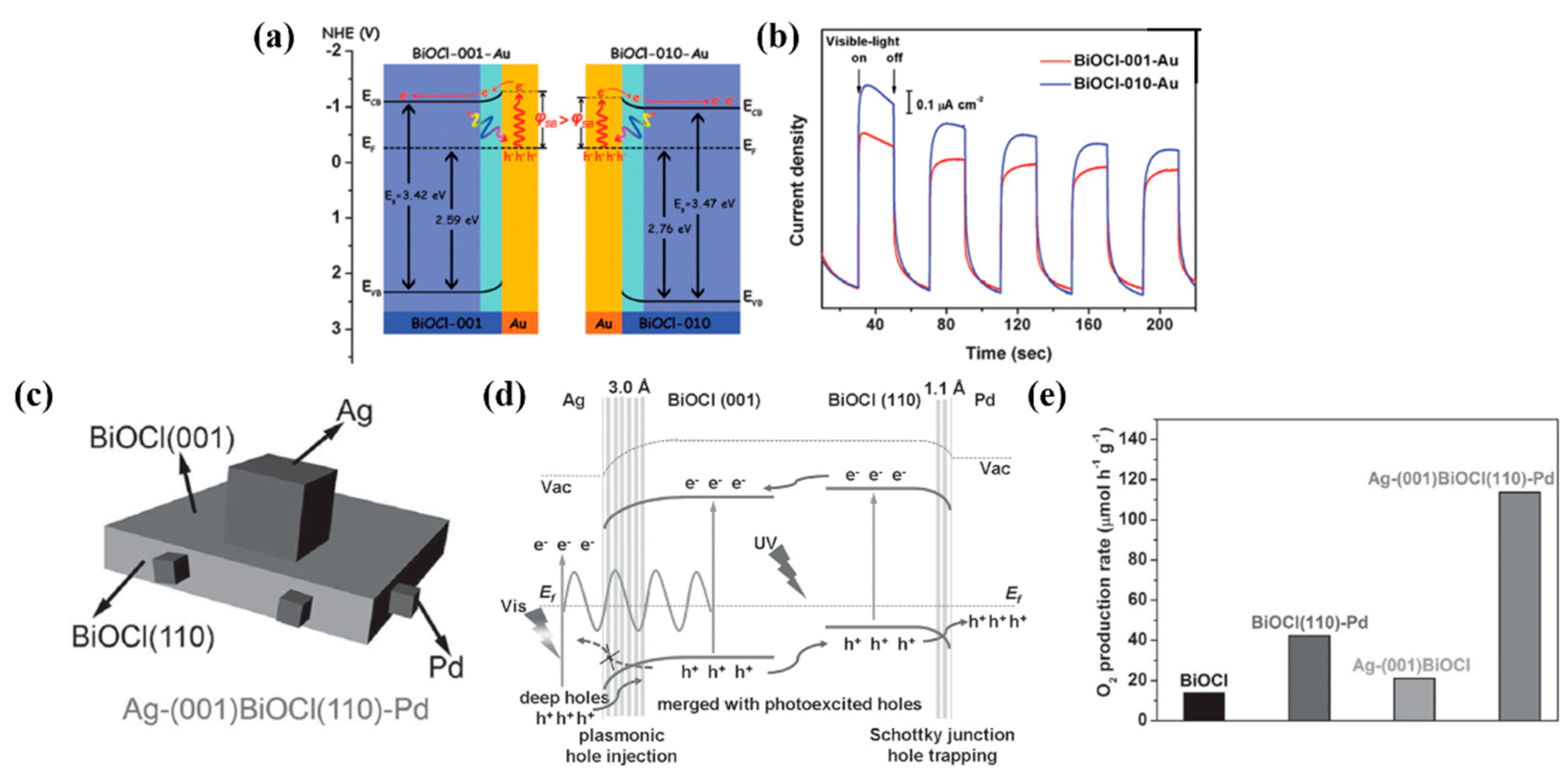
3.2.3. Crystal Orientation
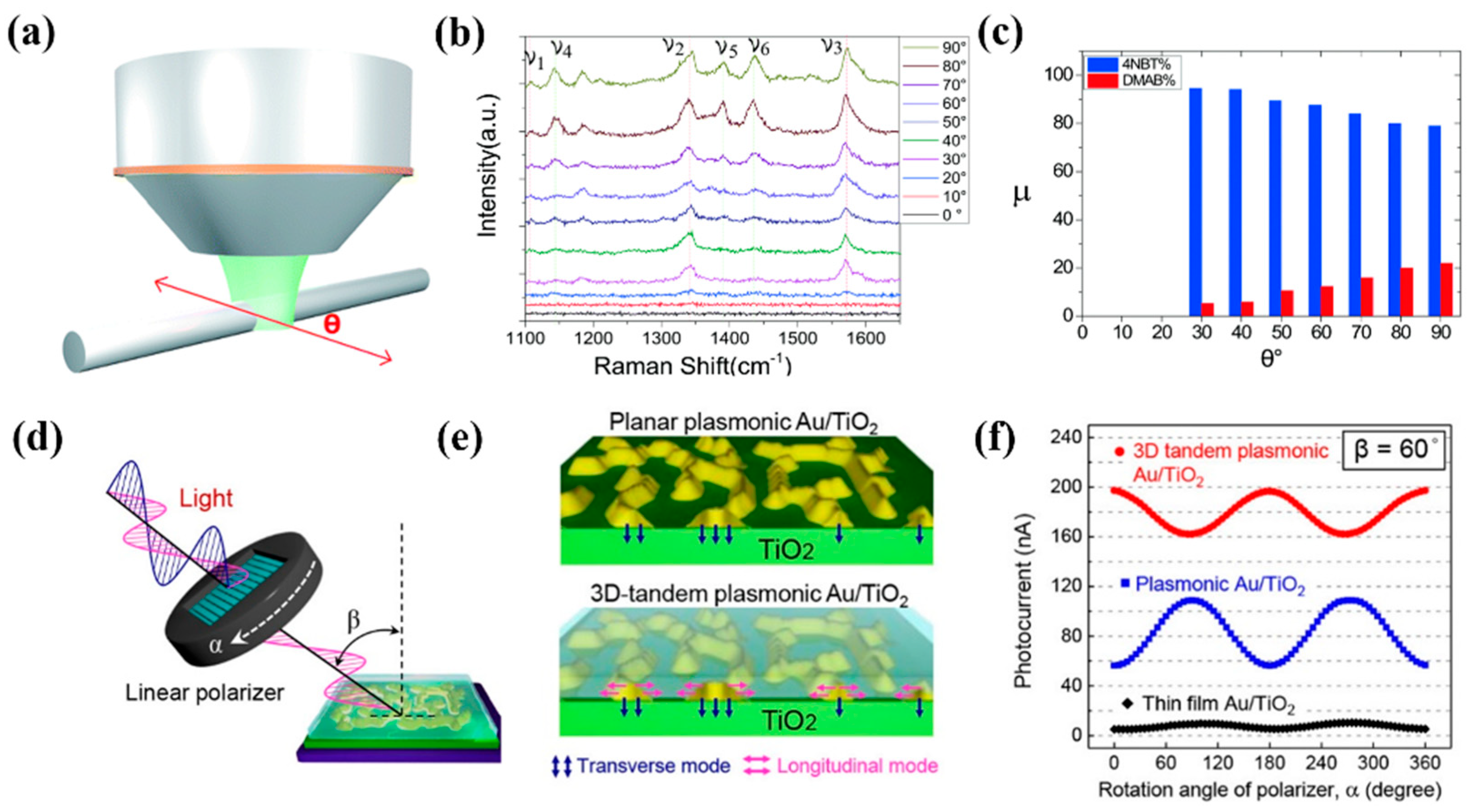
3.2.4. Optic Field Distribution Consideration
4. Applications of Plasmonic Photocatalysts in Environmental Protection
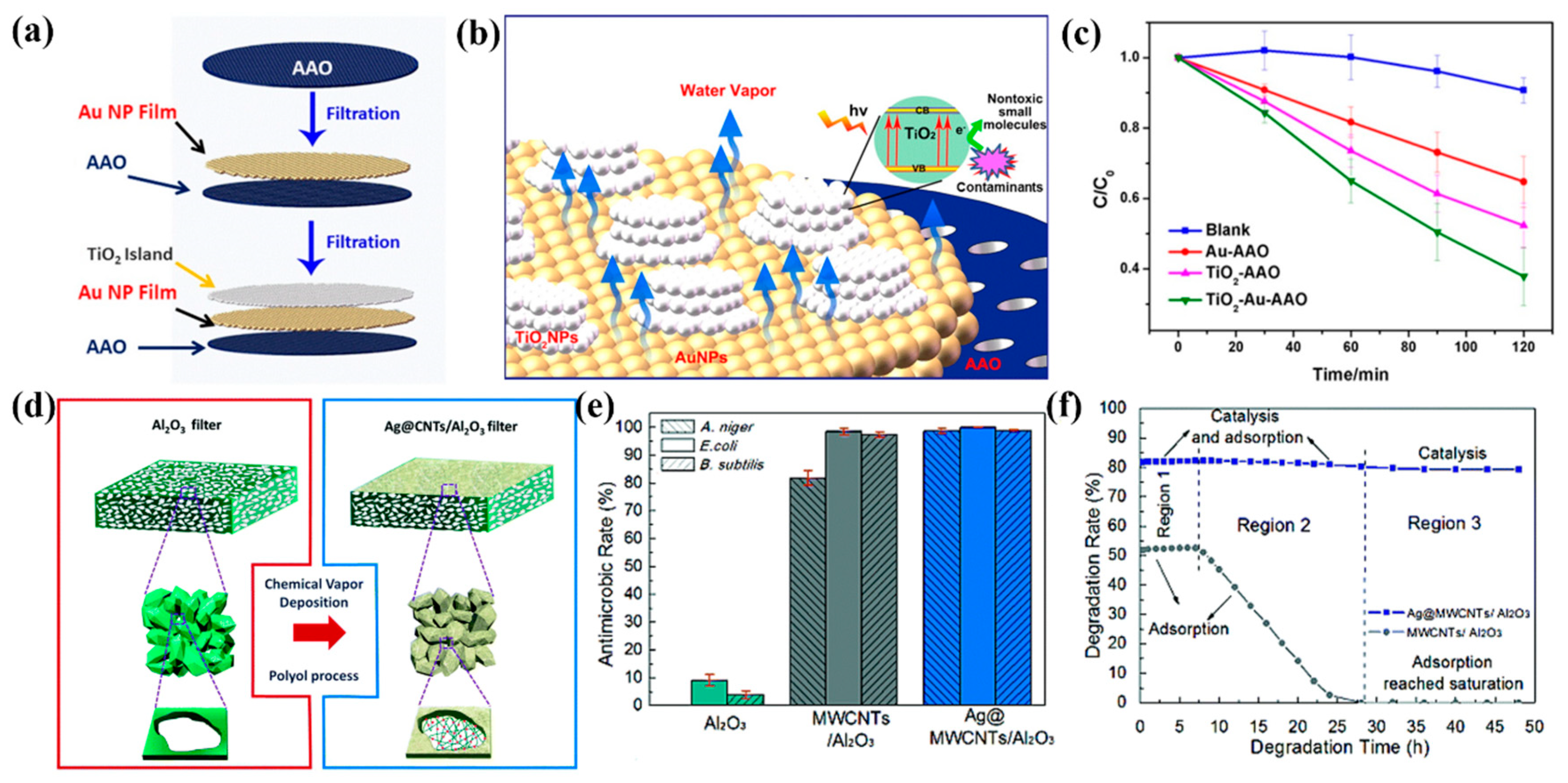
4.1. Water Treatment
4.2. Air Purification
4.3. Photochemical Reactions
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z.; et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Reece, S.Y.; Hamel, J.A.; Sung, K.; Jarvi, T.D.; Esswein, A.J.; Pijpers, J.J.H.; Nocera, D.G. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 2011, 334, 645–648. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Qiu, J.; Wei, W.D. Surface plasmon-mediated photothermal chemistry. J. Phys. Chem. C 2014, 118, 20735–20749. [Google Scholar] [CrossRef]
- Friedmann, D.; Hakki, A.; Kim, H.; Choi, W.; Bahnemann, D. Heterogeneous photocatalytic organic synthesis: State-of-the-art and future perspectives. Green Chem. 2016, 18, 5391–5411. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. C 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Ong, W.L.; Wang, J.; Ho, J.W. Structural design of TiO2-based photocatalyst for H2 production and degradation applications. Catal. Sci. Technol. 2015, 5, 4703–4726. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Di Paola, A.; García-López, E.; Marcì, G.; Palmisano, L. A survey of photocatalytic materials for environmental remediation. J. Hazard. Mater. 2012, 211, 3–29. [Google Scholar] [CrossRef]
- Maier, S.A.; Brongersma, M.L.; Kik, P.G.; Meltzer, S.; Requich, A.A.G.; Atwater, H.A. Plasmonics-a route to nanoscale optical devices. Adv. Mater. 2001, 13, 1501–1505. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Metal nanostructures for subwavelength photonic devices. IEEE J. Sel. Top. Quant. 2006, 12, 1214–1220. [Google Scholar] [CrossRef]
- Zhang, T.; Su, D.; Li, R.Z.; Wang, S.J.; Shan, F.; Xu, J.J.; Zhang, X.Y. Plasmonic nanostructures for electronic designs of photovoltaic devices: Plasmonic hot-carrier photovoltaic architectures and plasmonic electrode structures. J. Photonics Energy 2016, 6, 042504. [Google Scholar] [CrossRef]
- Ingram, D.B.; Linic, S. Water splitting on composite plasmonic-metal/semiconductor photoelectrodes: Evidence for selective plasmon-induced formation of charge carriers near the semiconductor surface. J. Am. Chem. Soc. 2011, 133, 5202–5205. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Jang, Y.H.; Kim, D.H. A study on the mechanism for the interaction of light with noble metal-metal oxide semiconductor nanostructures for various photophysical applications. Chem. Soc. Rev. 2013, 42, 8467–8493. [Google Scholar] [CrossRef]
- Li, J.; Cushing, S.K.; Meng, F.; Senty, T.R.; Bristow, A.D.; Wu, N. Plasmon-induced resonance energy transfer for solar energy conversion. Nat. Photonics 2015, 9, 601–607. [Google Scholar] [CrossRef]
- Maier, S.A.; Atwater, H.A. Plasmonics: Localization and guiding of electromagnetic energy in metal/dielectric structures. J. Appl. Phys. 2005, 98. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, W.; Pavaskar, P.; Aykol, M.; Cronin, S.B. Plasmon resonant enhancement of photocatalytic water splitting under visible illumination. Nano Lett. 2011, 11, 1111–1116. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Plasmoninduced photoelectrochemistry at metal nanoparticles supported on nanoporous TiO2. Chem. Commun. 2004, 1810–1811. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef]
- Christopher, P.; Moskovits, M. Hot charge carrier transmission from plasmonic nanostructures. Annu. Rev. Phys. Chem. 2017, 68, 379–398. [Google Scholar] [CrossRef]
- Zhan, C.; Chen, X.J.; Yi, J.; Li, J.F.; Wu, D.Y.; Tian, Z.Q. From plasmon-enhanced molecular spectroscopy to plasmon-mediated chemical reactions. Nat. Rev. Chem. 2018, 2, 216–230. [Google Scholar] [CrossRef]
- Aslam, U.; Rao, V.G.; Chavez, S.; Linic, S. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures. Nat. Catal. 2018, 1, 656–665. [Google Scholar] [CrossRef]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- O’Brien, K.; Lanzillotti-Kimura, N.D.; Rho, J.; Suchowski, H.; Yin, X.; Zhang, X. Ultrafast acousto-plasmonic control and sensing in complex nanostructures. Nat. Commun. 2014, 5, 4042. [Google Scholar] [CrossRef]
- Subramanian, P.; Meziane, D.; Wojcieszak, R.; Dumeignil, F.; Boukherroub, R.; Szunerits, S. Plasmon-Induced Electrocatalysis with Multi-Component Nanostructures. Materials 2019, 12, 43. [Google Scholar] [CrossRef]
- Tan, S.; Argondizzo, A.; Ren, J.; Liu, L.; Zhao, J.; Petek, H. Plasmonic coupling at a metal/semiconductor interface. Nat. Photonics 2017, 11, 806–812. [Google Scholar] [CrossRef]
- Cushing, S.K. Plasmonic hot carriers skip out in femtoseconds. Nat. Photonics 2017, 11, 748–749. [Google Scholar] [CrossRef]
- Valentine, J. Bridging the gap with hot electrons. Nat. Nanotechnol. 2018, 13, 96–97. [Google Scholar] [CrossRef]
- Yu, S.; Wilson, A.J.; Heo, J.; Jain, P.K. Plasmonic control of multi-electron transfer and C–C coupling in visible-light-driven CO2 reduction on Au nanoparticles. Nano Lett. 2018, 18, 2189–2194. [Google Scholar] [CrossRef]
- Achermann, M. Exciton-plasmon interactions in metal-semiconductor nanostructures. J. Phys. Chem. Lett. 2010, 1, 2837–2843. [Google Scholar] [CrossRef]
- DuChene, J.S.; Sweeny, B.C.; Johnston-Peck, A.C.; Su, D.; Stach, E.A.; Wei, W.D. Prolonged hot electron dynamics in plasmonic-metal/semiconductor heterostructures with implications for solar photocatalysis. Angew. Chem. Int. Edit. 2014, 53, 7887–7891. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Du, J.; Dong, X.; Jian, S.; Yan, L.; Gu, Z.; Zhao, Y. Enhanced Generation of Non-Oxygen Dependent Free Radicals by Schottky-Type Heterostructures of Au-Bi2S3 Nanoparticles via X-Ray-Induced Catalytic Reaction for Radiosensitization. ACS Nano 2019. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.X.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef]
- Nishi, H.; Torimoto, T.; Tatsuma, T. Wavelength-and efficiency-tunable plasmon-induced charge separation by the use of Au–Ag alloy nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 4042–4046. [Google Scholar] [CrossRef]
- Tung, R.T. The physics and chemistry of the Schottky barrier height. Appl. Phys. Rev. 2014, 1, 011304. [Google Scholar]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Petek, H.; Nagano, H.; Ogawa, S. Hot-electron dynamics in copper revisited: The d-band effect. Appl. Phys. B Lasers Opt. 1999, 68, 369–375. [Google Scholar] [CrossRef]
- Sundararaman, R.; Narang, P.; Jermyn, A.S.; Goddard, W.A.; Atwater, H.A. Theoretical predictions for hot-carrier generation from surface plasmon decay. Nat. Commun. 2014, 5, 5788. [Google Scholar] [CrossRef]
- Khurgin, J.B. How to deal with the loss in plasmonics and metamaterials. Nat. Nanotechnol. 2015, 10, 2–6. [Google Scholar] [CrossRef]
- Xue, J.; Elbanna, O.; Kim, S.; Fujitsuka, M.; Majima, T. Defect state-induced efficient hot electron transfer in Au nanoparticles/reduced TiO2 mesocrystal photocatalysts. Chem. Commun. 2018, 54, 6052–6055. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Yasumoto, N.; Imai, J.; Sakamoto, H.; Tanaka, S.; Ichikawa, S.; Ohtanie, B.; Hiraia, T. Quantum tunneling injection of hot electrons in Au/TiO2 plasmonic photocatalysts. Nanoscale 2017, 9, 8349–8361. [Google Scholar] [CrossRef]
- Sönnichsen, C.; Franzl, T.; Wilk, T.; von Plessen, G.; Feldmann, J.; Wilson, O.; Mulvaney, P. Drastic reduction of plasmon damping in gold nanorods. Phys. Rev. Lett. 2002, 88, 077402. [Google Scholar] [CrossRef]
- Hartland, G.V. Optical studies of dynamics in noble metal nanostructures. Chem. Rev. 2011, 111, 3858–3887. [Google Scholar] [CrossRef]
- Govorov, A.O.; Zhang, H.; Gun’ko, Y.K. Theory of photoinjection of hot plasmonic carriers from metal nanostructures into semiconductors and surface molecules. J. Phys. Chem. C 2013, 117, 16616–16631. [Google Scholar] [CrossRef]
- Fowler, R.H. The analysis of photoelectric sensitivity curves for clean metals at various temperatures. Phys. Rev. Lett. 1931, 38, 45. [Google Scholar] [CrossRef]
- Knight, M.W.; Sobhani, H.; Nordlander, P.; Halas, N.J. Photodetection with active optical antennas. Science 2011, 332, 702–704. [Google Scholar] [CrossRef]
- Wu, K.; Chen, J.; McBride, J.R.; Lian, T. Efficient hot-electron transfer by a plasmon-induced interfacial charge-transfer transition. Science 2015, 349, 632–635. [Google Scholar] [CrossRef]
- Christopher, P.; Xin, H.; Marimuthu, A.; Linic, S. Singular characteristics and unique chemical bond activation mechanisms of photocatalytic reactions on plasmonic nanostructures. Nat. Mater. 2012, 11, 1044–1050. [Google Scholar] [CrossRef]
- Cortés, E.; Xie, W.; Cambiasso, J.; Jermyn, A.S.; Sundararaman, R.; Narang, P.; Schlücker, S.; Maier, S.A. Plasmonic hot electron transport drives nano-localized chemistry. Nat. Commun. 2017, 8, 14880. [Google Scholar] [CrossRef]
- Hartland, G.V.; Besteiro, L.V.; Johns, P.; Govorov, A.O. What’s so hot about electrons in metal nanoparticles? ACS Energy Lett. 2017, 2, 1641–1653. [Google Scholar] [CrossRef]
- Szczerbiński, J.; Gyr, L.; Kaeslin, J.; Zenobi, R. Plasmon-driven photocatalysis leads to products known from E-beam and X-ray-induced surface chemistry. Nano Lett. 2018, 18, 6740–6749. [Google Scholar] [CrossRef]
- Besteiro, L.V.; Kong, X.T.; Wang, Z.; Hartland, G.; Govorov, A.O. Understanding hot-electron generation and plasmon relaxation in metal nanocrystals: Quantum and classical mechanisms. ACS Photonics 2017, 4, 2759–2781. [Google Scholar] [CrossRef]
- Zhang, H.; Govorov, A.O. Optical generation of hot plasmonic carriers in metal nanocrystals: The effects of shape and field enhancement. J. Phys. Chem. C 2014, 118, 7606–7614. [Google Scholar] [CrossRef]
- Kong, X.T.; Wang, Z.; Govorov, A.O. Plasmonic nanostars with hot spots for efficient generation of hot electrons under solar illumination. Adv. Opt. Mater. 2017, 5. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef]
- Langille, M.R.; Personick, M.L.; Zhang, J.; Mirkin, C.A. Defining rules for the shape evolution of gold nanoparticles. J. Am. Chem. Soc. 2012, 134, 14542–14554. [Google Scholar] [CrossRef]
- Sousa-Castillo, A.; Comesaña-Hermo, M.; Rodríguez-González, B.; Pérez-Lorenzo, M.; Wang, Z.; Kong, X.T.; Govorov, A.O.; Correa-Duarte, M.A. Boosting hot electron-driven photocatalysis through anisotropic plasmonic nanoparticles with hot spots in Au-TiO2 nanoarchitectures. J. Phys. Chem. C 2016, 120, 11690–11699. [Google Scholar] [CrossRef]
- Wu, B.; Liu, D.; Mubeen, S.; Chuong, T.T.; Moskovits, M.; Stucky, G.D. Anisotropic growth of TiO2 onto gold nanorods for plasmon-enhanced hydrogen production from water reduction. J. Am. Chem. Soc. 2016, 138, 1114–1117. [Google Scholar] [CrossRef]
- Hong, J.W.; Wi, D.H.; Lee, S.U.; Han, S.W. Metal-semiconductor heteronanocrystals with desired configurations for plasmonic photocatalysis. J. Am. Chem. Soc. 2016, 138, 15766–15773. [Google Scholar] [CrossRef]
- Ho, Y.L.; Tai, Y.H.; Clark, J.K.; Wang, Z.; Wei, P.K.; Delaunay, J.J. Plasmonic Hot-Carriers in Channel-Coupled Nanogap Structure for Metal-Semiconductor Barrier Modulation and Spectral-Selective Plasmonic Monitoring. ACS Photonics 2018, 5, 2617–2623. [Google Scholar] [CrossRef]
- Su, M.N.; Sun, Q.; Ueno, K.; Chang, W.S.; Misawa, H.; Link, S. Optical Characterization of Gold Nanoblock Dimers: From Capacitive Coupling to Charge Transfer Plasmons and Rod Modes. J. Phys. Chem. C 2018, 122, 18005–18011. [Google Scholar] [CrossRef]
- Flauraud, V.; Bernasconi, G.D.; Butet, J.; Alexander, D.T.L.; Martin, O.J.F.; Brugger, J. Mode coupling in plasmonic heterodimers probed with electron energy loss spectroscopy. ACS Nano 2017, 11, 3485–3495. [Google Scholar] [CrossRef]
- Besteiro, L.V.; Govorov, A.O. Amplified generation of hot electrons and quantum surface effects in nanoparticle dimers with plasmonic hot spots. J. Phys. Chem. C 2016, 120, 19329–19339. [Google Scholar] [CrossRef]
- Negrín-Montecelo, Y.; Comesaña-Hermo, M.; Kong, X.T.; Rodríguez-González, B.; Wang, Z.; Pérez-Lorenzo, M.; Govorov, A.O.; Correa-Duarte, M.A. Traveling Hot Spots in Plasmonic Photocatalysis: Manipulating Interparticle Spacing for Real-Time Control of Electron Injection. ChemCatChem 2018, 10, 1561–1565. [Google Scholar] [CrossRef]
- Li, X.; Guo, S.; Kan, C.; Zhu, J.; Tong, T.; Ke, S.; Choy, W.C.H.; Wei, B. Au Multimer@MoS2 hybrid structures for efficient photocatalytical hydrogen production via strongly plasmonic coupling effect. Nano Energy 2016, 30, 549–558. [Google Scholar] [CrossRef]
- Fang, Y.; Jiao, Y.; Xiong, K.; Ogier, R.; Yang, Z.J.; Gao, S.; Dahlin, A.B.; Käll, M. Plasmon enhanced internal photoemission in antenna-spacer-mirror based Au/TiO2 nanostructures. Nano Lett. 2015, 15, 4059–4065. [Google Scholar] [CrossRef]
- Sykes, M.E.; Stewart, J.W.; Akselrod, G.M.; Kong, X.T.; Wang, Z.; Gosztola, D.J.; Martinson, A.B.F.; Rosenmann, D.; Mikkelsen, M.H.; Govorov, A.O.; et al. Enhanced generation and anisotropic Coulomb scattering of hot electrons in an ultra-broadband plasmonic nanopatch metasurface. Nat. Commun. 2017, 8, 986. [Google Scholar] [CrossRef]
- Ng, C.; Cadusch, J.J.; Dligatch, S.; Roberts, A.; Davis, T.J.; Mulvaney, P.; Gómez, D.E. Hot carrier extraction with plasmonic broadband absorbers. ACS Nano 2016, 10, 4704–4711. [Google Scholar] [CrossRef]
- Ho, K.H.W.; Shang, A.; Shi, F.; Lo, T.W.; Yeung, P.H.; Yu, Y.S.; Zhang, X.; Wong, K.; Lei, D.Y. Plasmonic Au/TiO2-Dumbbell-On-Film Nanocavities for High-Efficiency Hot-Carrier Generation and Extraction. Adv. Funct. Mater. 2018, 28, 1800383. [Google Scholar] [CrossRef]
- Bernardi, M.; Mustafa, J.; Neaton, J.B.; Louie, S.G. Theory and computation of hot carriers generated by surface plasmon polaritons in noble metals. Nat. Commun. 2015, 6, 7044. [Google Scholar] [CrossRef]
- Ma, X.C.; Dai, Y.; Yu, L.; Huang, B.B. Energy transfer in plasmonic photocatalytic composites. Light Sci. Appl. 2016, 5, e16017. [Google Scholar] [CrossRef]
- Sedghi, M.; Rahimi, R.; Rabbani, M. Design of a Plasmonic Photocatalyst Structure Consisting of Metallic Nanogratings for Light-Trapping Enhancement. Plasmonics 2018. [Google Scholar] [CrossRef]
- Taghinejad, M.; Taghinejad, H.; Xu, Z.; Lee, K.T.; Rodrigues, S.P.; Yan, J.; Adibi, A.; Lian, T.; Cai, W. Ultrafast Control of Phase and Polarization of Light Expedited by Hot-Electron Transfer. Nano Lett. 2018, 18, 5544–5551. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, Z.; Lyu, M.; Luo, B.; Wang, S.; Liu, G.; Cheng, H.M.; Wang, L. Hollow nanostructures for Photocatalysis: Advantages and challenges. Adv. Mater. 2018. [Google Scholar] [CrossRef]
- Genç, A.; Patarroyo, J.; Sancho-Parramon, J.; Bastús, N.G.; Puntes, V.; Arbiol, J. Hollow metal nanostructures for enhanced plasmonics: Synthesis, local plasmonic properties and applications. Nanophotonics 2017, 6, 193–213. [Google Scholar] [CrossRef]
- Shi, X.; Lou, Z.; Zhang, P.; Fujitsuka, M.; Majima, T. 3D-Array of Au–TiO2 Yolk–Shell as Plasmonic Photocatalyst Boosting Multi-Scattering with Enhanced Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 31738–31745. [Google Scholar] [CrossRef]
- Dinh, C.T.; Yen, H.; Kleitz, F.; Do, T.O. Three-Dimensional Ordered Assembly of Thin-Shell Au/TiO2 Hollow Nanospheres for Enhanced Visible-Light-Driven Photocatalysis. Angew. Chem. Int. Edit. 2014, 53, 6618–6623. [Google Scholar] [CrossRef]
- Wen, L.; Xu, R.; Cui, C.; Tang, W.; Mi, Y.; Lu, X.; Zeng, Z.; Suib, S.L.; Gao, P.X.; Lei, Y. Template-Guided Programmable Janus Heteronanostructure Arrays for Efficient Plasmonic Photocatalysis. Nano Lett. 2018, 18, 4914–4921. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, X.; Ruan, X.; Song, H.; Lou, Z.; Ye, Z.; Zhu, L. Enhancing photocatalytic activity for visible-light-driven H2 generation with the surface reconstructed LaTiO2N nanostructures. Nano Energy 2015, 12, 775–784. [Google Scholar] [CrossRef]
- Hwang, S.H.; Yun, J.; Jang, J. Multi-Shell Porous TiO2 Hollow Nanoparticles for Enhanced Light Harvesting in Dye-sensitized Solar Cells. Adv. Funct. Mater. 2014, 24, 7619–7626. [Google Scholar] [CrossRef]
- Bai, S.; Ge, J.; Wang, L.; Gong, M.; Deng, M.; Kong, Q.; Song, L.; Jiang, J.; Zhang, Q.; Luo, Y.; et al. A unique semiconductor–metal–graphene stack design to harness charge flow for photocatalysis. Adv. Mater. 2014, 26, 5689–5695. [Google Scholar] [CrossRef]
- Tachikawa, T.; Yonezawa, T.; Majiama, T. Super-Resolution Mapping of Reactive Sites on Titania-Based Nanoparticles with Water-Soluble Fluorogenic Probes. ACS Nano 2013, 7, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Lambright, S.; Butaeva, E.; Razgoniaeva, N.; Hopkins, T.; Smith, B.; Perera, D.; Corbin, J.; Khon, E.; Thomas, R.; Moroz, P.; et al. Enhanced lifetime of excitons in nonepitaxial Au/CdS core/shell nanocrystals. ACS Nano 2014, 8, 352–361. [Google Scholar] [CrossRef]
- Zhang, T.; Song, Y.J.; Zhang, X.Y.; Wu, J.Y. Synthesis of silver nanostructures by multistep methods. Sensors 2014, 14, 5860–5889. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Lee, K.; Ouyang, M. Nonepitaxial growth of hybrid core-shell nanostructures with large lattice mismatches. Science 2010, 327, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, J.; Gui, J.; Chen, T.; Xu, M.; Wang, H.; Dong, H.; Chen, H.; Li, X.; Wang, L.; et al. Metal@semiconductor core-shell nanocrystals with atomically organized interfaces for efficient hot electron-mediated photocatalysis. Nano Energy 2018, 48, 44–52. [Google Scholar] [CrossRef]
- Lim, S.C.; Lo, W.F.; Yang, P.Y.; Lu, S.C.; Joplin, A.; Link, S.; Chang, W.S.; Tuan, H.Y. Au@CdSe heteroepitaxial nanorods: An example of metal nanorods fully covered by a semiconductor shell with strong photo-induced interfacial charge transfer effects. J. Colloid Interface Sci. 2018, 532, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ouyang, S.; Ye, J. Gold-Nanorod-Photosensitized Titanium Dioxide with Wide-Range Visible-Light Harvesting Based on Localized Surface Plasmon Resonance. Angew. Chem. Int. Edit. 2013, 52, 6689–6693. [Google Scholar] [CrossRef]
- Leschkies, K.S.; Divakar, R.; Basu, J.; Enache-Pommer, E.; Boercker, J.E.; Carter, C.B.; Kortshagen, U.R.; Norris, D.J.; Aydil, E.S. Photosensitization of ZnO nanowires with CdSe quantum dots for photovoltaic devices. Nano Lett. 2007, 7, 1793–1798. [Google Scholar] [CrossRef]
- Ding, D.; Liu, K.; He, S.; Gao, C.; Yin, Y. Ligand-exchange assisted formation of Au/TiO2 schottky contact for visible-light photocatalysis. Nano Lett. 2014, 14, 6731–6736. [Google Scholar] [CrossRef]
- Lopez-Sanchez, J.A.; Dimitratos, N.; Hammond, C.; Brett, G.L.; Kesavan, L.; White, S.; Miedziak, P.; Tiruvalam, R.; Jenkins, R.L.; Carley, A.F.; et al. Facile removal of stabilizer-ligands from supported gold nanoparticles. Nat. Chem. 2011, 3, 551–556. [Google Scholar] [CrossRef]
- Ge, Y.; Duan, X.; Zhang, M.; Mei, L.; Hu, J.; Hu, W.; Duan, X. Direct room temperature welding and chemical protection of silver nanowire thin films for high performance transparent conductors. J. Am. Chem. Soc. 2017, 140, 193–199. [Google Scholar] [CrossRef]
- Shan, F.; Zhang, X.Y.; Fu, X.C.; Zhang, L.J.; Su, D.; Wang, S.J.; Wu, J.Y.; Zhang, T. Investigation of simultaneously existed Raman scattering enhancement and inhibiting fluorescence using surface modified gold nanostars as SERS probes. Sci. Rep. 2017, 7, 6813. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xu, J.J.; Wu, J.Y.; Shan, F.; Ma, X.D.; Chen, Y.Z.; Zhang, T. Seeds triggered massive synthesis and multi-step room temperature post-processing of silver nanoink-application for paper electronics. RSC Adv. 2017, 7, 8–19. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, Y.; Yin, M.; Zhang, H.; Cheng, C.; Lu, L.; Xue, X.; Fan, H.J.; Chen, X.; Li, D. Understanding the Enhancement Mechanisms of Surface Plasmon-Mediated Photoelectrochemical Electrodes: A Case Study on Au Nanoparticle Decorated TiO2 Nanotubes. Adv. Mater. Interfaces 2015, 2, 1500169. [Google Scholar] [CrossRef]
- Bian, Z.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 superstructure-based plasmonic photocatalysts exhibiting efficient charge separation and unprecedented activity. J. Am. Chem. Soc. 2013, 136, 458–465. [Google Scholar] [CrossRef]
- Hao, C.H.; Guo, X.N.; Sankar, M.; Yang, H.; Ma, B.; Zhang, Y.F.; Tong, X.L.; Jin, G.Q.; Guo, X.Y. Synergistic Effect of Segregated Pd and Au Nanoparticles on Semiconducting SiC for Efficient Photocatalytic Hydrogenation of Nitroarenes. ACS Appl. Mater. Interfaces 2018, 10, 23029–23036. [Google Scholar] [CrossRef]
- Li, X.; McNaughter, P.D.; O’Brien, P.; Minamimoto, H.; Murakoshi, K. Plasmonically enhanced electromotive force of narrow bandgap PbS QD-based photovoltaics. Phys. Chem. Chem. Phys. 2018, 20, 14818–14827. [Google Scholar] [CrossRef]
- Liu, G.; Wang, T.; Zhou, W.; Meng, X.; Zhang, H.; Liu, H.; Kako, T.; Ye, J. Crystal-facet-dependent hot-electron transfer in plasmonic-Au/semiconductor heterostructures for efficient solar photocatalysis. J. Mater. Chem. C 2015, 3, 7538–7542. [Google Scholar] [CrossRef]
- Bai, S.; Li, X.; Kong, Q.; Long, R.; Wang, C.; Jiang, J.; Xiong, Y. Toward enhanced photocatalytic oxygen evolution: Synergetic utilization of plasmonic effect and schottky junction via interfacing facet selection. Adv. Mater. 2015, 27, 3444–3452. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, S.; Zhao, Z.; Shi, Z.; Yan, S.; Zou, Z. A Facet-Dependent Schottky-Junction Electron Shuttle in a BiVO4 {010}–Au–Cu2O Z-Scheme Photocatalyst for Efficient Charge Separation. Adv. Funct. Mater. 2018, 28, 1801214. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Zhang, L.; Fang, Y.; Wang, P. Polarization-dependent surface plasmon-driven catalytic reaction on a single nanowire monitored by SERS. Nanoscale 2018, 10, 18720–18727. [Google Scholar] [CrossRef]
- Lee, C.; Lee, Y.K.; Park, Y.; Park, J.Y. Polarization Effect of Hot Electrons in Tandem-Structured Plasmonic Nanodiode. ACS Photonics 2018, 5, 3499–3506. [Google Scholar] [CrossRef]
- Gong, T.; Munday, J.N. Angle-independent hot carrier generation and collection using transparent conducting oxides. Nano Lett. 2014, 15, 147–152. [Google Scholar] [CrossRef]
- Li, W.; Coppens, Z.J.; Besteiro, L.V.; Wang, W.; Govorov, A.O.; Valentine, J. Circularly polarized light detection with hot electrons in chiral plasmonic metamaterials. Nat. Commun. 2015, 6, 8379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhou, H.L.; Shan, F.; Xue, X.M.; Su, D.; Liu, Y.R.; Chen, Y.Z.; Wu, J.Y.; Zhang, T. Synthesis of silver nanoplate based two-dimension plasmonic platform from 25 nm to 40 μm: Growth mechanism and optical characteristic investigation in situ. RSC Adv. 2017, 7, 55680–55690. [Google Scholar] [CrossRef]
- Gao, M.; Connor, P.K.N.; Ho, G.W. Plasmonic photothermic directed broadband sunlight harnessing for seawater catalysis and desalination. Energy Environ. Sci. 2016, 9, 3151–3160. [Google Scholar] [CrossRef]
- Liu, Y.; Lou, J.; Ni, M.; Song, C.; Wu, J.; Dasgupta, N.P.; Tao, P.; Shang, W.; Deng, T. Bioinspired bifunctional membrane for efficient clean water generation. ACS Appl. Mater. Interfaces 2015, 8, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Shan, F.; Zhou, H.L.; Su, D.; Xue, X.M.; Wu, J.Y.; Chen, Y.Z.; Zhao, N.; Zhang, T. Silver nanoplate aggregation based multifunctional black metal absorbers for localization, photothermic harnessing enhancement and omnidirectional light antireflection. J. Mater. Chem. C 2018, 6, 989–999. [Google Scholar] [CrossRef]
- Souzandeh, H.; Johnson, K.S.; Wang, Y.; Bhamidipaty, K.; Zhong, W.H. Soy-protein-based nanofabrics for highly efficient and multifunctional air filtration. ACS Appl. Mater. Interfaces 2016, 8, 20023–20031. [Google Scholar] [CrossRef]
- Xiong, Z.C.; Yang, R.L.; Zhu, Y.J.; Chen, F.F.; Dong, L.Y. Flexible hydroxyapatite ultralong nanowire-based paper for highly efficient and multifunctional air filtration. J. Mater. Chem. A 2017, 5, 17482–17491. [Google Scholar] [CrossRef]
- Lv, D.; Wang, R.; Tang, G.; Mou, Z.; Lei, J.; Han, J.; Smedt, S.D.; Xiong, R.; Huang, C. Eco-friendly Electrospun Membranes Loaded with Visible-light Response Nano-particles for Multifunctional usages: High-efficient Air Filtration, Dye Scavenger and Bactericide. ACS Appl. Mater. Interfaces 2019, 11, 12880–12889. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Cho, H.; Han, S.; Won, P.; Lee, H.; Hong, S.; Yeo, J.; Kwon, J.; Ko, S.H. High efficiency, transparent, reusable, and active PM2. 5 filters by hierarchical Ag nanowire percolation network. Nano Lett. 2017, 17, 4339–4346. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xue, X.M.; Zhou, H.L.; Zhao, N.; Shan, F.; Su, D.; Liu, Y.R.; Zhang, T. Seeds screening aqueous synthesis, multiphase interfacial separation and in situ optical characterization of invisible ultrathin silver nanowires. Nanoscale 2018, 10, 15468–15484. [Google Scholar] [CrossRef] [PubMed]
- Björklund, K.; Li, L. Evaluation of low-cost materials for sorption of hydrophobic organic pollutants in stormwater. J. Environ. Manag. 2015, 159, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hsu, P.C.; Lee, H.W.; Ye, M.; Zheng, G.; Liu, N.; Li, W.; Cui, Y. Transparent air filter for high-efficiency PM2.5 capture. Nat. Commun. 2015, 6, 6205–6214. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, C.; Hsu, P.C.; Liu, K.; Zhang, R.; Liu, Y.; Cui, Y. Roll-to-Roll Transfer of Electrospun Nanofiber Film for High-Efficiency Transparent Air Filter. Nano Lett. 2016, 16, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Low, Z.X.; Feng, S.; Zhong, Z.; Wang, Y.; Yao, Z. Multifunctional hybrid porous filters with hierarchical structures for simultaneous removal of indoor VOCs, dusts and microorganisms. Nanoscale 2017, 9, 5433–5444. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, Y.; Ye, W.; Li, Y. CO2 reduction: From the electrochemical to photochemical approach. Adv. Sci. 2017, 4, 1700194. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.J.; Gong, J. Nanostructured materials for heterogeneous electrocatalytic CO2 reduction and their related reaction mechanisms. Angew. Chem. Int. Edit. 2017, 56, 11326–11353. [Google Scholar] [CrossRef]
- Lang, X.; Chen, X.; Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef]
- Corrigan, N.; Shanmugam, S.; Xu, J.; Boyer, C. Photocatalysis in organic and polymer synthesis. Chem. Soc. Rev. 2016, 45, 6165–6212. [Google Scholar] [CrossRef]
- Li, B.; Zhang, B.; Nie, S.; Shao, L.; Hu, L. Optimization of plasmon-induced photocatalysis in electrospun Au/CeO2 hybrid nanofibers for selective oxidation of benzyl alcohol. J. Catal. 2017, 348, 256–264. [Google Scholar] [CrossRef]
- Han, D.; Bao, Z.; Xing, H.; Yang, Y.; Ren, Q.; Zhang, Z. Fabrication of plasmonic Au–Pd alloy nanoparticles for photocatalytic Suzuki–Miyaura reactions under ambient conditions. Nanoscale 2017, 9, 6026–6032. [Google Scholar] [CrossRef]
- Yurdakal, S.; Tek, B.S.; Değirmenci, Ç.; Palmisano, G. Selective photocatalytic oxidation of aromatic alcohols in solar-irradiated aqueous suspensions of Pt, Au, Pd and Ag loaded TiO2 catalysts. Catal. Today 2017, 281, 53–59. [Google Scholar] [CrossRef]
- Zhao, J.; Nguyen, S.C.; Ye, R.; Ye, B.; Weller, H.; Somorjai, G.A.; Alivisatos, A.P.; Toste, F.D. A comparison of photocatalytic activities of gold nanoparticles following plasmonic and interband excitation and a strategy for harnessing interband hot carriers for solution phase photocatalysis. ACS Cent. Sci. 2017, 3, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Minutella, E.; Schulz, F.; Lange, H. Excitation-dependence of plasmon-induced hot electrons in gold nanoparticles. J. Phys. Chem. Lett. 2017, 8, 4925–4929. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Swearer, D.F.; Zhang, C.; Robatjazi, H.; Zhao, H.; Henderson, L.; Dong, L.; Christopher, P.; Carter, E.A.; Nordlander, P.; et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 2018, 362, 69–72. [Google Scholar] [CrossRef]
- Meng, X.; Liu, L.; Ouyang, S.; Xu, H.; Wang, D.; Zhao, N.; Ye, J. Nanometals for Solar-to-Chemical Energy Conversion: From Semiconductor-Based Photocatalysis to Plasmon-Mediated Photocatalysis and Photo-Thermocatalysis. Adv. Mater. 2016, 28, 6781–6803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Reish, M.E.; Zhang, D.; Su, N.Q.; Gutiérrez, Y.; Moreno, F.; Yang, W.; Everitt, H.O.; Liu, J. Plasmon-enhanced catalysis: Distinguishing thermal and nonthermal effects. Nano Lett. 2018, 18, 1714–1723. [Google Scholar] [CrossRef]
- Swearer, D.F.; Leary, R.K.; Newell, R.; Yazdi, S.; Robatjazi, H.; Zhang, Y.; Renard, D.; Nordlander, P.; Midgley, P.A.; Halas, N.J.; et al. Transition-metal decorated aluminum nanocrystals. ACS Nano 2017, 11, 10281–10288. [Google Scholar] [CrossRef]
- Shaik, F.; Peer, I.; Jain, P.K.; Amirav, L. Plasmon-Enhanced Multicarrier Photocatalysis. Nano Lett. 2018, 18, 4370–4376. [Google Scholar] [CrossRef]
- Wang, R.; Purdie, D.G.; Fan, Y.; Massabuau, F.C.-P.; Braeuninger-Weimer, P.; Burton, O.J.; Blume, R.; Schloegl, R.; Lombardo, A.; Weatherup, R.S.; et al. A Peeling Approach for Integrated Manufacturing of Large Monolayer h-BN Crystals. ACS Nano 2019, 13, 2114–2126. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Hao, B.; Waters, K.; Lee, C.H.; Idrobo, J.C.; Zhang, D.; Pandey, R.; Yap, Y.K. Two-Dimensional Gold Quantum Dots with Tunable Bandgaps. ACS Nano 2019, 13, 4347–4353. [Google Scholar] [CrossRef]
- Tom, K.B.; Lin, S.; Wan, L.F.; Wang, J.; Ahlm, N.; N’Diaye, A.T.; Bustillo, K.; Huang, J.; Liu, Y.; Lou, S.; et al. Solution-Based, Template-Assisted Realization of Large-Scale Graphitic ZnO. ACS Nano 2018, 12, 7554–7561. [Google Scholar] [CrossRef]
- Jang, Y.J.; Chung, K.; Lee, J.S.; Choi, C.H.; Lim, J.W.; Kim, D.H. Plasmonic Hot Carriers Imaging: Promise and Outlook. ACS Photonics 2018, 5, 4711–4723. [Google Scholar] [CrossRef]
- Keller, E.L.; Frontiera, R.R. Ultrafast nanoscale Raman thermometry proves heating is not a primary mechanism for plasmon-driven photocatalysis. ACS Nano 2018, 12, 5848–5855. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Kim, S.; Zhang, P.; Shi, X.; Fujitsuka, M.; Majima, T. In situ observation of single Au triangular nanoprism etching to various shapes for plasmonic photocatalytic hydrogen generation. ACS Nano 2016, 11, 968–974. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Wang, S.-J.; Zhang, X.-Y.; Su, D.; Yang, Y.; Wu, J.-Y.; Xu, Y.-Y.; Zhao, N. Progress in the Utilization Efficiency Improvement of Hot Carriers in Plasmon-Mediated Heterostructure Photocatalysis. Appl. Sci. 2019, 9, 2093. https://doi.org/10.3390/app9102093
Zhang T, Wang S-J, Zhang X-Y, Su D, Yang Y, Wu J-Y, Xu Y-Y, Zhao N. Progress in the Utilization Efficiency Improvement of Hot Carriers in Plasmon-Mediated Heterostructure Photocatalysis. Applied Sciences. 2019; 9(10):2093. https://doi.org/10.3390/app9102093
Chicago/Turabian StyleZhang, Tong, Shan-Jiang Wang, Xiao-Yang Zhang, Dan Su, Yi Yang, Jing-Yuan Wu, Yao-Yao Xu, and Ning Zhao. 2019. "Progress in the Utilization Efficiency Improvement of Hot Carriers in Plasmon-Mediated Heterostructure Photocatalysis" Applied Sciences 9, no. 10: 2093. https://doi.org/10.3390/app9102093
APA StyleZhang, T., Wang, S.-J., Zhang, X.-Y., Su, D., Yang, Y., Wu, J.-Y., Xu, Y.-Y., & Zhao, N. (2019). Progress in the Utilization Efficiency Improvement of Hot Carriers in Plasmon-Mediated Heterostructure Photocatalysis. Applied Sciences, 9(10), 2093. https://doi.org/10.3390/app9102093



