Early Therapeutic Prediction Based on Tumor Hemodynamic Response Imaging: Clinical Studies in Breast Cancer with Time-Resolved Diffuse Optical Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients, Settings, and Study Design
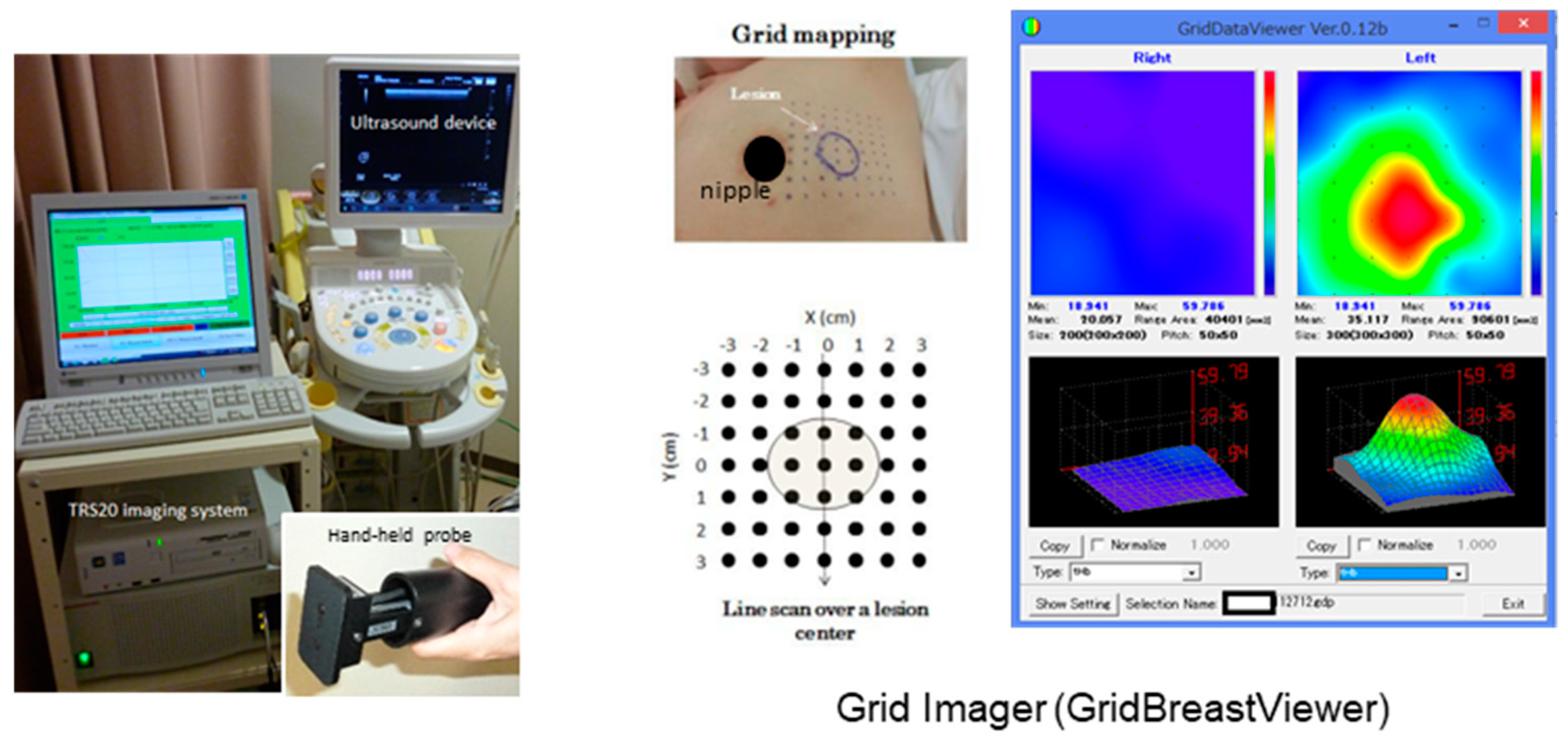
2.2. Establishment of the TRS Breast Imaging System
2.3. Procedure of TRS Measurements
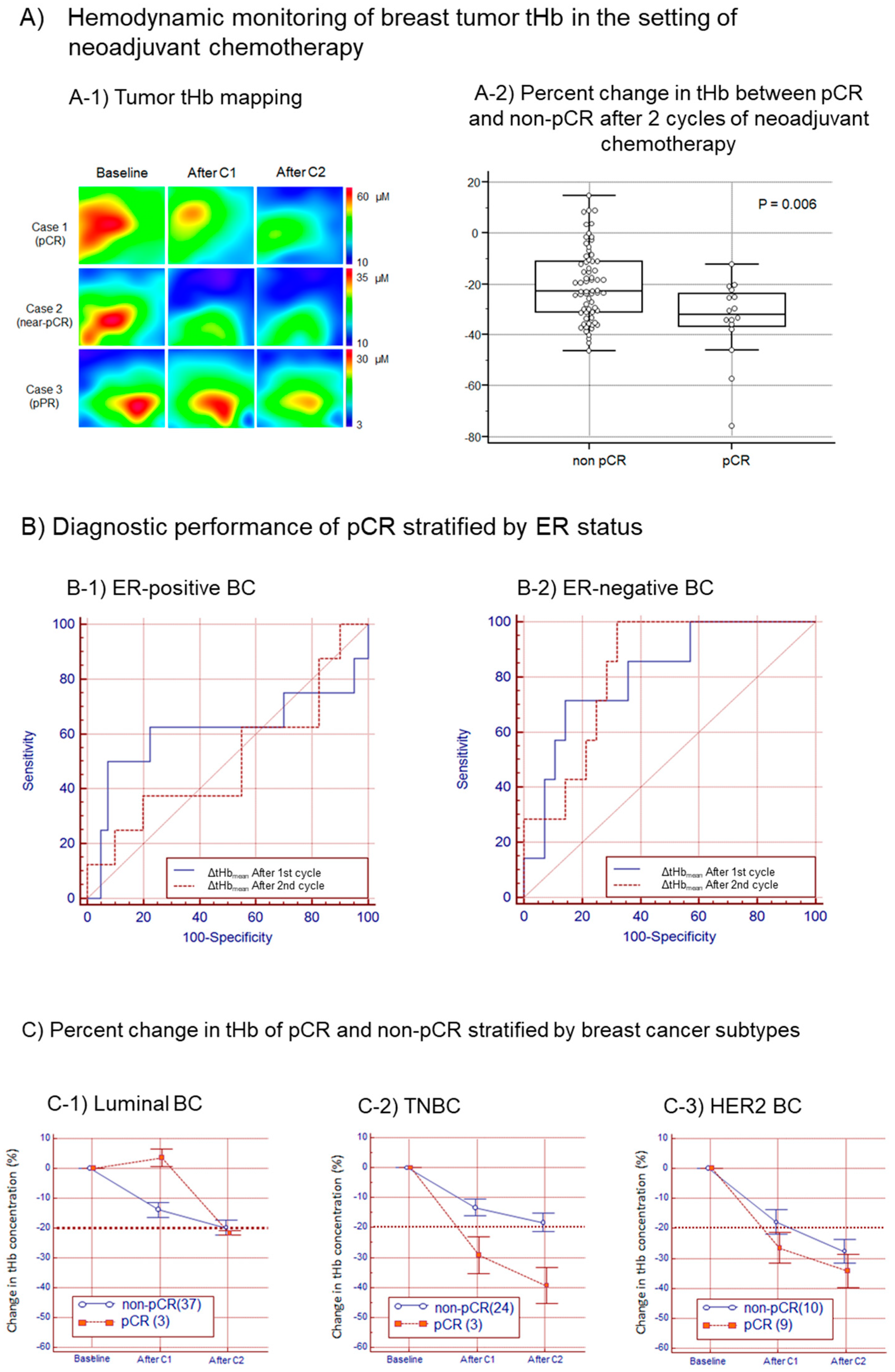
2.4. Monitoring Tumor Response to Neoadjuvant Chemotherapy
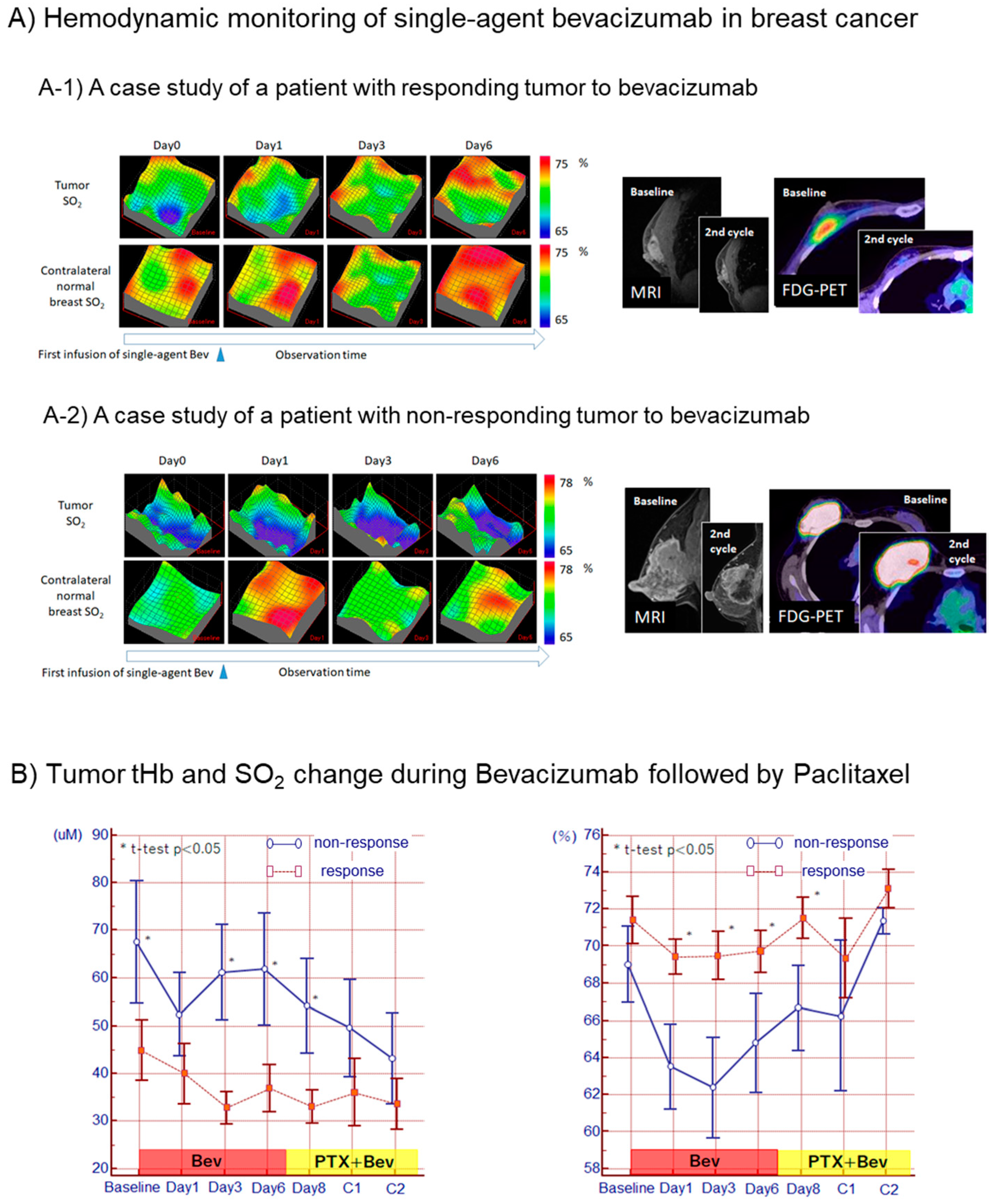
2.5. Optical Visualization of Cancer Vascular Remodeling after Antiangiogenic Therapy
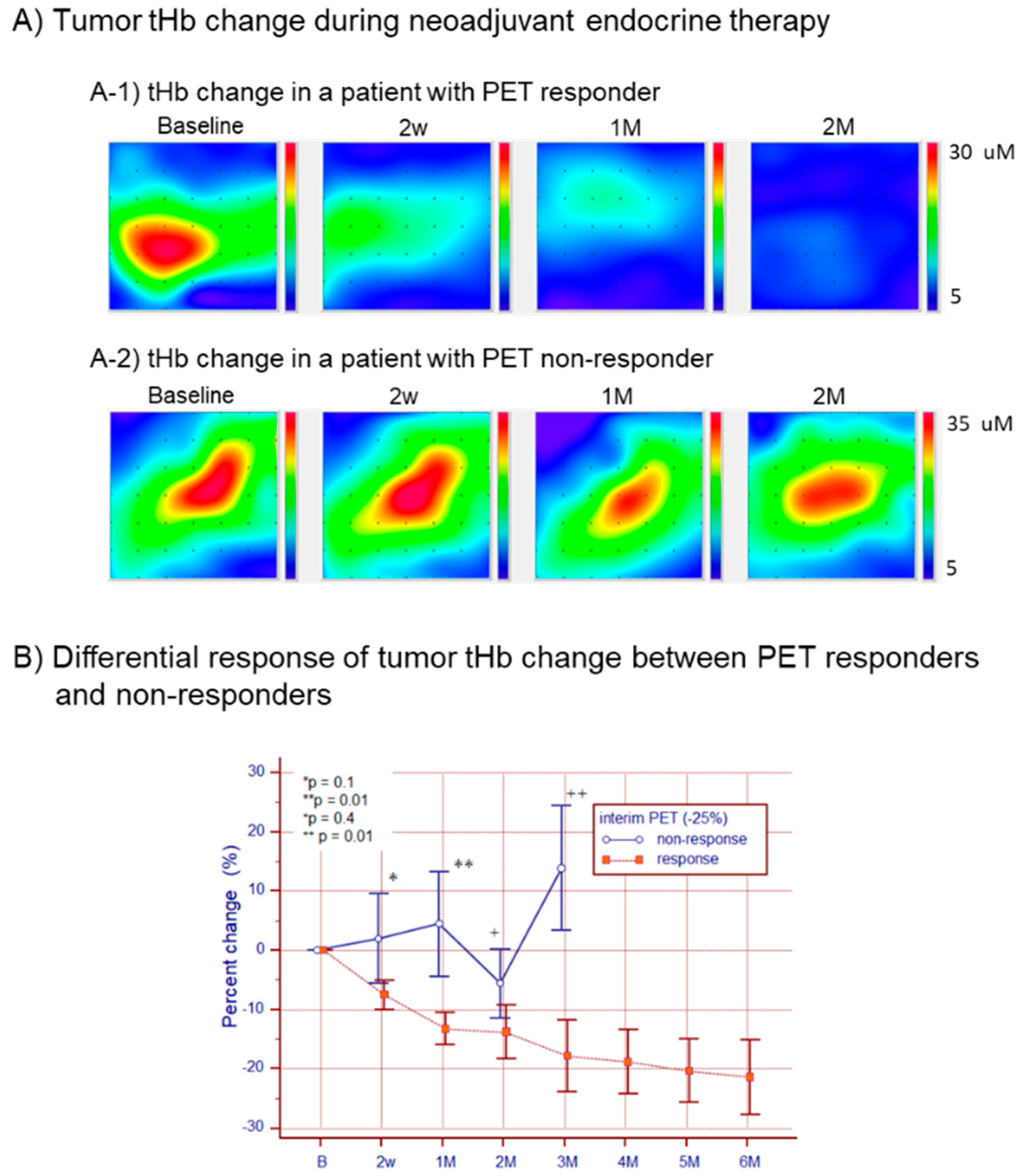
2.6. Monitoring Tumor Response to Neoadjuvant Endocrine Therapy
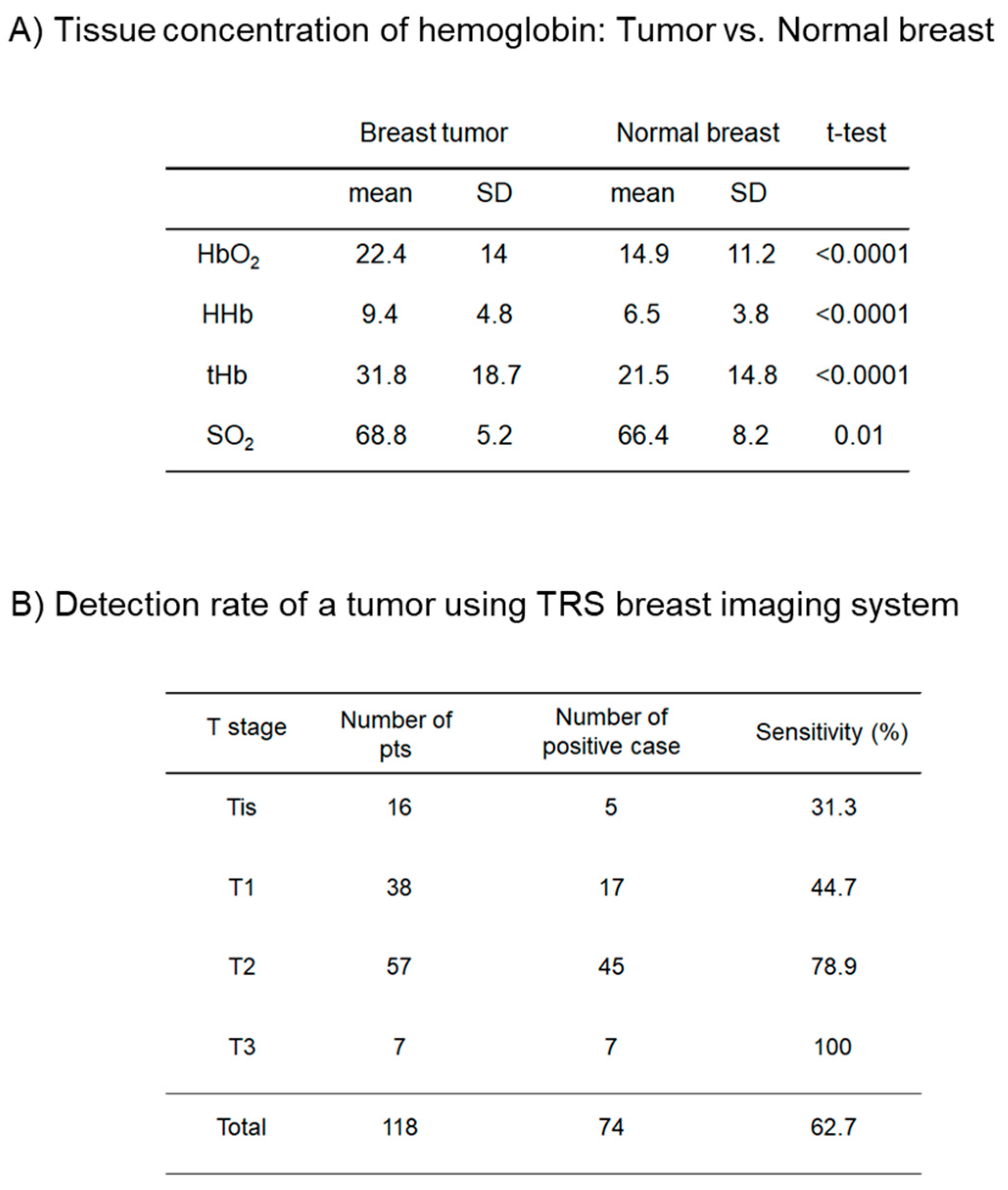
3. Results
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Machida, Y.; Tozaki, M.; Shimauchi, A.; Yoshida, T. Breast density: The trend in breast cancer screening. Breast Cancer 2015, 22, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Ishida, T.; Ohuchi, N. Controversies in breast cancer screening for women aged 40–49 years. Jpn. J. Clin. Oncol. 2014, 44, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Tromberg, B.J.; Cerussi, A.E. Imaging breast cancer chemotherapy response with light. Commentary on soliman et al., p. 2605. Clin. Cancer Res. 2010, 16, 2486–2488. [Google Scholar] [CrossRef] [PubMed]
- Tromberg, B.J.; Cerussi, A.; Shah, N.; Compton, M.; Durkin, A.; Hsiang, D.; Butler, J.; Mehta, R. Imaging in breast cancer: Diffuse optics in breast cancer: Detecting tumors in pre-menopausal women and monitoring neoadjuvant chemotherapy. Breast Cancer Res. 2005, 7, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Cerussi, A.E.; Klifa, C.; Baek, H.M.; Birgul, O.; Gulsen, G.; Merritt, S.I.; Hsiang, D.; Tromberg, B.J. In vivo water state measurements in breast cancer using broadband diffuse optical spectroscopy. Phys. Med. Biol. 2008, 53, 6713–6727. [Google Scholar] [CrossRef]
- Taroni, P.; Quarto, G.; Pifferi, A.; Abbate, F.; Balestreri, N.; Menna, S.; Cassano, E.; Cubeddu, R. Breast tissue composition and its dependence on demographic risk factors for breast cancer: Non-invasive assessment by time domain diffuse optical spectroscopy. PLoS ONE 2015, 10, e0128941. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, M.; Morrow, M.; von Minckwitz, G.; Harris, J.R.; Biedenkopf Expert Panel, M. Locoregional treatment of primary breast cancer: Consensus recommendations from an international expert panel. Cancer 2010, 116, 1184–1191. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef]
- Li, H.; Yao, L.; Jin, P.; Hu, L.; Li, X.; Guo, T.; Yang, K. Mri and pet/ct for evaluation of the pathological response to neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. Breast 2018, 40, 106–115. [Google Scholar] [CrossRef]
- Zhu, Q.; Ricci, A., Jr.; Hegde, P.; Kane, M.; Cronin, E.; Merkulov, A.; Xu, Y.; Tavakoli, B.; Tannenbaum, S. Assessment of functional differences in malignant and benign breast lesions and improvement of diagnostic accuracy by using us-guided diffuse optical tomography in conjunction with conventional us. Radiology 2016, 280, 387–397. [Google Scholar] [CrossRef]
- Zhao, Y.; Mastanduno, M.A.; Jiang, S.; Ei-Ghussein, F.; Gui, J.; Pogue, B.W.; Paulsen, K.D. Optimization of image reconstruction for magnetic resonance imaging-guided near-infrared diffuse optical spectroscopy in breast. J. Biomed. Opt. 2015, 20, 56009. [Google Scholar] [CrossRef] [PubMed]
- Leproux, A.; O’Sullivan, T.D.; Cerussi, A.; Durkin, A.; Hill, B.; Hylton, N.; Yodh, A.G.; Carp, S.A.; Boas, D.; Jiang, S.; et al. Performance assessment of diffuse optical spectroscopic imaging instruments in a 2-year multicenter breast cancer trial. J. Biomed. Opt. 2017, 22, 121604. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.G. Breast tumor hemodynamic response during a breath-hold as a biomarker to predict chemotherapeutic efficacy: Preclinical study. J. Biomed. Opt. 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Nakamiya, N.; Matsuura, K.; Shigekawa, T.; Sano, H.; Hirokawa, E.; Shimada, H.; Suzuki, H.; Oda, M.; Yamashita, Y.; et al. Optical imaging of tumor vascularity associated with proliferation and glucose metabolism in early breast cancer: Clinical application of total hemoglobin measurements in the breast. BMC Cancer 2013, 13, 514. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.S.; Chance, B.; Wilson, B.C. Time resolved reflectance and transmittance for the non-invasive measurement of tissue optical properties. Appl. Opt. 1989, 28, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Ijichi, S.; Kusaka, T.; Isobe, K.; Okubo, K.; Kawada, K.; Namba, M.; Okada, H.; Nishida, T.; Imai, T.; Itoh, S. Developmental changes of optical properties in neonates determined by near-infrared time-resolved spectroscopy. Pediatr. Res. 2005, 58, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From recist to percist: Evolving considerations for pet response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. 1), S122–S150. [Google Scholar] [CrossRef] [PubMed]
- Drisis, S.; Metens, T.; Ignatiadis, M.; Stathopoulos, K.; Chao, S.L.; Lemort, M. Quantitative dce-mri for prediction of pathological complete response following neoadjuvant treatment for locally advanced breast cancer: The impact of breast cancer subtypes on the diagnostic accuracy. Eur. Radiol. 2016, 26, 1474–1484. [Google Scholar] [CrossRef]
- Xu, C.; Vavadi, H.; Merkulov, A.; Li, H.; Erfanzadeh, M.; Mostafa, A.; Gong, Y.; Salehi, H.; Tannenbaum, S.; Zhu, Q. Ultrasound-guided diffuse optical tomography for predicting and monitoring neoadjuvant chemotherapy of breast cancers: Recent progress. Ultrason. Imaging 2016, 38, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Tromberg, B.J.; Zhang, Z.; Leproux, A.; O’Sullivan, T.D.; Cerussi, A.E.; Carpenter, P.M.; Mehta, R.S.; Roblyer, D.; Yang, W.; Paulsen, K.D.; et al. Predicting responses to neoadjuvant chemotherapy in breast cancer: Acrin 6691 trial of diffuse optical spectroscopic imaging. Cancer Res. 2016, 76, 5933–5944. [Google Scholar] [CrossRef]
- Roblyer, D.; Ueda, S.; Cerussi, A.; Tanamai, W.; Durkin, A.; Mehta, R.; Hsiang, D.; Butler, J.A.; McLaren, C.; Chen, W.P.; et al. Optical imaging of breast cancer oxyhemoglobin flare correlates with neoadjuvant chemotherapy response one day after starting treatment. Proc. Natl. Acad. Sci. USA 2011, 108, 14626–14631. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Saeki, T.; Takeuchi, H.; Shigekawa, T.; Yamane, T.; Kuji, I.; Osaki, A. In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: A comparison to bevacizumab. Br. J. Cancer 2016, 114, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [PubMed]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Jain, R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Kuji, I.; Shigekawa, T.; Takeuchi, H.; Sano, H.; Hirokawa, E.; Shimada, H.; Suzuki, H.; Oda, M.; Osaki, A.; et al. Optical imaging for monitoring tumor oxygenation response after initiation of single-agent bevacizumab followed by cytotoxic chemotherapy in breast cancer patients. PLoS ONE 2014, 9, e98715. [Google Scholar] [CrossRef]
- Miller, K.; Wang, M.; Gralow, J.; Dickler, M.; Cobleigh, M.; Perez, E.A.; Shenkier, T.; Cella, D.; Davidson, N.E. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007, 357, 2666–2676. [Google Scholar] [CrossRef]
- Robert, N.J.; Dieras, V.; Glaspy, J.; Brufsky, A.M.; Bondarenko, I.; Lipatov, O.N.; Perez, E.A.; Yardley, D.A.; Chan, S.Y.; Zhou, X.; et al. Ribbon-1: Randomized, double-blind, placebo-controlled, phase iii trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J. Clin. Oncol. 2011, 29, 1252–1260. [Google Scholar] [CrossRef]
- Ueda, S.; Saeki, T.; Osaki, A.; Yamane, T.; Kuji, I. Bevacizumab induces acute hypoxia and cancer progression in patients with refractory breast cancer: Multimodal functional imaging and multiplex cytokine analysis. Clin. Cancer Res. 2017. [Google Scholar] [CrossRef]
- Cataliotti, L.; Buzdar, A.U.; Noguchi, S.; Bines, J.; Takatsuka, Y.; Petrakova, K.; Dube, P.; de Oliveira, C.T. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: The pre-operative “arimidex” compared to tamoxifen (proact) trial. Cancer 2006, 106, 2095–2103. [Google Scholar] [CrossRef]
- Smith, I.E.; Dowsett, M.; Ebbs, S.R.; Dixon, J.M.; Skene, A.; Blohmer, J.U.; Ashley, S.E.; Francis, S.; Boeddinghaus, I.; Walsh, G.; et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: The immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (impact) multicenter double-blind randomized trial. J. Clin. Oncol. 2005, 23, 5108–5116. [Google Scholar] [CrossRef] [PubMed]
- Chia, Y.H.; Ellis, M.J.; Ma, C.X. Neoadjuvant endocrine therapy in primary breast cancer: Indications and use as a research tool. Br. J. Cancer 2010, 103, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Tsuda, H.; Saeki, T.; Omata, J.; Osaki, A.; Shigekawa, T.; Ishida, J.; Tamura, K.; Abe, Y.; Moriya, T.; et al. Early metabolic response to neoadjuvant letrozole, measured by fdg pet/ct, is correlated with a decrease in the ki67 labeling index in patients with hormone receptor-positive primary breast cancer: A pilot study. Breast Cancer 2011, 18, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Nakamiya, N.; Ueda, S.; Shigekawa, T.; Takeuchi, H.; Sano, H.; Hirokawa, E.; Shimada, H.; Suzuki, H.; Oda, M.; Osaki, A.; et al. Clinicopathological and prognostic impact of imaging of breast cancer angiogenesis and hypoxia using diffuse optical spectroscopy. Cancer Sci. 2014, 105, 833–839. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, S.; Saeki, T. Early Therapeutic Prediction Based on Tumor Hemodynamic Response Imaging: Clinical Studies in Breast Cancer with Time-Resolved Diffuse Optical Spectroscopy. Appl. Sci. 2019, 9, 3. https://doi.org/10.3390/app9010003
Ueda S, Saeki T. Early Therapeutic Prediction Based on Tumor Hemodynamic Response Imaging: Clinical Studies in Breast Cancer with Time-Resolved Diffuse Optical Spectroscopy. Applied Sciences. 2019; 9(1):3. https://doi.org/10.3390/app9010003
Chicago/Turabian StyleUeda, Shigeto, and Toshiaki Saeki. 2019. "Early Therapeutic Prediction Based on Tumor Hemodynamic Response Imaging: Clinical Studies in Breast Cancer with Time-Resolved Diffuse Optical Spectroscopy" Applied Sciences 9, no. 1: 3. https://doi.org/10.3390/app9010003
APA StyleUeda, S., & Saeki, T. (2019). Early Therapeutic Prediction Based on Tumor Hemodynamic Response Imaging: Clinical Studies in Breast Cancer with Time-Resolved Diffuse Optical Spectroscopy. Applied Sciences, 9(1), 3. https://doi.org/10.3390/app9010003




