Preparation and in Vitro Bioactivity of Micron-sized Bioactive Glass Particles Using Spray Drying Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Structural Characterization
2.3. Bioactivity Test
3. Results
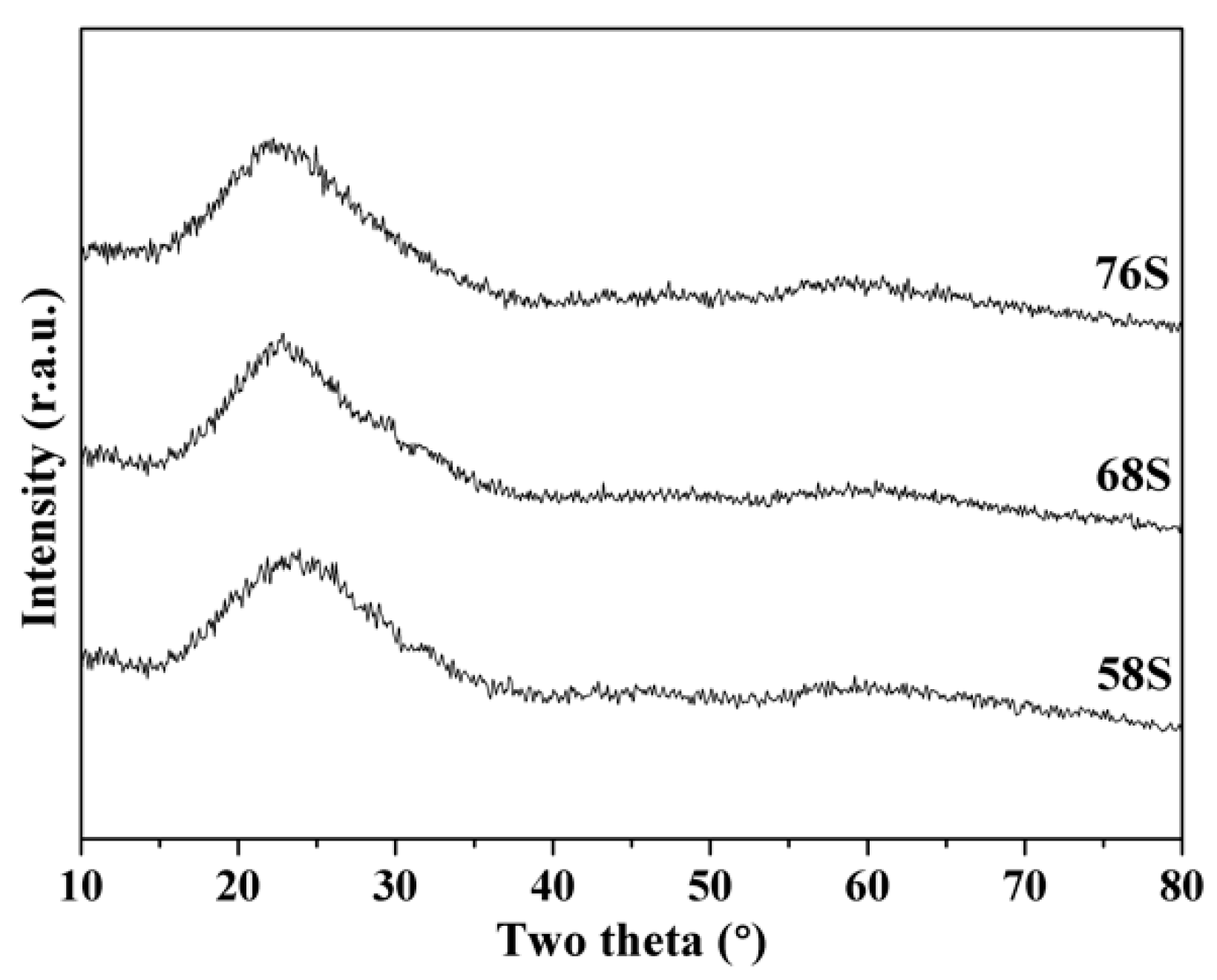
3.1. Phase Composition
3.2. Morphology and Composition
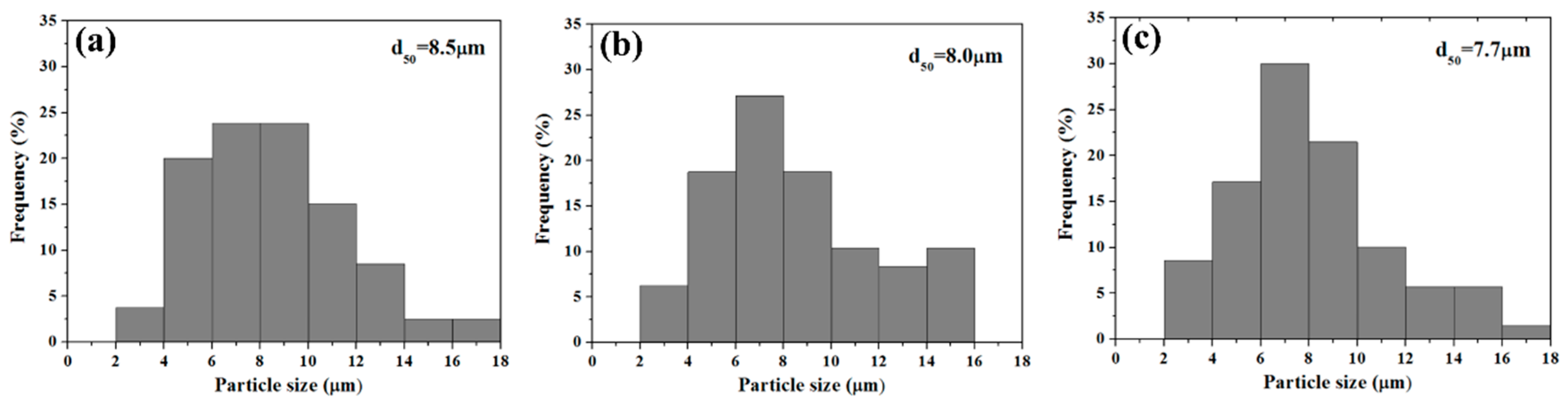
3.3. Particle Size, Pore Size and Specific Surface area
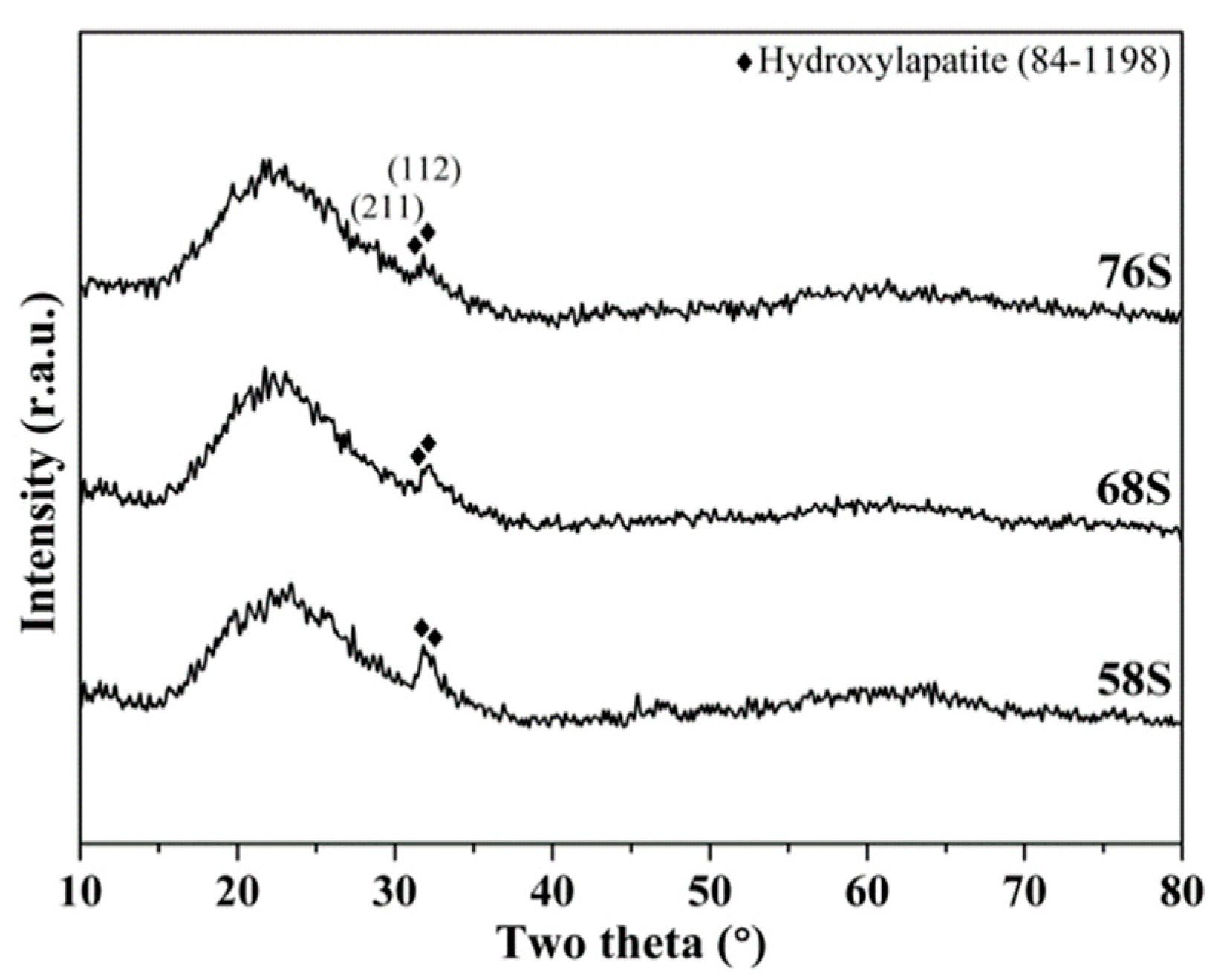
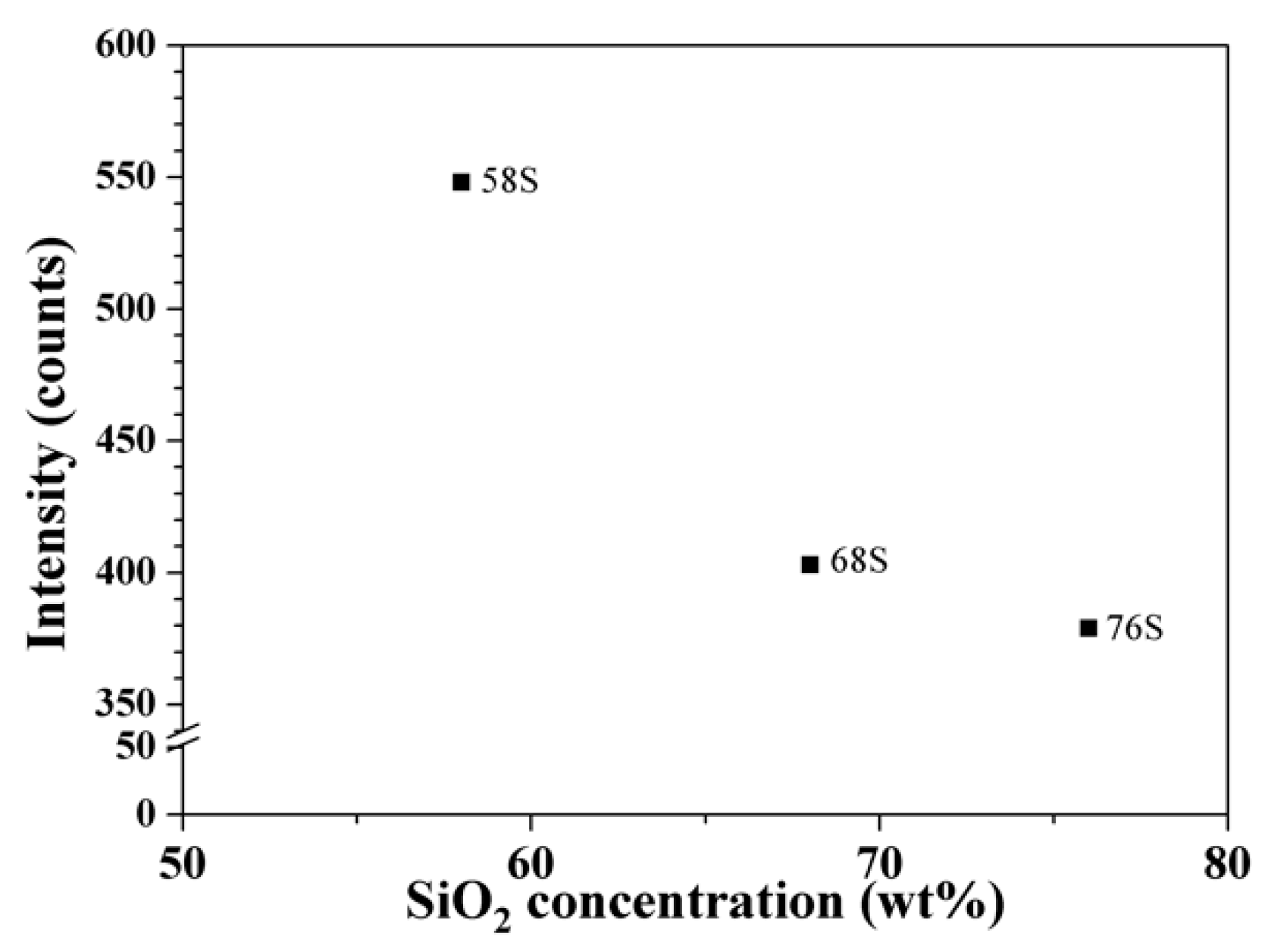
3.4. In Vitro Bioactivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hench, L.L. Bioactive ceramics: Theory and clinical applications. Bioceramics 1994, 7, 3–14. [Google Scholar]
- Cao, W.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- De Groot, K. Bioceramics of calcium phosphate. J. Clin. Eng. 1984, 9, 52. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Ceramics for medical applications. J. Chem. Soc. Dalton 2001, 0, 97–108. [Google Scholar] [CrossRef]
- Kirsten, A.; Hausmann, A.; Weber, M.; Fischer, J.; Fischer, H. Bioactive and thermally compatible glass coating on zirconia dental implants. J. Dent. Res. 2015, 94, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.J.; Shruti, S.; Malavasi, G.; Menabue, L.; Vallet-Regí, M. Substitutions of cerium, gallium and zinc in ordered mesoporous bioactive glasses. Acta Biomater. 2011, 7, 3452–3458. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J. Well-ordered mesoporous bioactive glasses (MBG): A promising bioactive drug delivery system. J. Control. Release 2006, 110, 522–530. [Google Scholar] [CrossRef]
- Christie, J.K.; Malik, J.; Tilocca, A. Bioactive glasses as potential radioisotope vectors for in situ cancer therapy: Investigating the structural effects of yttrium. Phys. Chem. Chem. Phys. 2011, 13, 17749–17755. [Google Scholar] [CrossRef]
- Tilocca, A. Realistic models of bioactive glass radioisotope vectors in practical conditions: Structural effects of ion exchange. J. Phys. Chem. C 2015, 119, 27442–27448. [Google Scholar] [CrossRef]
- Hench, L.L. The story of bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.E.; Pantano, C.G.; Hench, L.L. Auger spectroscopic analysis of bioglass corrosion films. J. Am. Ceram. Soc. 1976, 59, 37–39. [Google Scholar] [CrossRef]
- Li, R.; Clark, A.; Hench, L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.J.; Chen, H.T.; Huang, L.F.; Lu, P.S.; Chang, H.F.; Chang, I.L. Synthesis and in vitro bioactivity of mesoporous bioactive glass scaffolds. Mater. Sci. Eng. C 2010, 30, 657–663. [Google Scholar] [CrossRef]
- Shih, S.-J.; Chou, Y.-J.; Chien, I.-C. One-step synthesis of bioactive glass by spray pyrolysis. J. Nanopart. Res. 2012, 14, 1–8. [Google Scholar] [CrossRef]
- Messing, G.L.; Zhang, S.-C.; Jayanthi, G.V. Ceramic powder synthesis by spray pyrolysis. J. Am. Ceram. Soc. 1993, 76, 2707–2726. [Google Scholar] [CrossRef]
- Chou, Y.-J.; Hong, B.-J.; Lin, Y.-C.; Wang, C.-Y.; Shih, S.-J. The correlation of pore size and bioactivity of spray-pyrolyzed mesoporous bioactive glasses. Materials 2017, 10, 488. [Google Scholar] [CrossRef]
- Morhenn, V.B.; Lemperle, G.; Gallo, R.L. Phagocytosis of different particulate dermal filler substances by human macrophages and skin cells. Dermatol. Surg. 2002, 28, 484–490. [Google Scholar]
- Tamjid, E.; Bagheri, R.; Vossoughi, M.; Simchi, A. Effect of particle size on the in vitro bioactivity, hydrophilicity and mechanical properties of bioactive glass-reinforced polycaprolactone composites. Mater. Sci. Eng. C 2011, 31, 1526–1533. [Google Scholar] [CrossRef]
- Ghidelli, M.; Idrissi, H.; Gravier, S.; Blandin, J.-J.; Raskin, J.-P.; Schryvers, D.; Pardoen, T. Homogeneous flow and size dependent mechanical behavior in highly ductile Zr65Ni35 metallic glass films. Acta Mater. 2017, 131, 246–259. [Google Scholar] [CrossRef]
- Vehring, R.; Foss, W.R.; Lechuga-Ballesteros, D. Particle formation in spray drying. J. Aerosol Sci. 2007, 38, 728–746. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Okuyama, K. Progress in developing spray-drying methods for the production of controlled morphology particles: From the nanometer to submicrometer size ranges. Adv. Powder Technol. 2011, 22, 1–19. [Google Scholar] [CrossRef]
- Ostomel, T.A.; Shi, Q.; Tsung, C.-K.; Liang, H.; Stucky, G.D. Spherical bioactive glass with enhanced rates of hydroxyapatite deposition and hemostatic activity. Small 2006, 2, 1261–1265. [Google Scholar] [CrossRef]
- Molino, G.; Bari, A.; Baino, F.; Fiorilli, S.; Vitale-Brovarone, C. Electrophoretic deposition of spray-dried Sr-containing mesoporous bioactive glass spheres on glass–ceramic scaffolds for bone tissue regeneration. J. Mater. Sci. 2017, 52, 9103–9114. [Google Scholar] [CrossRef]
- Kokubo, T.; Ito, S.; Huang, Z.T.; Hayashi, T.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Ca, P-rich layer formed on high-strength bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 331–343. [Google Scholar] [CrossRef]
- Lintingre, E.; Lequeux, F.; Talini, L.; Tsapis, N. Control of particle morphology in the spray drying of colloidal suspensions. Soft Matter 2016, 12, 7435–7444. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Shih, S.-J.; Tzeng, W.-L. Manipulation of morphology of strontium titanate particles by spray pyrolysis. Powder Technol. 2014, 264, 291–297. [Google Scholar] [CrossRef]
- Iskandar, F.; Gradon, L.; Okuyama, K. Control of the morphology of nanostructured particles prepared by the spray drying of a nanoparticle sol. J. Colloid Interface Sci. 2003, 265, 296–303. [Google Scholar] [CrossRef]
- Shih, S.-J.; Chou, Y.-J.; Panjaitan, L.V.P. Synthesis and characterization of spray pyrolyzed mesoporous bioactive glass. Ceram. Int. 2013, 39, 8773–8779. [Google Scholar] [CrossRef]
- Hench, L.L.; Wilson, J. An Introduction to Bioceramics; Word Scientific: Singapore, 1999. [Google Scholar]



| Specimen | Si | Ca | P |
|---|---|---|---|
| 58S | 52.47 ± 2.26 | 37.71 ± 1.62 | 9.81 ± 0.65 |
| 68S | 63.64 ± 3.09 | 26.68 ± 3.90 | 9.68 ± 1.15 |
| 76S | 74.18 ± 0.90 | 16.33 ± 1.51 | 9.49 ± 0.83 |
| Unit: mol% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, Y.-J.; Hsiao, C.-W.; Tsou, N.-T.; Wu, M.-H.; Shih, S.-J. Preparation and in Vitro Bioactivity of Micron-sized Bioactive Glass Particles Using Spray Drying Method. Appl. Sci. 2019, 9, 19. https://doi.org/10.3390/app9010019
Chou Y-J, Hsiao C-W, Tsou N-T, Wu M-H, Shih S-J. Preparation and in Vitro Bioactivity of Micron-sized Bioactive Glass Particles Using Spray Drying Method. Applied Sciences. 2019; 9(1):19. https://doi.org/10.3390/app9010019
Chicago/Turabian StyleChou, Yu-Jen, Chih-Wei Hsiao, Nien-Ti Tsou, Meng-Huang Wu, and Shao-Ju Shih. 2019. "Preparation and in Vitro Bioactivity of Micron-sized Bioactive Glass Particles Using Spray Drying Method" Applied Sciences 9, no. 1: 19. https://doi.org/10.3390/app9010019
APA StyleChou, Y.-J., Hsiao, C.-W., Tsou, N.-T., Wu, M.-H., & Shih, S.-J. (2019). Preparation and in Vitro Bioactivity of Micron-sized Bioactive Glass Particles Using Spray Drying Method. Applied Sciences, 9(1), 19. https://doi.org/10.3390/app9010019





