Abstract
The livestock productivity in Ethiopia is seriously constrained by the shortage of fodder due to increasing soil salinization. Therefore, restoration of salt-affected lands into productive soils through salt-tolerant forages and improved irrigation and crop management practices is crucial for enhancing the productivity of the livestock sector in Ethiopia. In this three-year study, pot trials were conducted to evaluate the impact of five different soil salinity levels (i.e., 0, 5, 10, 15, and 20 dS m−1) on plant growth, biomass production, and nutrient quality attributes of three Rhodes grass (Chloris gayana) genotypes (ILRI-6633, ILRI-7384, CV-massaba). Increasing soil salinity negatively affected germination percentage (GP) and mean germination time (MGT) of all genotypes. For all salinity levels, the highest GP was observed for ILRI-6633 and the lowest for CV-massaba. Plant height and chlorophyll content for ILRI-6633 was higher than the other two genotypes. The crude protein (CP) content was higher in low dry matter-producing genotype (ILRI-7384). The performance of ILRI-6633 at all salinity levels was superior to the other two genotypes. CV-massaba genotype performed better under low to medium soil salinity conditions. Therefore, ILRI-6633 and CV-massaba genotypes have excellent potential to increase forage production in salt-affected areas of Ethiopia.
Keywords:
soil salinity; amelioration; forage grass; Ethiopia; livestock productivity; soil salinity 1. Introduction
Saline soils are now becoming a growing threat to sustainable crop production in the irrigated lands of the semi-arid areas of the world [1,2]. Currently, over 1000 million ha (Mha) are inflicted with the different levels of salinity problems [3], and an additional 0.25–0.5 Mha of agricultural land is lost annually [4]. The salinity development is mainly caused by the presence of soluble salts in the water used for irrigation, hot and dry weather conditions, and excessive use of poor quality groundwater for irrigation [5,6]. Salinity problems are wide-spread in the Asian and African regions. In Ethiopia, approximately 11 Mha of land (i.e., 9% of the country’s total landmass and 13% of the irrigated area) is exposed to salinity, of which 8 Mha have combined salinity and sodicity problems whereas the remaining 3 Mha are only sodic in nature [7,8].
In Ethiopia, saline soils are more dominant in the Awash Valley and semi-arid lowlands where 9% of the total population lives [8]. The growing prevalence of salt-affected soils is undermining the sustainability of irrigated agriculture in Ethiopia, as it reduces natural biodiversity as well as farm and livestock productivity. The situation is expected to aggravate in the future due to factors induced by climate change. The ongoing land degradation has grave implications for future food security and economic development of Ethiopia. With an annual growth rate of 2.48%, securing food and improving the livelihood of the increasing population will be the biggest challenge for the government. Even today, food shortages are widespread, and since 1970, the country is in the grip of consistent famines. It is reported that, among children aged up to five years, around 25% are underweight and 40% are stunted as a result of chronic malnutrition [9].
To meet future food security challenges, transforming salt-affected soils into productive lands and preventing the spread of salinity in other areas is of critical importance. In the low to moderate saline areas, degraded soils can be improved by excessive leaching and installing effective drainage systems [10,11]. In the high saline areas, where the growth of normal field crops is problematic, soil reclamation can be done by using chemicals and/or by growing suitable salt-tolerant plant species (bioremediation approach) [12,13,14]. This approach is based on integrated crop-livestock feeding systems, which can enhance the resilience of smallholder farmers in the salt-affected areas. For the success of bioremediation approaches, the selection of suitable genotypes that have the potential to tolerate abiotic stresses is of paramount importance.
The introduction of salt-tolerant forage grasses can increase fodder production in the degraded lands because they have the capacity to prevent salinity development by creating a mulching layer on the soil surface. This mulching layer prevents the upward capillary movement of water thereby forcing plants to use water from the lower layers of the soil through their root system [15,16]. One such forage grass is Chloris gayana, which can successfully produce good biomass yield in marginal soil and water conditions [14,16]. Since salt-tolerant forage plants are variable in biomass production and nutritive value, they need to be tested locally for their (a) edible biomass production, (b) nutritive value of edible biomass (i.e., the response in animal production per unit of voluntary feeding intake), and (c) the use of micronutrients and nutraceutical properties. This study was designed to evaluate the salt tolerance, biomass production, and nutrient composition of selected Rhodes grass (Chloris gayana) genotypes under Ethiopian conditions. It is anticipated that the developed crop-livestock value chain system will enhance the livestock productivity in Ethiopia.
2. Materials and Methods
2.1. Study Area
The field trials were conducted for three years (2016–2018) at the Werer Agricultural Research Center (WARC), Amibara district of the Afar region of Ethiopia. WARC is located at an altitude of 740 m above mean sea level between 9°12′8″ N latitude and 40°15′21″ E longitude (Figure 1). The study area is mainly characterized as semi-arid with mean annual temperatures ranging between 19 °C and 34 °C. The mean annual Class A pan evaporation is 2800 mm, which is five times higher than the mean annual rainfall (570 mm) [17]. The study area consists of an extensive alluvial plain, which was historically formed by the deposits of the Awash River. The area has flat topography with a slope gradient ranging between 1–2%. The predominant soil types are weak-structured Vertisols and Fluvisols. The Vertisols are silty clay to clay whereas Fluvisols are sandy loam to silty loam in texture [18]. The constituents of Fluvisols are muscovite/illite clay minerals while Vertisols are dominated by montmorillonite clay minerals.
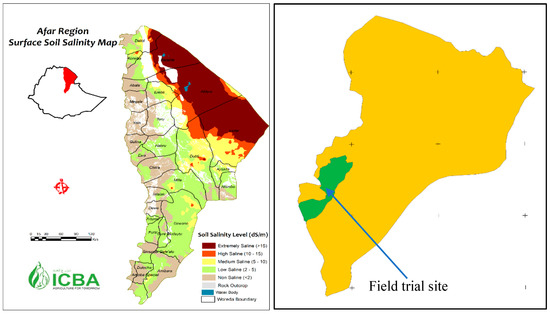
Figure 1.
Geographical location of the field trial site.
2.2. Experimental Design and Salinity Treatments
The pot trials were conducted under a controlled environment. NaCl weighing 7.3, 14.6, 21.4 and 29.2 g was mixed with 6 kg soil packed in each pot to accomplish soil salinity levels of 5, 10, 15 and 20 dS m−1, respectively. The control pot was established without any addition of NaCl. Two Chloris gayana genotypes (ILRI-6633; ILRI-7384) obtained from the International Livestock Research Institute (ILRI) and one local genotype from WARC (CV-massaba) were used for this study. Farmers in Ethiopia widely use these genotypes without much knowledge about their quality and quantity parameters. Treatments were factorially combined and arranged in a completely randomized design with three replications. Irrigations were applied according to crop evapotranspiration (ETc = ETo × Kc) values plus 10% for leaching requirements using good quality canal water (EC = 0.2 dS m−1). The reference evapotranspiration (ETo) was calculated with the modified Penman-Monteith equation (FAO-56) using climatic data collected from the field trial site. The crop coefficient (Kc) values were taken from the FAO-56 publication.
2.3. Data Collection and Analysis
Five seeds of each genotype were planted in each pot. Seeds that produced full radicle were considered as germinated. The data regarding mean germination time, germination percentage, number of tillers per plant, and plant height were collected from all pots. Chloris gayana grasses were sustained for up to 75 days after planting. At the time of harvesting, the shoot and root dry matter weight was recorded. The first germination count was done after five days of plantation. Because a saline environment often causes a delay in seed germination, the counting of the seedlings was done on the 10th and 15th day after plantation. The Germination Percentage (GP) was calculated according to Ashraf et al. (2005) [19].
Mean Germination Time (MGT) was calculated using the following equation [20].
where n = number of germinated seeds on day D, and D = number of days from the start of germination.
The plant samples were dried and ground for analyzing crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber (ADF), and ash content (AC) using standard procedures [21,22]. Chlorophyll (SPAD units) content was also measured. The data were analyzed using analysis of variance (ANOVA) technique [23] for factorial CRD using SAS 9.3 software (SAS Institute, Cary, NC, USA). The least significant test was used to decipher the main and interaction effects of treatments at 5% level of significance (P < 0.05).
3. Results and Discussion
3.1. Effect of Soil Salinity on Germination Percentage (GP) and Mean Germination Time (MGT)
Increased salinity negatively affected the germination percentage (GP) and mean germination time (MGT) of three Chloris gayana genotypes. The GP showed a decreasing trend with the increasing salinity and ranged between 68.3–71.7% in control (0 dS m−1), 63.2–67.0% at 5 dS m−1, 53.3–56.7% at 10 dS m−1, 50.0–51.7% at 15 dS m−1, and 45.0–48.3% at 20 dS m−1 (Figure 2a). The highest GP at each salinity level was obtained for ILRI-6633, followed by ILRI-7384 except for higher salinity level of 20 dS m−1 where CV-massaba performed better than ILRI-7384.
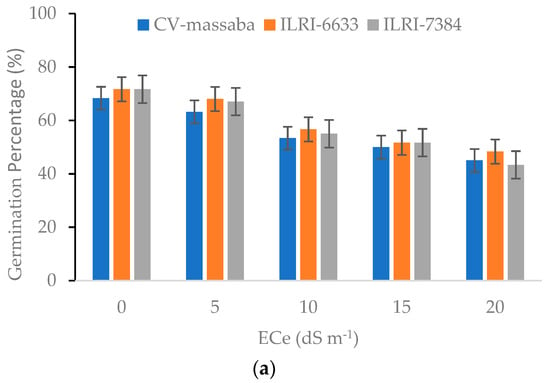
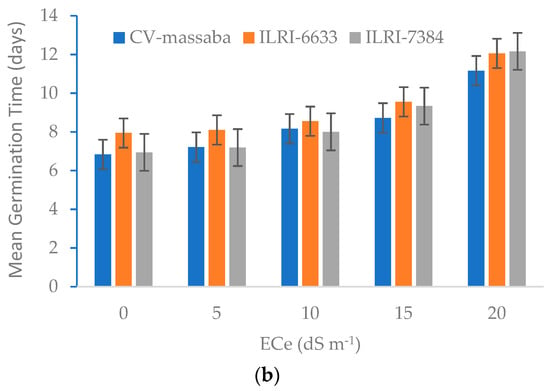
Figure 2.
Effect of different salinity levels on (a) Germination Percentage (LSD = 6.69; CV = 12.32) and (b) Mean Germination Time (LSD = 0.86; CV = 9.68) of three selected Chloris gayana genotypes.
The germination of all three genotypes was delayed with the increasing salinity, i.e., from eight days in control (0 dS m−1) to more than 12 days for higher salinity (20 dS m−1) (Figure 2b). The local genotype CV-massaba germinated faster than the other two genotypes under all salinity levels. This shows that CV-massaba is more tolerant to salinity as far as germination is concerned. On average, the delay in MGT was 4, 13.3, 21.7, and 39.1% at 5, 10, 15, and 20 dS m−1, respectively. The average MGT for all genotypes was 7.2 days in control, with ILRI-6633 being the maximum (7.9 days) and CV-massaba the minimum (6.8 days). At the highest salinity (20 dS m−1), the average MGT for ILRI-7384 was 12.2 days, followed by 12.1 days for ILRI-6633 and 11.2 days for CV-massaba. This shows that although CV-massaba germinated faster, its germination percentage was lower than the other two genotypes. In conclusion, the GP and MGT of all genotypes was negatively influenced by increasing salt stress in the soil. These results agree with the findings of Horst et al. (1989) [24] for perennial ryegrass and Iqbal et al. (2006) [25] for the teff grass.
Crop genotypes that can effectively germinate under salt stress conditions do not necessarily qualify for a good seedling growth [26]. However, in our case, ILRI-6633 and CV-massaba genotypes showed promising results not only in terms of germination percentage (GP) and mean germination time (MGT) but also seedling growth. Studies have shown that the crops with higher germination percentage have the capacity to establish themselves effectively on moderate to high saline soils (ECe = 5–12 dS m−1) [17]. Although it is difficult to generalize pot results for field conditions, ILRI-6633 and CV-massaba genotypes can potentially germinate and establish in moderate to high saline areas. This will help in producing high biomass because yield losses due to poor germination and inadequate crop stand establishment will be minimum [27,28,29].
3.2. Plant Height, Number of Tillers and Chlorophyll SPAD Value
The plant growth is affected by salinity because of a number of factors, including osmotic stress, ionic toxicity, lack of minerals, and physiological and biochemical responses [30]. This study reveals that at the seedling stage, there was a significant reduction in plant height, number of tillers per plant, and plant fresh and dry matter weight of three Chloris gayana genotypes with the increasing salinity levels. However, high chlorophyll content (SPAD value) was detected in salt-tolerant genotypes. These higher chlorophyll values can be ascribed to an improved photosynthetic rate and higher dry matter production [31,32]. The reduction in chlorophyll content under marginal environments has been observed in many previous studies [19,25,31].
The plant height and the total number of tillers of three Chloris gayana genotypes under different soil salinity conditions is given in Table 1. A linear decrease in plant height with increasing soil salinity was observed for all genotypes. The plant height of Chloris gayana genotypes ranged between 93.0–120.8 cm for control (0 dS m−1), 92.5–117.8 cm at 5 dS m−1, 81.4–103.6 cm at 10 dS m−1, 60.3–78.3 cm at 15 dS m−1, and 52.0–69.0 at 20 dS m−1. The average plant height for all Chloris gayana genotypes at 0 dS m−1 was 105 cm with ILRI-6633 being the highest (120.8 cm). Likewise, the average plant height for all genotypes at the highest salinity level (20 dS m−1) was 61.6 cm with ILRI-6633 taking the lead (68.9 cm). The highest decrease in plant height with an increase in salinity level from 0 to 20 dS m−1 was noted in ILRI-6633 (51.8 cm) followed by ILRI-7384 (41.0 cm) and CV-massaba (37.5 cm).

Table 1.
Effect of different salinity levels on plant height and the number of tillers of three selected Chloris gayana genotypes.
The number of tillers per plant is one of the most important agronomic traits because it determines the biomass yield of the forage grasses. The results indicate that higher salinity conditions significantly affect the tiller production at maturity. The average number of tillers per plant was found to be 7.2, 7.1, 6.6, 5.9, and 4.8 at 0, 5, 10, 15, and 20 dS m−1, respectively (Table 1). At 0 dS m−1, ILRI-7384 gave maximum number of tillers per plant (9.4) followed by CV-massaba (6.9), and ILRI-6633 (5.4). A similar pattern was observed at higher salinity conditions (20 dS m−1) where the maximum and the minimum number of tillers per plant were recorded in ILRI-7384 (5.8) and ILRI-6633 (3.1), respectively. These results coincide with the earlier findings which show that morphological and physiological traits of plants such as the plant height, number of tillers per plant, and fresh and dry biomass yields tend to decrease with the increasing levels of salinity [31,33,34].
Figure 3 shows the chlorophyll content (SPAD value) of three Chloris gayana genotypes. The SPAD values were found to be consistent up to a salinity level of 10 dSbm−1. However, a significant reduction in SPAD values at salinity levels of 15 dSbm−1 and 20 dSbm−1 was observed. For all salinity levels, highest SPAD values were found in ILRI-6633 followed by ILRI-7384 and CV-massaba. The highest SPAD value (63.6) was recorded in ILRI-6633 at moderate salinity level (10 dSbm−1). However, for 20 dSbm−1, SPAD values decreased significantly for all three genotypes except for ILRI-6633. Similar trends of chlorophyll content reductions with the increasing salinity have also been observed for different crops [31,34].
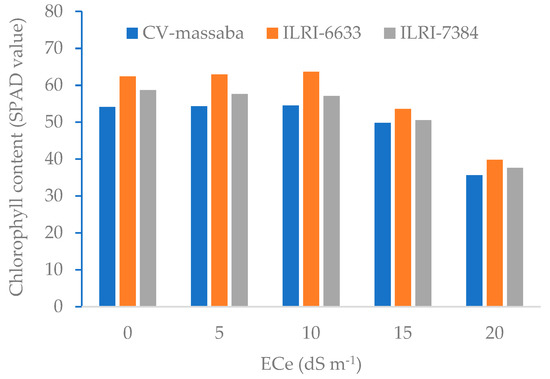
Figure 3.
Effect of different salinity levels on chlorophyll content (SPAD value) of three selected Chloris gayana genotypes.
3.3. Root and Shoot Dry Matter
Figure 4 shows the impact of different salinity levels on shoot and root dry matter of three Chloris gayana genotypes. The highest shoot dry matter (71.2 g/pot) was obtained in ILRI-6633 genotype whereas the lowest (60.3 g/pot) was recorded in ILRI-7384. The reduction in shoot dry matter was more pronounced at salinity levels of 15 dSbm−1 and 20 dSbm−1. The overall reduction in shoot dry matter from 0 to 20 dSbm−1 was found to be 18.0 g/pot for ILRI-6633, 21.5 g/pot for ILRI-7384, and 27.5 g/pot for CV-massaba. This demonstrates that ILRI-6633 genotype has the lowest reduction in shoot dry matter with the increasing salinity.
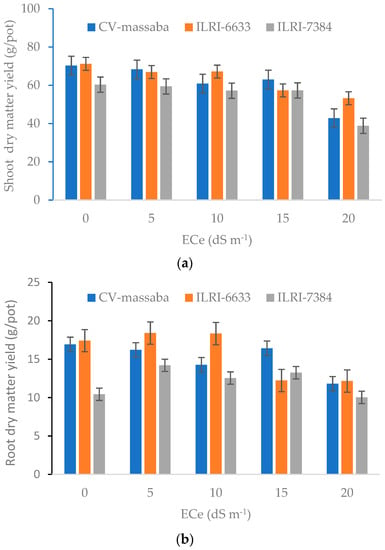
Figure 4.
Effect of different salinity levels on (a) shoot dry matter (LSD = NS; CV = 23.75) and (b) root dry matter (LSD = 3.53; CV = 26.15) of three selected Chloris gayana genotypes.
The root dry matter ranged between 10.4–17.4 g/pot at 0 dS m−1, 14.2–18.4 g/pot at 5 dS m−1, 12.5–18.3 g/pot at 10 dS m−1, 12.2–16.4 g/pot at 15 dS m−1, and 10.0–12.2 g/pot at 20 dS m−1 (Figure 4b). For all salinity levels, the highest root dry matter was achieved for ILRI-6633 followed by CV-massaba. The maximum root dry matter for ILRI-6633 and CV-massaba was obtained at low to medium salinity levels (5–10 dS m−1). This suggests that the performance of ILRI-6633 with regard to root dry matter was superior to the other two genotypes. The average reduction in root dry matter ranged from 11.3–16.3 g/pot with an increase in salinity from 5–20 dS m−1. The declining trends in the root dry matter with increasing salinity levels can be ascribed by the fact that in saline soils, plants spend more energy to get water and nutrients from the soil. This situation negatively affects the yield and the quality of the plant [3,35,36,37,38].
3.4. Nutrient Composition
Forage grasses are considered to be the most remunerative form of animal feed in semi-arid regions. The nourishing value of forages mainly depends on their nutritional composition such as crude protein (CP) and fiber and ash contents [2]. Crude protein is an important element of the animal diet that enhances their milk-producing capacity and maintains meat quality [16]. Both CP and ash contents of three Chloris gayana genotypes exhibited a decreasing trend with increasing soil salinity. The highest CP (6.1%) and ash (15.5%) contents were obtained in ILRI-6633 and ILRI-7384 genotypes at low salinity level (5 dS m−1), whereas CV-massaba reported the lowest crude protein (3.8%) and ash content (12.4) at the same salinity level (Figure 5). However, CV-massaba genotype produced a higher CP content at salinity levels of 10–15 dS m−1. Generally, low dry matter-producing genotypes are known to have higher CP content whereas high dry matter producing accessions are low in nutrition at low salinity levels [30,39]. All three genotypes produced high CP and ash content at low to medium salinity (0–10 dS m−1) levels while a falling trend was observed at higher salinity levels (15–20 dS m−1). This shows that these varieties are more suitable for low to moderate salinity conditions.
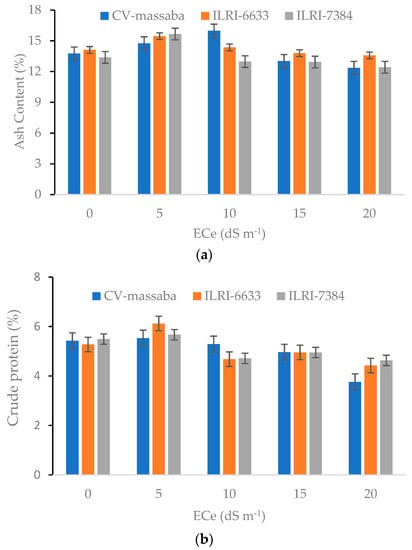
Figure 5.
Effect of different salinity levels on (a) Ash content (LSD = 1.71; CV = 9.75) and (b) Crude protein (LSD = 1.05; CV = 13.65) on three Chloris gayana genotypes.
Neutral detergent fiber (NDF) mainly consists of hemicellulose, cellulose, and lignin. From the feeding point of view, lower NDF values are desirable for fodder as well as grains [40,41]. The NDF values for all three genotypes showed an increasing trend with the growing salt stress. Differences in NDF among the Chloris gayana genotypes show a similar trend as of CP. ILRI-6633 reported the highest NDF (70.9–73.9%) value whereas the lowest NDF values (67.5–72.9%) were observed in CV-massaba for all salinity levels (Figure 6). The average Invitro dry matter digestibility content (IvDMDC) was 42.9% for control, 41% at 5 dS m−1, 41.6% at 10 dS m−1, 40.2% at 15 dS m−1, and 36.1% at 20 dS m−1 (Figure 6). The highest IvDMDC was obtained in ILRI-6633 at control while the lowest was observed in CV-massaba at 20 dS m−1.
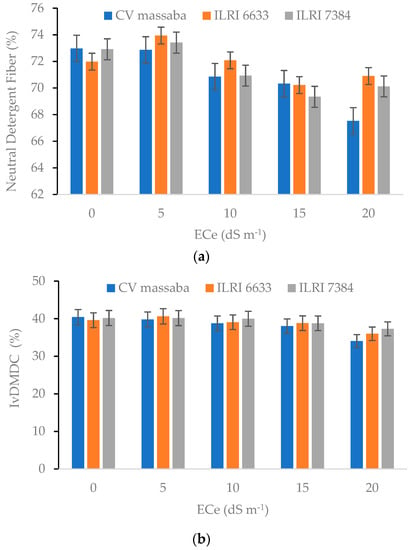
Figure 6.
Effect of different salinity levels on (a) Neutral Detergent Fiber (NDF) (LSD = 2.72; CV = 21.85) and (b) Invitro dry matter digestibility content (IvDMDC) (LSD = 3.19; CV = 20.83) on three Chloris gayana genotypes.
4. Conclusions and Recommendations
The effects of five soil salinity levels on the agronomic and nutritional quality parameters of three Chloris gayana genotypes (ILRI-6633, ILRI-7384, and CV-massaba) for dry and hot conditions of Ethiopia were evaluated. The agronomic parameters such as the germination rate, germination time, plant height, and shoot and root dry matter weights showed a decreasing trend with the increasing soil salinity levels for all Chloris gayana genotypes. The highest germination rate, lowest germination time, maximum plant height, and the number of tillers per plant were observed in ILRI-6633 compared to ILRI-7384 and CV-massaba genotypes. For all three genotypes, the root length was more severely affected than the shoot length at all salinity levels. The increasing soil salinity negatively affected the shoot and root dry matter of three Chloris gayana genotypes. The reduction in shoot dry matter was more noticeable at the higher salinity levels (i.e., 15–20 dS m−1).
The chlorophyll content values were consistent up to 10 dS m−1, whereas significant reduction was observed at higher salinity levels (15–20 dS m−1). The highest crude protein (CP) and ash content (AC) values were obtained for ILRI-6633 and ILRI-7384 genotypes at 5 dS m−1, whereas CV-massaba reported lowest CP value (3.8%) and ash content (12.4) at the same salinity level.
From the feeding point of view, lower values of neutral detergent fiber (NDF) are desirable. NDF values showed an increasing trend with the growing salt stress. The genotypes with lower CP values tend to have higher NDF values. On average, the maximum NDF values were found in ILRI-6633 and the lowest in CV-massaba genotype for all salinity levels. The average in vitro dry matter digestibility content (IvDMDC) also showed a decreasing trend with the increasing salinity levels. This means Chloris gayana forage grass has higher digestibility.
In conclusion, ILRI-6633 is identified as the most salt-tolerant, high yielding and better nutritive forage grass than the other two genotypes. CV-massaba is superior to the other two genotypes in terms of germination under saline conditions. Therefore, these two genotypes can potentially be grown to increase farm productivity in salt-affected areas of Ethiopia.
Author Contributions
Methodology, A.W.D. and A.S.Q.; field experiment and data collection, A.W.D. and B.N.N.; writing—original draft preparation, A.W.D. and A.S.Q.; writing—review and editing, A.S.Q.
Funding
This work was supported by the International Fund for Agricultural Development (IFAD). Rome, Italy. (Grant No. 2000001100).
Acknowledgments
This study was conducted as part of the project “Rehabilitation and management of salt-affected soils to improve agricultural productivity (RAMSAP)” in Ethiopia and South Sudan. The authors gratefully acknowledge the financial grant received for the implementation of this project. We are also thankful to the field team of the Werer Agricultural Research Center in Ethiopia for conducting field trials and collecting field data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhowmik, P.C.; Hossain, M.A.; Rahman, M.M.; Prasad, M.N.V.; Ozturk, M.; Fujita, M. Potential use of halophytes to remediate saline soils. BioMed Res. Int. 2014. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Wicke, B.; Smeets, E.; Dornburg, V.; Vashev, B.; Gaiser, T.; Turkenburg, W.; Faaij, A. The global technical and economic potential of bioenergy from salt-affected soils. Energy Environ. Sci. 2011, 8, 2669–2681. [Google Scholar] [CrossRef]
- Qadir, M.; Quillerou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J. Economics of salt-induced land degradation and restoration. Nat. Res. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Munns, R. Plant adaptations to salt and water stress: Differences and commonalities. Adv. Bot. Res. 2011, 57, 1–32. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Kidane, G.; Abebe, F.; Heluf, G.; Fentaw, A.; Wondimadegne, C.; Hibstu, A.; Asegid, A.; Messele, F.; Mohammed, B. Report of the National Task Force on Assessment of Salt Affected Soils and Recommendations on Management Options for Sustainable Utilization; Ethiopia, A.A., Ed.; Minister of Agriculture and Rural Development: Addis Ababa, Ethiopia, 2006; 120p.
- Frew, A.; Tena, A.; Fentaw, A. Appraisal and mapping of soil salinity problems in Amibara area of Middle Awash Basin Ethiopia. Int. J. Innov. Sci. Res. 2015, 13, 298–314. [Google Scholar]
- UNICEF (United Nations Children’s Fund). The State of the World’s Children 2016: A Fair Chance for Every Child; UNICEF: New York, NY, USA, 2016; Available online: www.unicef.org/publications/files/UNICEF_SOWC_2016.pdf (accessed on 22 July 2016).
- Jacobsen, S.E.; Mujica, A.; Jensen, C.R. The resistance of quinoa (Chenopodium quinoa Wild.) to adverse abiotic factors. Food Rev. Int. 2003, 19, 99–109. [Google Scholar] [CrossRef]
- Rosa, M.; Hilal, M.; González, J.A.; Prado, F.E. Low-temperature effect on enzyme activities involved in sucrose-starch partitioning in salt-stressed and salt-acclimated cotyledons of quinoa (Chenopodium quinoa Willd.) seedlings. Plant Physiol. Biochem. 2009, 47, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Bazile, D.; Baudron, F. The dynamics of the global expansion of quinoa growing in view of its high biodiversity. In State of the Art Report on Quinoa Around the World in 2013; Bazile, D., Bertero, H.D., Nieto, C., Eds.; FAO: Rome, Italy, 2015; pp. 42–55. [Google Scholar]
- Akil, H. Biological Improvement of Lime-Saline-Sodic Soil in Harran Plain. Master’s Thesis, Harran Universdity Institute of Science, Sanliurfa, Turkey, 2008. [Google Scholar]
- Temel, S.; Keskin, B.; Simsek, U.; Ibrahim, H.Y. Performance of Some Forage Grass Species in Halomorphic Soil. Turk. J. Field Crops 2015, 20, 131–141. [Google Scholar] [CrossRef]
- Koc, A.; Tan, M. Role of Forage Crops Against Salinity Problem Arising in the GAP region. In Proceedings of the GAP I. Agricultural Congress, Sanliurfa, Turkey, 26–28 May 1999. [Google Scholar]
- Al-Dakheel, A.J.; Hussain, M.I.; Abdul Rahman, A.Q. Impact of irrigation water salinity on agronomical and quality attributes of Cenchrus ciliaris L. accessions. Agric. Water Manag. 2015, 159, 148–154. [Google Scholar] [CrossRef]
- Hassen, A.; Souguir, M.; Hannachi, C. Effect of Salt Stress (NaCl) on Germination and Early Seedling Parameters of Three Pepper Genotypes (Capsicum annuum L.). J. Stress Physiol. Biochem. 2014, 10, 15–25. [Google Scholar]
- Hailay, T.; Tadele, G.S.; Tekalign, M. Assessment of salinity/sodicity problems in Abaya State Farm, Southern Rift Valley of Ethiopia. Ethiop. J. Nat. Res. 2000, 2, 151–163. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Pre-sowing seed treatment-a shotgun approach to improve germination growth and crop yield under saline and none-saline conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar]
- Ellis, R.A.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Kitcherside, M.A.; Glen, E.F.; Webster, A.J.F. FibreCap: An improved methodfor the rapid analysis of fibre in feeding stuffs. Anim. Feed Sci. Technol. 2000, 86, 125–132. [Google Scholar] [CrossRef]
- Gomez, A.; Gomez, H. Statistical Analysis for Agricultural Research; John Willy and Sons Inc.: Hoboken, NJ, USA, 1984; pp. 120–155. [Google Scholar]
- Horst, G.L.; Dunning, N.B. Germination and seedling growth of perennial ryegrasses in soluble salts. J. Am. Soc. Hort. Sci. 1989, 114, 338–342. [Google Scholar]
- Iqbal, N.M.Y.; Ashraf, F.; Javed, V.; Martinez, K. Nitrate reduction and nutrient accumulation in wheat (Triticum aestivum L.) grown in soil salinization with four different salts. J. Plant Nutr. 2006, 29, 409–421. [Google Scholar] [CrossRef]
- Azhar, M.F.; McNeilly, T. Variability for salt tolerance in Sorghumn bicolor (L.) Moench under hydroponic conditions. J. Agron. Crop Sci. 1987, 159, 269–277. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Vicente, O.; Boscaiu, M.; Naranjo, M.A.; Estrelles, E.; Belles, J.M.; Soriana, P. Responses to salt stress in the halophyte Plantago crassifolia (Plantaginaceae). J. Arid Environ. 2004, 58, 463–481. [Google Scholar] [CrossRef]
- Almodares, A.; Hadi, M.R.; Dosti, B. Effects of salt stress on germination percentage and seedling growth in sweet sorghum genotypes. J. Biol. Sci. 2007, 7, 1492–1495. [Google Scholar]
- Al-Dakheel, A.J.; Hussain, M.I. Genotypic Variation for Salinity Tolerance in Cenchrus ciliaris L. Front. Plant Sci. 2016, 7, 1090. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Shirazi, M.U.; Khan, M.A.; Mujtaba, S.M.; Islam, E.; Mumtaz, S.; Shereen, A.; Ansari, R.U.; Ashraf, M.Y. Role of proline, K+/Na+ ratio and chlorophyll content in salt tolerance of wheat (Triticum aestivum L.). Pak. J. Bot. 2009, 41, 633–638. [Google Scholar]
- Harinasut, P.; Srisunak, S.; Pitukchaisopol, S.; Charoensataporn, R. Mechanisms of adaptation to increasing salinity of mulberry: Proline content and ascorbate peroxidase activity in leaves of multiple shoots. Sci. Asia 2000, 26, 207–211. [Google Scholar] [CrossRef]
- McConnell, J.S.; Francis, P.B.; Stark, C.R.; Glover, R.E. Plant responses of ultra-narrow row cotton to nitrogen fertilization. J. Plant Nutr. 2008, 31, 1005–1017. [Google Scholar] [CrossRef]
- Kumari, P.; Pahuja, S.K.; Sheoran, R.S.; Satyawan, A.; Joshi, U.N. Effect of Varying Levels of Salinity on Growth, Yield and Quality of Forage Sorghum Genotypes. Forage Res. 2017, 43, 64–66. [Google Scholar]
- Robinson, P.H.; Grattan, S.R.; Getachew, G.; Grieve, C.M.; Poss, J.A.; Suarez, D.L.; Benes, S.E. Biomass accumulation and potential nutritive value of some forages irrigated with saline-sodic drainage water. Anim. Feed Sci. Technol. 2004, 111, 175–189. [Google Scholar] [CrossRef]
- Masters, D.G.; Benesand, S.E.; Norman, H.C. Biosaline Agriculture for Forage and Livestock Production. Agric. Ecosyst. Environ. 2007, 119, 234–248. [Google Scholar] [CrossRef]
- Qadir, M.; Khan, Z.H.; Majeed, A.; Yaqoob, S.; Khan, R.A.; Anjum, K. Effect of salinity on forage production of range grasses. Pak. J. Sci. 2008, 60, 59–63. [Google Scholar]
- Kandil, A.A.; Sharif, A.E.; Abido, W.A.E.; İbrahim, M.M. Effect of salinity on seed germination and seedling characters of some forage sorghum genotypes. Int. J. Agric. Sci. 2012, 4, 306–311. [Google Scholar]
- Suyama, H.; Benes, S.B.; Robinson, P.H.; Grattan, S.R.; Grieve, C.M.; Getachew, G. Forage yield and quality under irrigation with saline-sodic drainage water: Greenhouse evaluation. Agric. Water Manag. 2007, 88, 159–173. [Google Scholar] [CrossRef]
- Ashenafi, W.; Bobe, B. Studies on Soil Physical Properties of Salt Affected Soil in Amibara Area, Central Rift Valley of Ethiopia. Int. J. Agric. Sci. Nat. Resour. 2016, 3, 8–17. [Google Scholar]
- Sima, N.A.; Ahmad, S.T.; Pessarakli, M. Comparative Study of Different Salts (Sodium Chloride, Sodium Sulfate, Potassium Chloride, and Potassium Sulfate) on Growth of Forage Species. J. Plant Nutr. 2013, 36, 214–230. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).