Ferroelectric Materials: A Novel Pathway for Efficient Solar Water Splitting
Abstract
1. Introduction
2. Overview of Solar Water Splitting
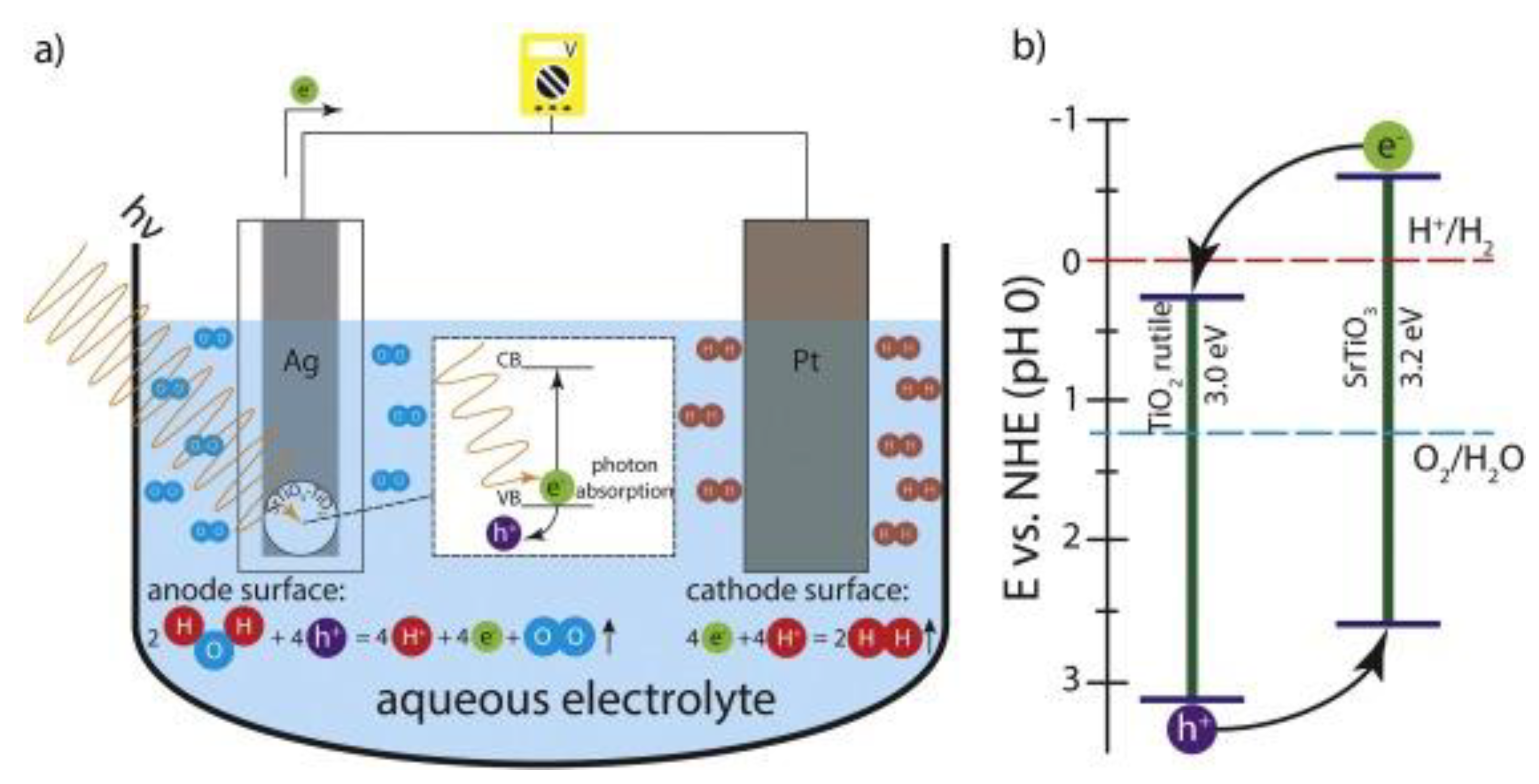
2.1. Basic Mechanism for Solar Energy to Hydrogen Conversion System
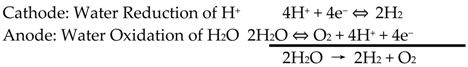
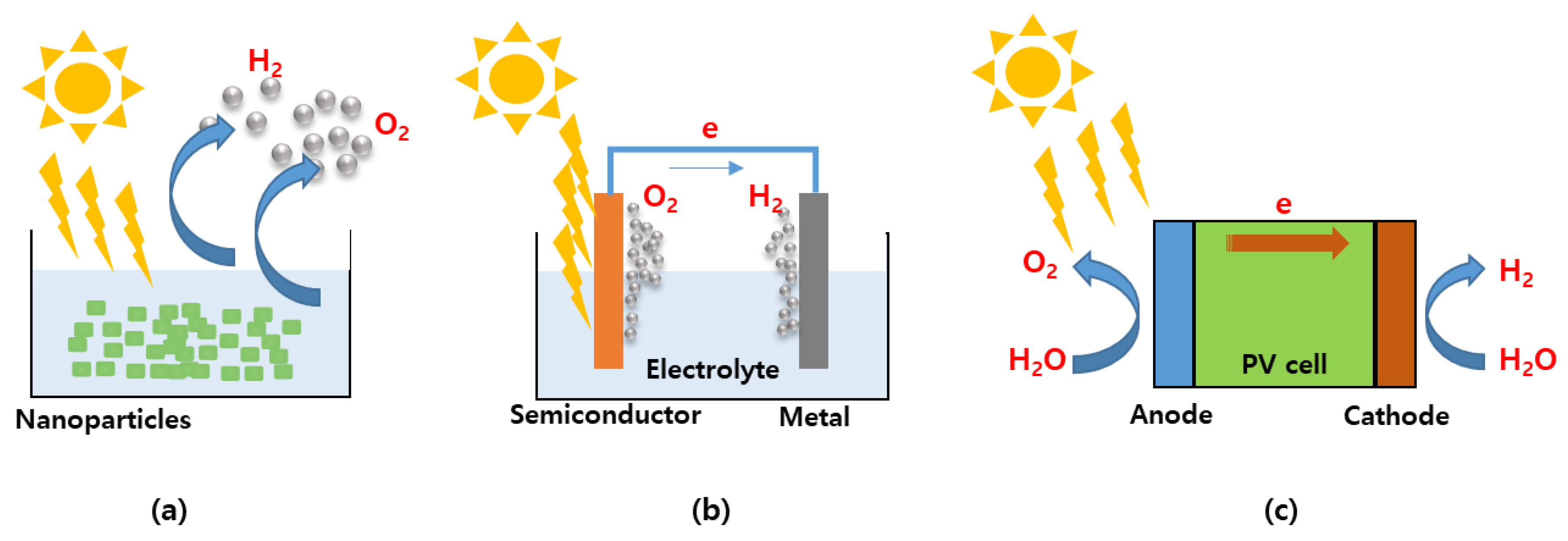
2.2. Solar Energy to Hydrogen Conversion System for Solar Water Splitting
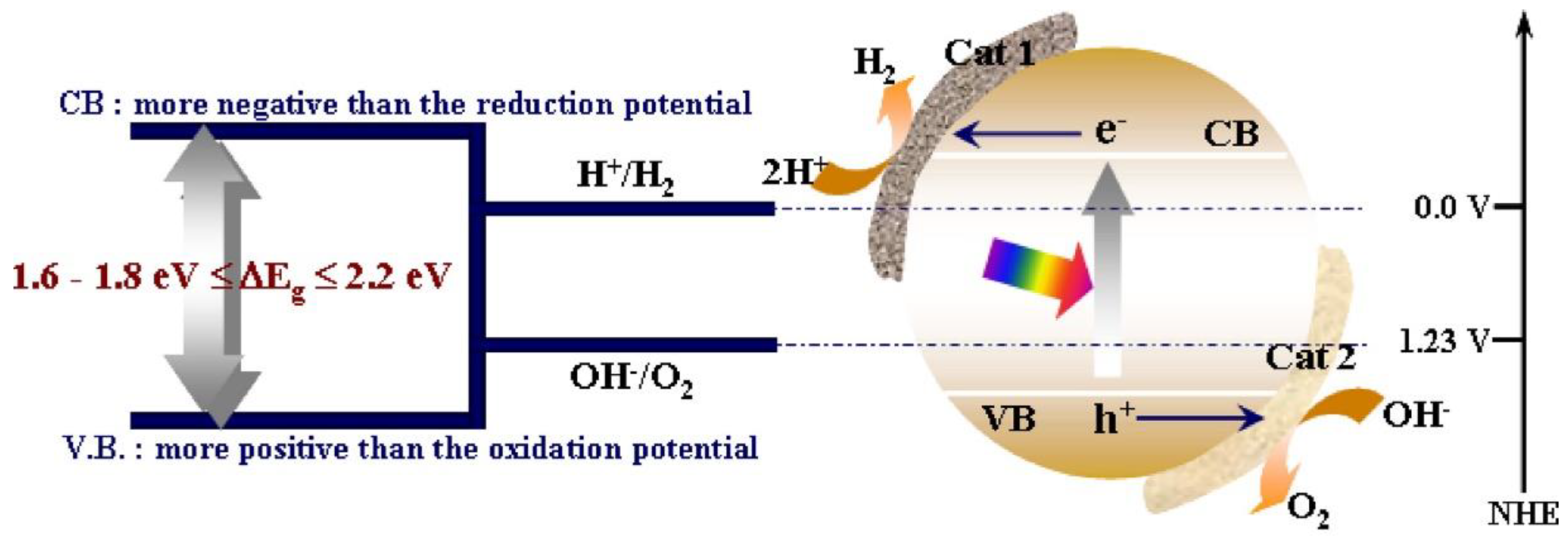
2.3. Strategy for High Solar-to-Hydrogen (STH) Efficiencies
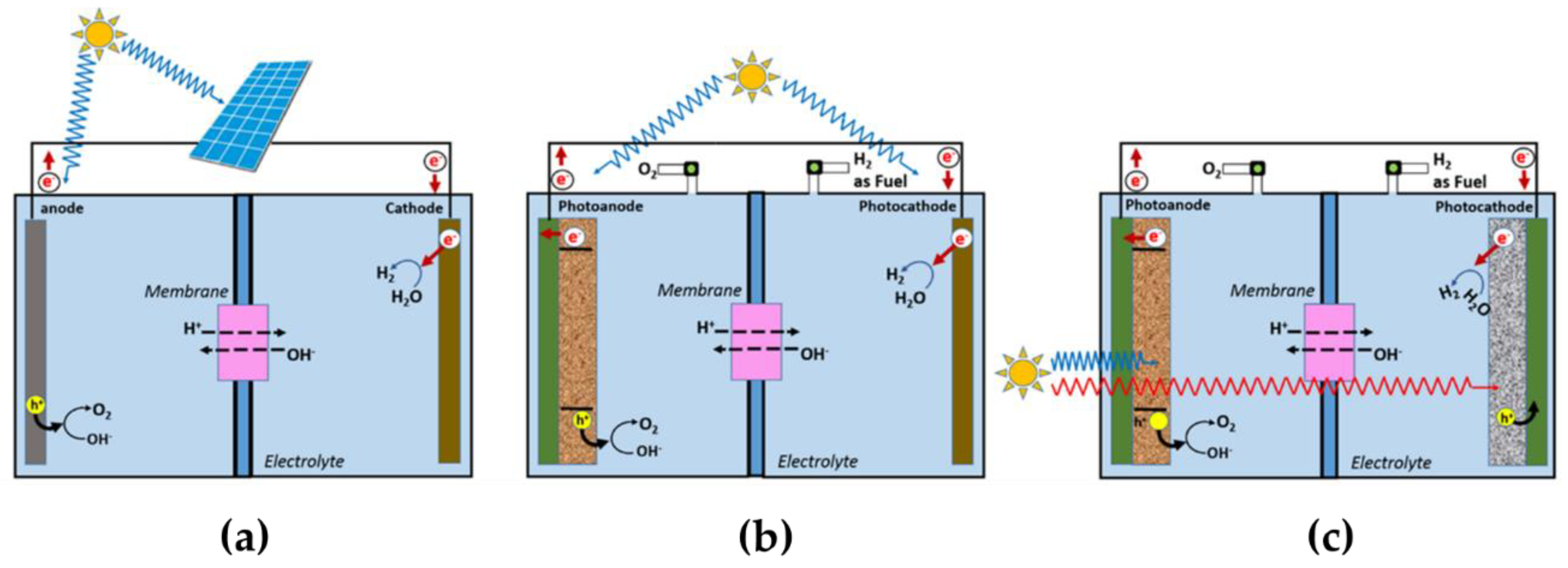
2.4. Strategy to Improve High STH Efficiency for Water Splitting
3. Solar Energy Conversion of Ferroelectric Materials
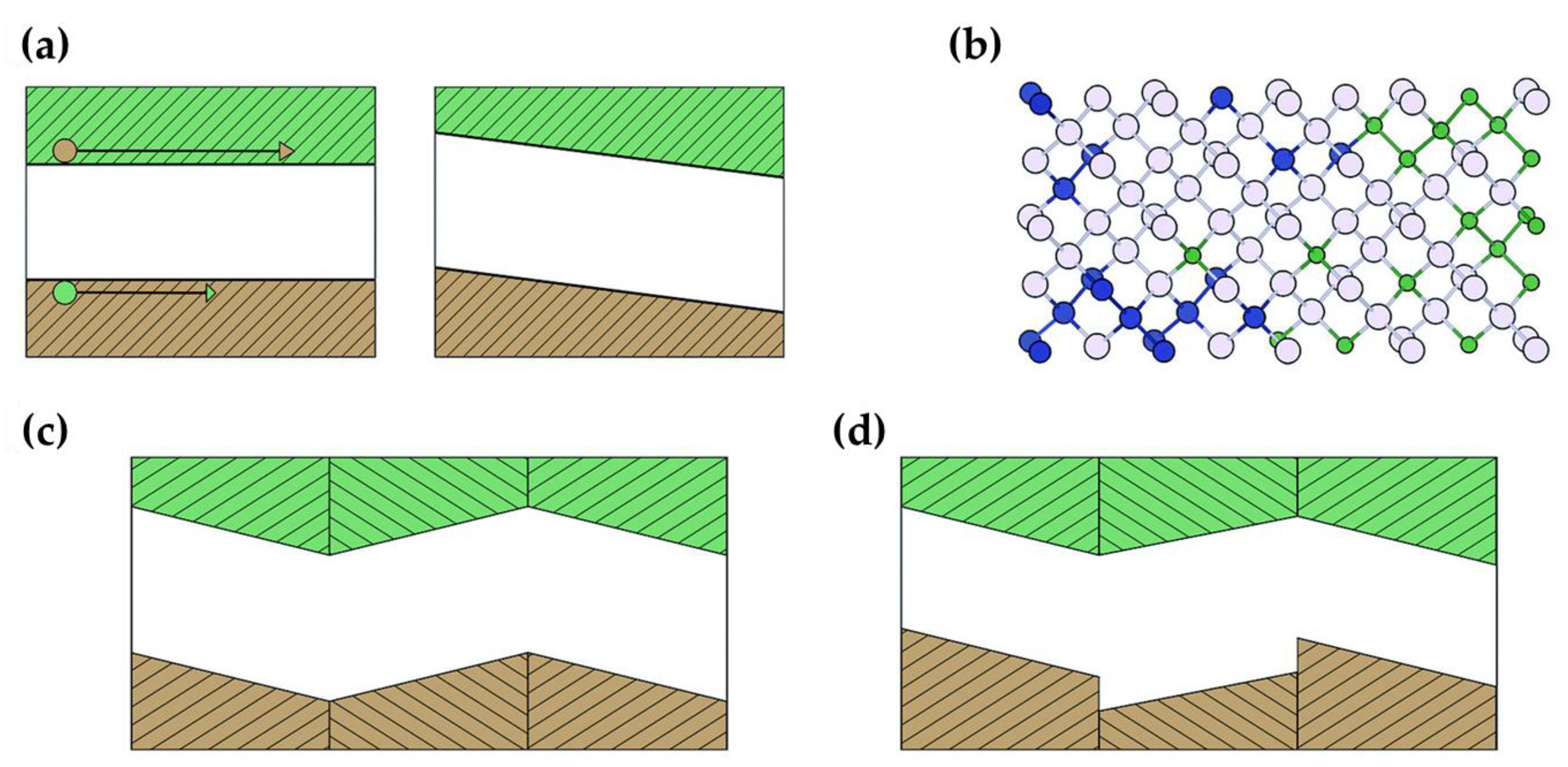
3.1. Ferroelectric Effect and Materials
3.2. Ferroelectric Photovoltaics—Phenomenology of Ferroelectric Solar Cells
4. Ferroelectric Materials for Photoelectrochemical (PEC) Water-Splitting Devices
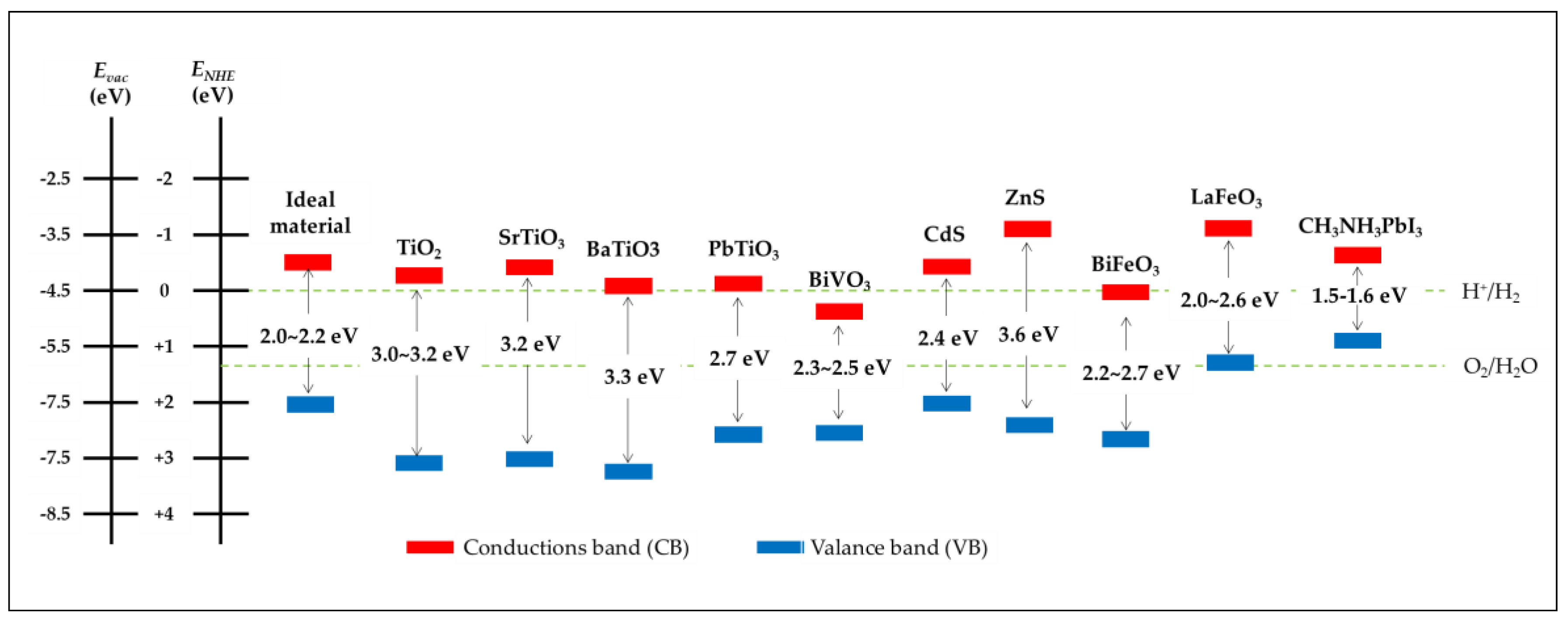
4.1. General Requirements for Photoelectrode Materials
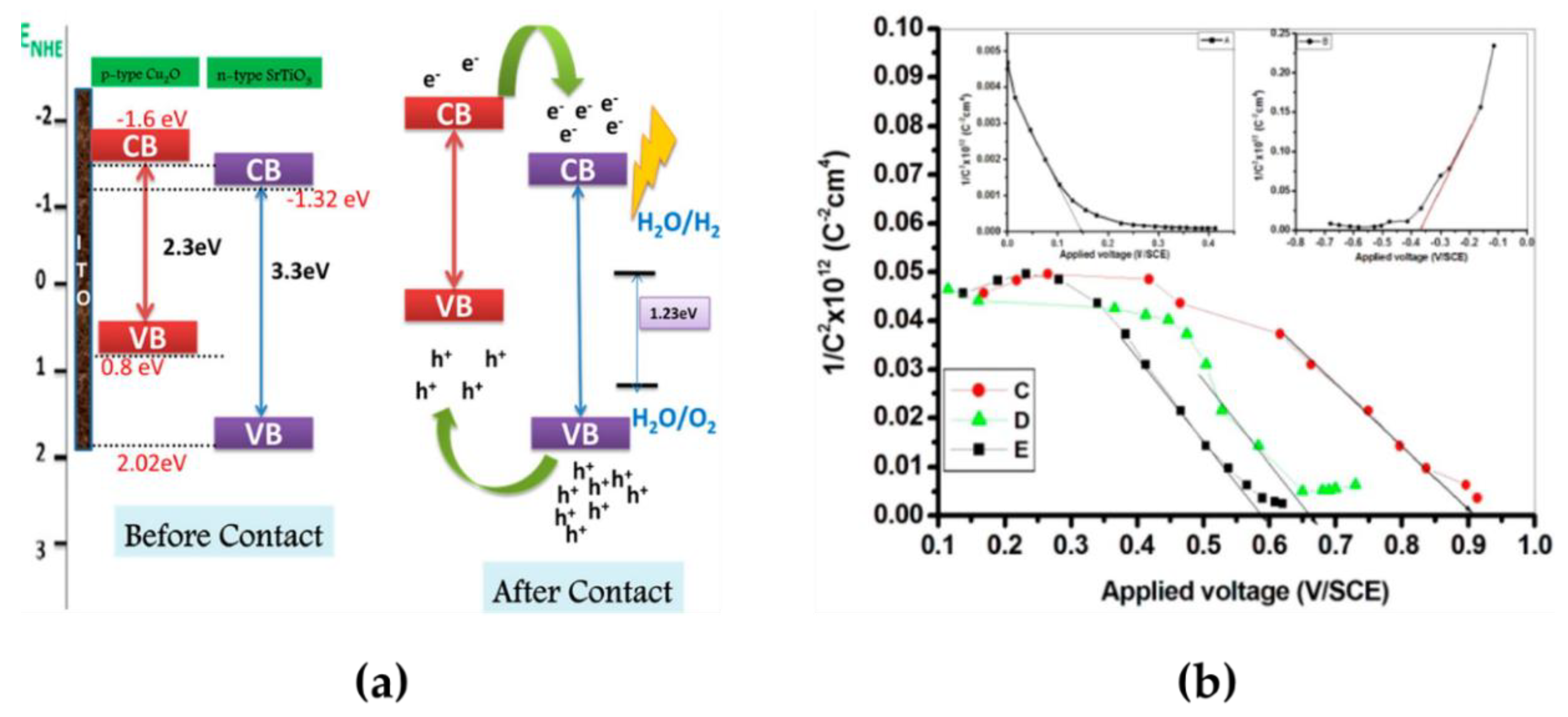
4.2. Ferroelectric-Based Materials in Photocathodes for Hydrogen
4.2.1. Ferroelectric Oxide Perovskites
Quaternary Metal Oxides
4.3. Ferroelectric-Based Materials in Photoanodes for Water
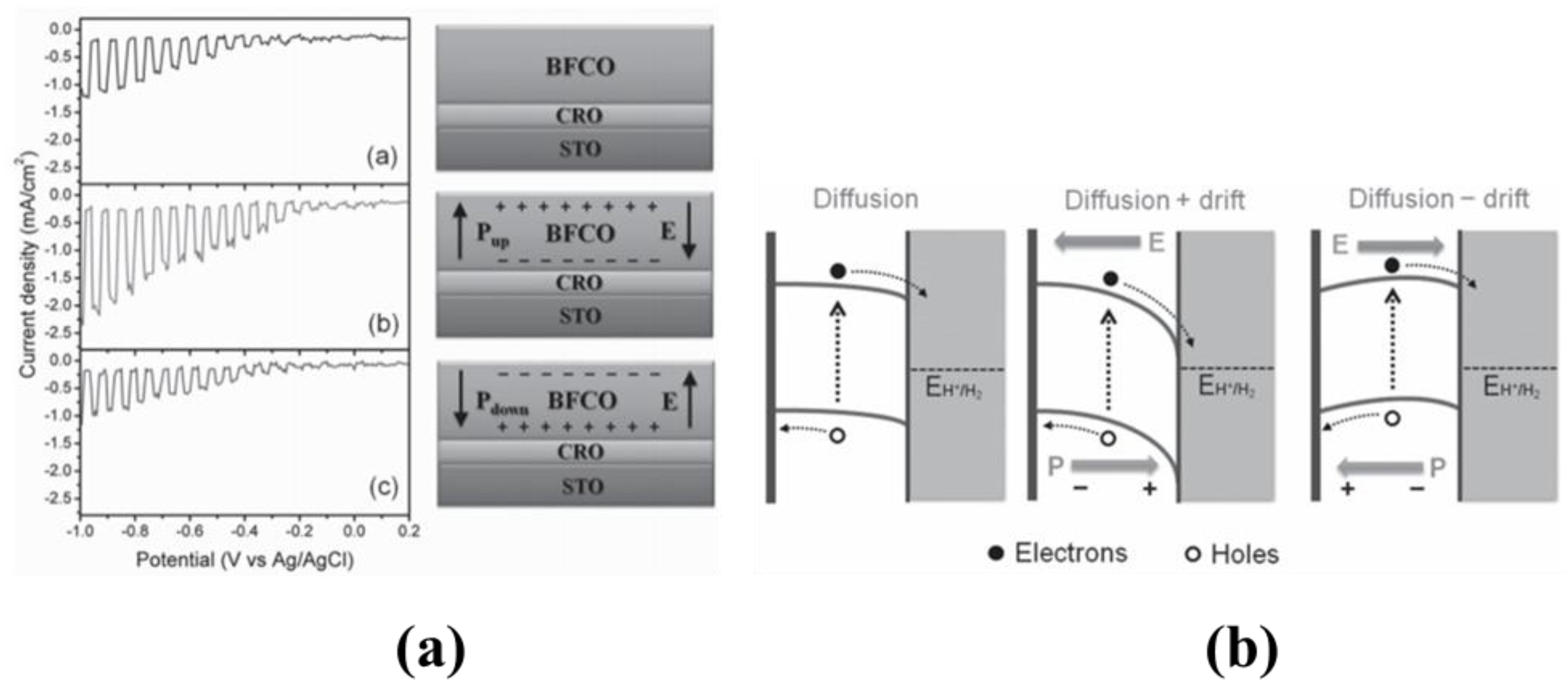
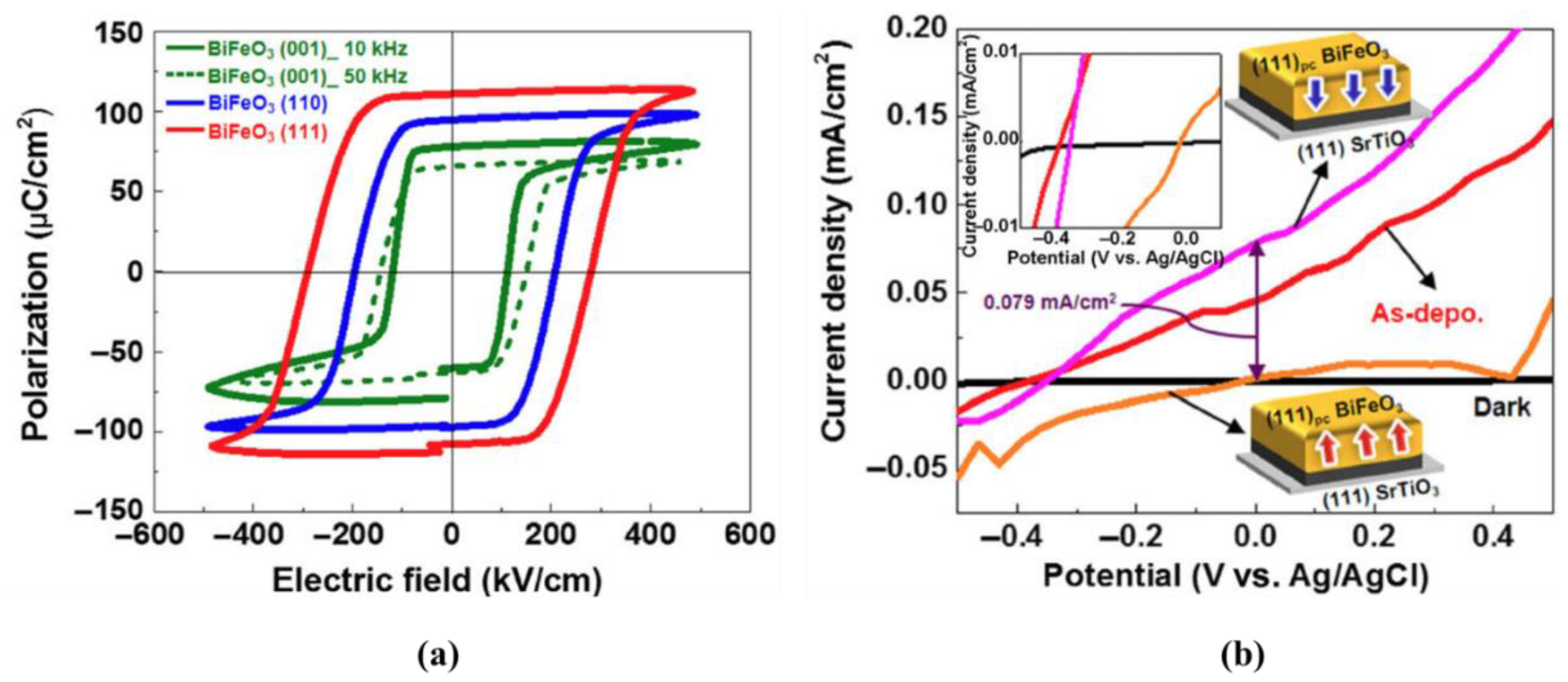
4.3.1. Ferroelectric Oxide Perovskites
4.3.2. Ferroelectric Chalcogenides
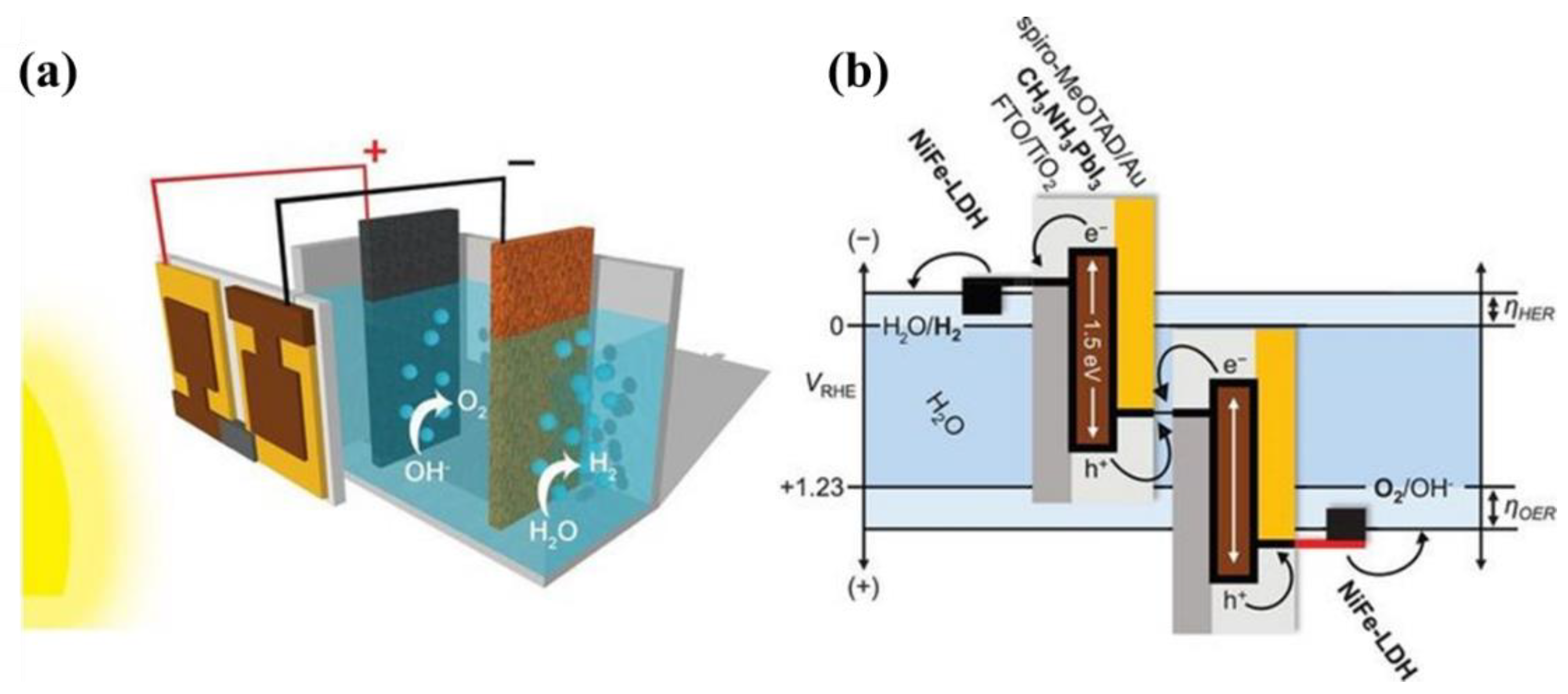
4.3.3. Hybrid Halide Ferroelectric CH3NH3PbI3 Perovskite Tandem System Approach
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Lopes, T.; Dias, P.; Andrade, L.; Mendes, A. An innovative photoelectrochemical lab device for solar water splitting. Sol. Energy Mater. Sol. Cells 2014, 128, 399–410. [Google Scholar] [CrossRef]
- Ngoh, S.K.; Njomo, D. An overview of hydrogen gas production from solar energy. Renew. Sustain. Energy Rev. 2012, 16, 6782–6792. [Google Scholar] [CrossRef]
- Sun, J.W.; Zhong, D.K.; Gamelin, D.R. Composite photoanodes for photoelectrochemical solar water splitting. Energy Environ. Sci. 2010, 3, 1252–1261. [Google Scholar] [CrossRef]
- Song, J.; Kim, T.L.; Lee, J.; Cho, S.Y.; Cha, J.; Jeong, S.Y.; An, H.; Kim, W.S.; Jung, Y.S.; Park, J.; et al. Domain-engineered BiFeO3 thin-film photoanodes for highly enhanced ferroelectric solar water splitting. Nano Res. 2018, 11, 642–655. [Google Scholar] [CrossRef]
- Pan, H.; Zhu, S.; Lou, X.; Mao, L.; Lin, J.; Tian, F.; Zhang, D. Graphene-based photocatalysts for oxygen evolution from water. RSC Adv. 2015, 5, 6543–6552. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Xu, Z.C.; Loo, J.S.C. Hybrid catalysts for photoelectrochemical reduction of carbon dioxide: A prospective review on semiconductor/metal complex co-catalyst systems. J. Mater. Chem. A 2014, 2, 15228–15233. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, H.G.; Lee, J.S. Heterojunction semiconductors: A strategy to develop efficient photocatalytic materials for visible light water splitting. Catal. Today 2012, 185, 270–277. [Google Scholar] [CrossRef]
- Vilanova, A.; Lopes, T.; Spenke, C.; Wullenkord, M.; Mendes, A. Optimized photoelectrochemical tandem cell for solar water splitting. Energy Storage Mater. 2018, 13, 175–188. [Google Scholar] [CrossRef]
- Tentu, R.D.; Basu, S. Photocatalytic water splitting for hydrogen production. Curr. Opin. Electrochem. 2017, 5, 56–62. [Google Scholar] [CrossRef]
- Li, R. Latest progress in hydrogen production from solar water splitting via photocatalysis, photoelectrochemical, and photovoltaic-photoelectrochemical solutions. Chin. J. Catal. 2017, 38, 5–12. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Jiang, C.R.; Moniz, S.J.A.; Wang, A.G.; Zhang, T.; Tang, J.W. Photoelectrochemical devices for solar water splitting-materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nat. Commun. 1972, 238, 37–38. [Google Scholar]
- Alfaifi, B.Y.; Ullah, H.; Alfaifi, S.; Tahir, A.A.; Mallick, T.K. Photoelectrochemical Solar Water Splitting: From Basic Principles to Advanced Devices. Veruscr. Funct. Nanomater. 2018, 2, 1–26. [Google Scholar] [CrossRef]
- Chen, Z.; Dinh, D.; Miller, E. Chapter 3 Experimental Considerations. In Photoelectrochemical Water Splitting, 1st ed.; Springer: New York, NY, USA, 2013; pp. 17–44. ISBN 978-1-4614-8298-7. [Google Scholar]
- Paillard, C.; Bai, X.F.; Infante, I.C.; Guennou, M.; Geneste, G.; Alexe, M.; Kreisel, J.; Dkhil, B. Photovoltaics with ferroelectrics: Current status and beyond. Adv. Mater. 2016, 28, 5153–5168. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.; Tilley, S.D. Photovoltaic and photoelectrochemical solar energy conversion with Cu2O. J. Phys. Chem. C 2015, 119, 26243–26257. [Google Scholar] [CrossRef]
- Tablero, C. Photovoltaic application of the multiferroic Bi2FeCrO6 double perovskite. Sol. Energy 2016, 137, 173–178. [Google Scholar] [CrossRef]
- Archer, M.D. Photovoltaics and photoelectrochemistry: Similarities and differences. Physica E 2002, 14, 61–64. [Google Scholar] [CrossRef]
- Lopez-Varo, P.; Bertoluzzi, L.; Bisquert, J.; Alexe, M.; Coll, M.; Huang, J.S.; Juan, A.J.T.; Kirchartz, T.; Nechache, R.; Rosei, F.; et al. Physical aspects of ferroelectric semiconductors for photovoltaic solar energy conversion. Phys. Rep. 2016, 653, 1–40. [Google Scholar] [CrossRef]
- Spanier, J.E.; Fridkin, V.M.; Rappe, A.M.; Akbashev, A.R.; Polemi, A.; Qi, Y.B.; Gu, Z.Q.; Young, S.M.; Hawley, C.J.; Imbrenda, D.; et al. Power conversion efficiency exceeding the shockley-queisser limit in a ferroelectric insulator. Nat. Photonics 2016, 10, 611–616. [Google Scholar] [CrossRef]
- Bonke, S.A.; Wiechen, M.; MacFarlane, D.R.; Spiccia, L. Renewable fuels from concentrated solar power: Towards practical artificial photosynthesis. Energy Environ. Sci. 2015, 8, 2791–2796. [Google Scholar] [CrossRef]
- Jia, J.Y.; Seitz, L.C.; Benck, J.D.; Huo, Y.J.; Chen, Y.S.; Ng, J.W.D.; Bilir, T.; Harris, J.S.; Jaramillo, T.F. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Li, W.; Yan, Y.; Hamann, T.; Shin, I.; Wang, D.; Mi, Z. Roadmap on solar water splitting: Current status and future prospects. Nano Futures 2017, 1, 1–29. [Google Scholar] [CrossRef]
- Jacobsson, T.J.; Fjallstrom, V.; Edoff, M.; Edvinsson, T. Sustainable solar hydrogen production: From photoelectrochemical cells to pv-electrolyzers and back again. Energy Environ. Sci. 2014, 7, 2056–2070. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Rick, J.; Dubale, A.A.; Su, W.N.; Hwang, B.J. Using hematite for photoelectrochemical water splitting: A review of current progress and challenges. Nanoscale Horiz. 2016, 1, 243–267. [Google Scholar] [CrossRef]
- Butler, K.T.; Frost, J.M.; Walsh, A. Ferroelectric materials for solar energy conversion: Photoferroics revisited. Energy Environ. Sci. 2015, 8, 838–848. [Google Scholar] [CrossRef]
- Khan, M.A.; Nadeem, M.A.; Idrissn, H. Ferroelectric polarization effect on surface chemistry and photo-catalytic activity: A review. Surf. Sci. Rep. 2016, 71, 1–31. [Google Scholar] [CrossRef]
- Li, L.; Salvador, P.A.; Rohrer, G.S. Photocatalysts with internal electric fields. Nanoscale 2014, 6, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Sivula, K.; Krol, R.V.D. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; Mckone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.S.; Enman, L.J.; Batchellor, A.S.; Zou, S.; Boettcher, S.W. Oxygen Evolution Reaction Electrocatalysis on Transition Metal Oxides and (Oxy)hydroxides: Activity Trends and Design Principles. Chem. Mater. 2015, 27, 7549–7558. [Google Scholar] [CrossRef]
- Jin, J.; Walczak, K.; Singh, M.R.; Karp, C.; Lewis, N.S.; Xiang, C. An experimental and modeling/simulation-based evaluation of the efficiency and operational performance characteristics of an integrated, membrane-free, neutral pH solar-driven water-splitting system. Energy Environ. Sci. 2014, 7, 3371–3380. [Google Scholar] [CrossRef]
- Seger, B.; Castelli, I.E.; Vesborg, P.C.K.; Jacobsen, K.W.; Hansen, O.; Chorkendorff, I. 2-Photon tandem device for water splitting: Comparing photocathode first versus photoanode first designs. Energy Environ. Sci. 2014, 7, 2397–2413. [Google Scholar] [CrossRef]
- Hara, M.; Kondo, T.; Komoda, M.; Ikeda, S.; Shinohara, K.; Tanaka, A.; Kondo, J.N.; Domen, K. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chem. Commun. 1998, 357–358. [Google Scholar] [CrossRef]
- Jongh, P.E.D.; Vanmaekelbergh, D.; Kelly, J.J. Cu2O: A catalyst for the photochemical decomposition of water? Chem. Commun. 1999, 1069–1070. [Google Scholar] [CrossRef]
- Chauhan, D.; Satsangi, V.R.; Dass, S.; Shrivastav, R. Preparation and characterization of nanostructured CuO thin films for photoelectrochemical splitting of water. Bull. Mater. Sci. 2006, 29, 709–716. [Google Scholar]
- Koffyberg, F.P.; Benko, F.A. A photoelectrochemical determination of the position of the conduction and valence band edges of p-type CuO. J. Appl. Phys. 1982, 53, 1173–1177. [Google Scholar] [CrossRef]
- Hsu, Y.K.; Yu, C.H.; Lin, H.H.; Chen, Y.C.; Lin, Y.G. Template synthesis of copper oxide nanowires for photoelectrochemical hydrogen generation. J. Electroanal. Chem. 2013, 704, 19–23. [Google Scholar] [CrossRef]
- Gupta, R.K.; Ghosh, K.; Kahol, P.K. Fabrication and characterization of NiO/ZnO p-n junctions by pulsed laser deposition. Physica E 2009, 41, 617–620. [Google Scholar] [CrossRef]
- Dong, Y.M.; Chen, Y.M.; Jiang, P.P.; Wang, G.L.; Wu, X.M.; Wu, R.X.; Zhang, C. Efficient and stable MoS2/CdSe/NiO photocathode for photoelectrochemical hydrogen generation from water. Chem. Asian J. 2015, 10, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Yeo, D.; Chang, H.; Loh, L.; Dunn, S. Perovskite BiFeO3 thin film photocathode performance with visible light activity. Nanotechnology 2016, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.T.; Choi, T.; Choi, S.G.; Oh, Y.S.; Cheong, S.W. Mechanism of the switchable photovoltaic effect in ferroelectric BiFeO3. Adv. Mater. 2011, 23, 3403–3407. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Nan, F.; You, L.; Zhou, Y.; Wang, Y.Y.; Wang, J.L.; Su, X.D.; Shen, M.R.; Fang, L. Enhanced photoelectrochemical performance in reduced graphene oxide/BiFeO3 heterostructures. Small 2017, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Quynh, L.T.; Van, C.N.; Bitla, Y.; Chen, J.W.; Do, T.H.; Tzeng, W.Y.; Liao, S.C.; Tsai, K.A.; Chen, Y.C.; Wu, C.L.; et al. Self-assembled BiFeO3-ε-Fe2O3 vertical heteroepitaxy for visible light photoelectrochemistry. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; You, L.; Wang, J.L.; Shen, M.R.; Fang, L. Enhanced ferroelectric photoelectrochemical properties of polycrystalline BiFeO3 film by decorating with ag nanoparticles. Appl. Phys. Lett. 2016, 108, 1–5. [Google Scholar] [CrossRef]
- Ji, W.; Yao, K.; Lim, Y.F.; Liang, Y.C.; Suwardi, A. Epitaxial ferroelectric BiFeO3 thin films for unassisted photocatalytic water splitting. Appl. Phys. Lett. 2013, 103, 1–4. [Google Scholar] [CrossRef]
- Hauser, A.J.; Zhang, J.; Mier, L.; Ricciardo, R.A.; Woodward, P.M.; Gustafson, T.L.; Brillson, L.J.; Yang, F.Y. Characterization of electronic structure and defect states of thin epitaxial BiFeO3 films by UV-visible absorption and cathodoluminescence spectroscopies. Appl. Phys. Lett. 2008, 92, 1–3. [Google Scholar] [CrossRef]
- Gupta, S.; Medwal, R.; Limbu, T.B.; Katiyar, R.K.; Pavunny, S.P.; Tomar, M.; Morell, G.; Gupta, V.; Katiyar, R.S. Graphene/semiconductor silicon modified BiFeO3/indium tin oxide ferroelectric photovoltaic device for transparent self-powered windows. Appl. Phys. Lett. 2015, 107. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.W.; Zhang, W.R.; Suwardi, A.; Wang, H.Y.; Wang, D.W.; MacManus-Driscoll, J.L. Single-crystalline thin films for studying intrinsic properties of BiFeO3-SrTiO3 solid solution photoelectrodes in solar energy conversion. Chem. Mater. 2015, 27, 6635–6641. [Google Scholar] [CrossRef]
- Chen, X.Y.; Yu, T.; Gao, F.; Zhang, H.T.; Liu, L.F.; Wang, Y.M.; Li, Z.S.; Zou, Z.G.; Liu, J.M. Application of weak ferromagnetic BiFeO3 films as the photoelectrode material under visible-light irradiation. Appl. Phys. Lett. 2007, 91. [Google Scholar] [CrossRef]
- Mohan, S.; Subramanian, B.; Bhaumik, I.; Gupta, P.K.; Jaisankar, S.N. Nanostructured Bi1−xGdxFeO3—A multiferroic photocatalyst on its sunlight driven photocatalytic activity. RSC Adv. 2014, 4, 16871–16878. [Google Scholar] [CrossRef]
- Gu, S.L.; Zhou, X.X.; Zheng, F.G.; Fang, L.; Dong, W.; Shen, M.R. Improved photocathodic performance in Pt catalyzed ferroelectric BiFeO3 films sandwiched by a porous carbon layer. Chem. Commun. 2017, 53, 7052–7055. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.R.; Shen, H.Y.; Dong, W.; Zheng, F.G.; Fang, L.A.; Su, X.D.; Shen, M.R. Nano-au and ferroelectric polarization mediated Si/ITO/BiFeO3 tandem photocathode for efficient H2 production. Adv. Mater. Interfaces 2016, 3, 1–7. [Google Scholar] [CrossRef]
- Kang, D.H.; Kim, T.W.; Kubota, S.R.; Cardiel, A.C.; Cha, H.G.; Choi, K.S. Electrochemical synthesis of photoelectrodes and catalysts for use in solar water splitting. Chem. Rev. 2015, 115, 12839–12887. [Google Scholar] [CrossRef] [PubMed]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Takai, A.; Kamat, P.V. Capture, store, and discharge. Capture, store, and discharge. Shuttling photogenerated electrons across TiO2-silver interface. ACS Nano 2011, 5, 7369–7376. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.Y.; Zhou, X.X.; Dong, W.; Su, X.D.; Fang, L.A.; Wu, X.; Shen, M.R. Dual role of TiO2 buffer layer in Pt catalyzed BiFeO3 photocathodes: Efficiency enhancement and surface protection. Appl. Phys. Lett. 2017, 111. [Google Scholar] [CrossRef]
- Prévot, M.S.; Sivula, K. Photoelectrochemical tandem cells for solar water splitting. J. Phys. Chem. C 2013, 117, 17879–17893. [Google Scholar] [CrossRef]
- Hu, S.; Xiang, C.X.; Haussener, S.; Berger, A.D.; Lewis, N.S. An analysis of the optimal band gaps of light absorbers in integrated tandem photoelectrochemical water-splitting systems. Energy Environ. Sci. 2013, 6, 2984–2993. [Google Scholar] [CrossRef]
- Fan, R.L.; Dong, W.; Fang, L.A.; Zheng, F.G.; Su, X.D.; Zou, S.; Huang, J.; Wang, X.S.; Shen, M.R. Stable and efficient multi-crystalline n(+)p silicon photocathode for H2 production with pyramid-like surface nanostructure and thin Al2O3 protective layer. Appl. Phys. Lett. 2015, 106. [Google Scholar] [CrossRef]
- Fan, R.L.; Min, J.W.; Li, Y.; Su, X.D.; Zou, S.; Wang, X.S.; Shen, M.R. N-type silicon photocathodes with Al-doped rear p(+) emitter and Al2O3-coated front surface for efficient and stable H2 production. Appl. Phys. Lett. 2015, 106. [Google Scholar] [CrossRef]
- Yu, Q.; Meng, X.G.; Wang, T.; Li, P.; Liu, L.Q.; Chang, K.; Liu, G.G.; Ye, J.H. A highly durable p-LaFeO3/n-Fe2O3 photocell for effective water splitting under visible light. Chem. Commun. 2015, 51, 3630–3633. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, G.P.; Choi, K.S. Photoelectrochemical properties and stability of nanoporous p-type LaFeO3 photoelectrodes prepared by electrodeposition. ACS Energy Lett. 2017, 2, 2378–2382. [Google Scholar] [CrossRef]
- Ding, J.L.; Lu, X.M.; Shu, H.M.; Xie, J.M.; Zhang, H. Microwave-assisted synthesis of perovskite ReFeO3 (Re: La, sm, eu, gd) photocatalyst. Mater. Sci. Eng. B 2010, 171, 31–34. [Google Scholar] [CrossRef]
- Parida, K.M.; Reddy, K.H.; Martha, S.; Das, D.P.; Biswal, N. Fabrication of nanocrystalline LaFeO3: An efficient sol-gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int. J. Hydrogen Energy 2010, 35, 12161–12168. [Google Scholar] [CrossRef]
- Tijare, S.N.; Joshi, M.V.; Padole, P.S.; Mangrulkar, P.A.; Rayalu, S.S.; Labhsetwar, N.K. Photocatalytic hydrogen generation through water splitting on nano-crystalline LaFeO3 perovskite. Int. J. Hydrogen Energy 2012, 37, 10451–10456. [Google Scholar] [CrossRef]
- Celorrio, V.; Bradley, K.; Weber, O.J.; Hall, S.R.; Fermin, D.J. Photoelectrochemical properties of LaFeO3 nanoparticles. ChemElectroChem 2014, 1, 1667–1671. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, J.; Feng, Z.J.; Du, C.; Wen, Y.W.; Shan, B.; Chen, R. Enhanced photoelectrochemical water oxidation by fabrication of p-LaFeO3/n-Fe2O3 heterojunction on hematite nanorods. J. Phys. Chem. C 2017, 121, 12991–12998. [Google Scholar] [CrossRef]
- Diez-Garcia, M.I.; Gómez, R. Metal doping to enhance the photoelectrochemical behavior of LaFeO3 photocathodes. ChemSusChem 2017, 10, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.D.; Paudel, T.R.; Zakutayev, A.; Ndione, P.F.; Parilla, P.A.; Young, D.L.; Lany, S.; Ginley, D.S.; Zunger, A.; Perry, N.H.; et al. Inverse design approach to hole doping in ternary oxides: Enhancing p-type conductivity in cobalt oxide spinels. Phys. Rev. B 2011, 84, 1–8. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, J.; Wen, Y.W.; Shan, B.; Chen, R. Surface modification of LaFeO3 by co-pi electrochemical deposition as an efficient photoanode under visible light. RCS Adv. 2016, 6, 26192–26198. [Google Scholar] [CrossRef]
- Pawar, G.S.; Tahir, A.A. Unbiased spontaneous solar fuel production using stable LaFeO3 photoelectrode. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cardona, M. Optical Properties and Band Structure of SrTiO3 and BaTiO3. Phys. Rev. 1965, 140, A651–A655. [Google Scholar] [CrossRef]
- Wrighton, M.S.; Ellis, A.B.; Wolczanski, P.T.; Morse, D.L.; Abrahamson, H.B.; Ginley, D.S. Strontium titanate photoelectrodes. Efficient photoassisted electrolysis of water at zero applied potential. J. Am. Chem. Soc. 1976, 98, 2774–2779. [Google Scholar] [CrossRef]
- Yu, H.G.; Irie, H.; Hashimoto, K. Conduction band energy level control of titanium dioxide: Toward an efficient visible-light-sensitive photocatalyst. J. Am. Chem. Soc. 2010, 132, 6898–6899. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.X.; Ye, J.H. β-AgAl1−xGaxO2 solid-solution photocatalysts: Continuous modulation of electronic structure toward high-performance visible-light photoactivity. J. Am. Chem. Soc. 2011, 133, 7757–7763. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.F.; Pierre, A.; Kibria, M.G.; Cui, K.; Han, X.G.; Bevan, K.H.; Guo, H.; Paradis, S.; Hakima, A.R.; Mi, Z.T. Wafer-level photocatalytic water splitting on GaN nanowire arrays grown by molecular beam epitaxy. Nano Lett. 2011, 11, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M. Surface modification of TiO2 with metal oxide nanoclusters: A route to composite photocatalytic materials. Chem. Commun. 2011, 47, 8617–8619. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Jin, Q.; Nishijima, H.; Yamamoto, H.; Fujishima, M.; Okuoka, S.; Hattori, T.; Sumida, Y.; Kobayashi, H. Titanium(iv) dioxide surface-modified with iron oxide as a visible light photocatalyst. Angew. Chem. Int. Ed. 2011, 50, 3501–3505. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Irie, H.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; Miyauchi, M.; Hashimoto, K. An efficient visible-light-sensitive Fe(iii)-grafted TiO2 photocatalyst. J. Phys. Chem. C 2010, 114, 16481–16487. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhao, Z.H.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhu, H.L.; Zheng, S.K.; Pan, F.; Wang, T.M. TiO2 film/Cu2O microgrid heterojunction with photocatalytic activity under solar light irradiation. ACS Appl. Mater. Interfaces 2009, 1, 2111–2114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bang, J.H.; Tang, C.C.; Kamat, P.V. Tailored TiO2-SrTiO3 heterostructure nanotube arrays for improved photoelectrochemical performance. ACS Nano 2010, 4, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Upadhyay, S.; Satsangi, V.R.; Shrivastav, R.; Waghmare, U.V.; Dass, S. Improved photoelectrochemical water splitting performance of Cu2O/SrTiO3 heterojunction photoelectrode. J. Phys. Chem. C 2014, 118, 25320–25329. [Google Scholar] [CrossRef]
- Liu, C.B.; Li, P.; Wu, G.L.; Luo, B.F.; Lin, S.; Ren, A.; Shi, W.D. Enhanced photoelectrochemical and photocatalytic activity by Cu2O/SrTiO3 p-n heterojunction via a facile deposition-precipitation technique. RSC Adv. 2015, 5, 33938–33945. [Google Scholar] [CrossRef]
- Arney, D.; Watkins, T.; Maggard, P.A. Effects of particle surface areas and microstructures on photocatalytic H2 and O2 production over PbTiO3. J. Am. Ceram. Soc. 2011, 94, 1483–1489. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.H.; Zhong, C.F.; Sun, C.K.; Yao, G.F.; Li, L.T. Synthesis and growth mechanism of lead titanate nanotube arrays by hydrothermal method. J. Am. Ceram. Soc. 2008, 91, 3388–3390. [Google Scholar] [CrossRef]
- Reddy, K.H.; Parida, K. Fabrication, characterization, and photoelectrochemical properties of Cu-doped PbTiO3 and its hydrogen production activity. ChemCatChem 2013, 5, 3812–3820. [Google Scholar] [CrossRef]
- Hu, Y.X.; Dong, W.; Zheng, F.G.; Fang, L.; Shen, M.R. Fe(iii) doped and grafted PbTiO3 film photocathode with enhanced photoactivity for hydrogen production. Appl. Phys. Lett. 2014, 105, 1–4. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, W.; Zheng, F.; Fang, L.; Shen, M. Improved Photocathodic Properties of Ferroelectric PbTiO3 Films Using Ag-Pt Bimetallic Catalyst. Energy Environ. Focus 2015, 4, 95–100. [Google Scholar] [CrossRef]
- Tabari, T.; Singh, D.; Calisan, A.; Ebadi, M.; Tavakkoli, H.; Caglar, B. Microwave assisted synthesis of La1−xCaxMnO3 (x = 0, 0.2 and 0.4): Structural and capacitance properties. Ceram. Int. 2017, 43, 15970–15977. [Google Scholar] [CrossRef]
- Jamali, S.S.; Singh, D.; Tavakkoli, H.; Kaveh, F.; Tabari, T. Microwave-assisted synthesis of nanostructured perovskite-type oxide with efficient photocatalytic activity against organic reactants in gaseous and aqueous phases. Mater. Sci. Semicond. Process 2017, 64, 47–54. [Google Scholar] [CrossRef]
- Tabari, T.; Ebadi, M.; Singh, D.; Caglar, B.; Yagci, M.B. Efficient synthesis of perovskite-type oxide photocathode by nonhydrolytic sol-gel method with an enhanced photoelectrochemical activity. J. Alloys Compd. 2018, 750, 248–257. [Google Scholar] [CrossRef]
- Tang, P.S.; Chen, H.F.; Cao, F.; Pan, G.X. Magnetically recoverable and visible-light-driven nanocrystalline YFeO3 photocatalysts. Catal. Sci. Technol. 2011, 1, 1145–1148. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Yang, J.X.; Xu, J.F.; Gao, Q.Y.; Hong, Z.L. Controllable synthesis of hexagonal and orthorhombic YFeO3 and their visible-light photocatalytic activities. Mater. Lett. 2012, 81, 1–4. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Yang, J.; Wang, X.L.; Feng, F.Y.; Zhang, Y.M.; Tang, Y. Synthesis YFeO3 by salt-assisted solution combustion method and its photocatalytic activity. J. Ceram. Soc. Jpn. 2014, 122, 146–150. [Google Scholar] [CrossRef]
- Lu, X.M.; Xie, J.M.; Shu, H.M.; Liu, J.; Yin, C.Q.; Lin, J.M. Microwave-assisted synthesis of nanocrystalline YFeO3 and study of its photoactivity. Mater. Sci. Eng. B 2007, 138, 289–292. [Google Scholar] [CrossRef]
- Díez-García, M.I.; Celorrio, V.; Calvillo, L.; Tiwari, D.; Gomez, R.; Fermin, D.J. YFeO3 photocathodes for hydrogen evolution. Electrochim. Acta 2017, 246, 365–371. [Google Scholar] [CrossRef]
- Glass, A.M.; von der Linde, D.; Auston, D.H.; Negran, T.J. Excited state polarization, bulk photovoltaic effect and the photorefractive effect in electrically polarized media. J. Electron. Mater. 1975, 4, 915–943. [Google Scholar] [CrossRef]
- Fridkin, V.M. Bulk photovoltaic effect in noncentrosymmetric crystals. Crystallogr. Rep. 2001, 46, 654–658. [Google Scholar] [CrossRef]
- Li, S.; AlOtaibi, B.; Huang, W.; Mi, Z.T.; Serpone, N.; Nechache, R.; Rosei, F. Epitaxial Bi2FeCrO6 multiferroic thin film as a new visible light absorbing photocathode material. Small 2015, 11, 4018–4026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, C.X.; Dong, W.; Zheng, F.G.; Fang, L.; Su, X.D.; Shen, M.R. Composition dependence of the photochemical reduction of Ag+ by as-grown Pb(ZrxTi1−x)O3 films on indium tin oxide electrode. Appl. Phys. Lett. 2013, 103, 1–4. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cao, D.W.; Zheng, F.G.; Dong, W.; Fang, L.; Su, X.D.; Shen, M.R. Photocathodic behavior of ferroelectric Pb(Zr,Ti)O3 films decorated with silver nanoparticles. Chem. Commun. 2013, 49, 3769–3771. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.R.; Dong, W.; Zheng, F.G.; Fang, L.; Shen, M.R. Enhanced photocathodic behaviors of Pb(Zr0.20Ti0.80)O3 films on Si substrates for hydrogen production. Appl. Phys. Lett. 2015, 106, 1–4. [Google Scholar] [CrossRef]
- Wul, B.; Goldman, J.M. Ferroelectric switching in BaTiO3 ceramics. CR Acad. Sci. URSS 1946, 51, 21. [Google Scholar]
- Kennedy, J.H.; Frese, K.W. Photo-oxidation of water at Barium Titanate electrodes. J. Electrochem. Soc. 1976, 123, 1683–1686. [Google Scholar] [CrossRef]
- Zielinska, B.; Borowiak-Palen, E.; Kalenczuk, R.J. Photocatalytic hydrogen generation over alkaline-earth titanates in the presence of electron donors. Int. J. Hydrogen Energy 2008, 33, 1797–1802. [Google Scholar] [CrossRef]
- Dholam, R.; Patel, N.; Adami, M.; Miotello, A. Hydrogen production by photocatalytic water-splitting using Cr- or Fe-doped TiO2 composite thin films photocatalyst. Int. J. Hydrogen Energy 2009, 39, 5337–5346. [Google Scholar] [CrossRef]
- Toupin, J.; Strub, H.; Kressmann, S.; Boudot, M.; Artero, V.; Laberty-Robert, C. Engineering n-p junction for photoelectrochemical hydrogen production. Phys. Chem. Chem. Phys. 2017, 19, 30675–30682. [Google Scholar] [CrossRef] [PubMed]
- Maruska, H.P.; Ghosh, A.K. Transition-metal dopants for extending the response of titanate photoelectrolysis anodes. Sol. Energy Mater. Sol. Cells 1979, 1, 237–247. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Sharma, D. Fe doped BaTiO3 sensitized by Fe3O4 nanoparticles for improved photoelectrochemical response. Mater. Res. Express 2018, 5, 1–9. [Google Scholar] [CrossRef]
- Upadhyay, S.; Shrivastava, J.; Solanki, A.; Choudhary, S.; Sharma, V.; Kumar, P.; Singh, N.; Satsangi, V.R.; Shrivastav, R.; Waghmare, U.V.; et al. Enhanced photoelectrochemical response of BaTiO3 with Fe doping: Experiments and first-principles analysis. J. Phys. Chem. C 2011, 115, 24373–24380. [Google Scholar] [CrossRef]
- Yang, W.G.; Yu, Y.H.; Starr, M.B.; Yin, X.; Li, Z.D.; Kvit, A.; Wang, S.F.; Zhao, P.; Wang, X.D. Ferroelectric polarization-enhanced photoelectrochemical water splitting in TiO2-BaTiO3 core-shell nanowire photoanodes. Nano Lett. 2015, 15, 7574–7580. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.F.; van de Krol, R. Nature and light dependence of bulk recombination in co-pi-catalyzed BiVO4 photoanodes. J. Phys. Chem. C 2012, 116, 9398–9404. [Google Scholar] [CrossRef]
- Tokunaga, S.; Kato, H.; Kudo, A. Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Ping, Y.; Galli, G.A.; Choi, K.S. Simultaneous enhancements in photon absorption and charge transport of bismuth vanadate photoanodes for solar water splitting. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.C.; Liu, W.; Chen, W.; Chen, W.; Zhou, G.M.; Hsu, P.C.; Zhang, R.F.; Liang, Z.; Fan, S.S.; Zhang, Y.G.; et al. Efficient solar-driven water splitting by nanocone BiVO4-perovskite tandem cells. Sci. Adv. 2016, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.M.; Cai, L.L.; Liu, C.; Cho, I.S.; Lee, C.H.; Weisse, J.M.; Yang, P.D.; Zheng, X.L. Simultaneously efficient light absorption and charge separation in WO3/BiVO4 core/shell nanowire photoanode for photoelectrochemical water oxidation. Nano Lett. 2014, 14, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Asai, T.; Hisatomi, T.; Uemura, J.; Tosa, M.; Shimamura, K.; Kubota, J.; Domen, K.; et al. Nanostructured WO3/BiVO4 photoanodes for efficient photoelectrochemical water splitting. Small 2014, 10, 3692–3699. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef]
- Van, C.N.; Do, T.H.; Chen, J.W.; Tzeng, W.Y.; Tsai, K.A.; Song, H.L.; Liu, H.J.; Lin, Y.C.; Chen, Y.C.; Wu, C.L.; et al. WO3 mesocrystal-assisted photoelectrochemical activity of BiVO4. NPG Asia Mater. 2017, 9. [Google Scholar] [CrossRef]
- Kuang, Y.B.; Jia, Q.X.; Nishiyama, H.; Yamada, T.; Kudo, A.; Domen, K. A front-illuminated nanostructured transparent BiVO4 photoanode for > 2% efficient water splitting. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Song, J.; Cha, J.; Lee, M.G.; Jeong, H.W.; Seo, S.; Yoo, J.A.; Kim, T.L.; Lee, J.; No, H.; Kim, D.H.; et al. Template-engineered epitaxial BiVO4 photoanodes for efficient solar water splitting. J. Mater. Chem. A 2017, 5, 18831–18838. [Google Scholar] [CrossRef]
- Liu, Z.K.; Yan, F. The application of bismuth-based oxides in organic-inorganic hybrid photovoltaic devices. J. Am. Ceram. Soc. 2012, 95, 1944–1948. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.K.; Misra, M. Enhanced photoelectrochemical generation of hydrogen from water by 2,6-dihydroxyantraquinone-functionalized titanium dioxide nanotubes. J. Phys. Chem. C 2007, 111, 11506–11510. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; Misra, M.; Mahajan, V.K.; Raja, K.S. Design of a Highly Efficient Photoelectrolytic Cell for Hydrogen Generation by Water Splitting: Application of TiO2−xCx Nanotubes as a Photoanode and Pt/TiO2 Nanotubes as a Cathode. J. Phys. Chem. C 2007, 111, 8677–8685. [Google Scholar] [CrossRef]
- Ruan, C.M.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Enhanced photo electrochemical-response in highly ordered TiO2 nanotube-arrays anodized in boric acid containing electrolyte. Sol. Energy Mater. Sol. Cells 2006, 90, 1283–1295. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Enhanced Photocleavage of Water Using Titania Nanotube Arrays. Nano Lett. 2005, 5, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Haeni, J.H.; Irvin, P.; Chang, W.; Uecker, R.; Reiche, P.; Li, Y.L.; Choudhury, S.; Tian, W.; Hawley, M.E.; Craigo, B.; et al. Room-temperature ferroelectricity in strained SrTiO3. Nature 2004, 430, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Selloni, A. TiO2/Ferroelectric Heterostructures as Dynamic Polarization-Promoted Catalysts for Photochemical and Electrochemical Oxidation of Water. Phys. Rev. Lett. 2014, 112, 196102. [Google Scholar] [CrossRef] [PubMed]
- Wysmulek, K.; Sar, J.; Osewski, P.; Orlinski, K.; Kolodziejak, K.; Trenczek-Zajac, A.; Radecka, M.; Pawlak, D.A. A SrTiO3-TiO2 eutectic composite as a stable photoanode material for photoelectrochemical hydrogen production. Appl. Catal. B 2017, 206, 538–546. [Google Scholar] [CrossRef]
- Kawasaki, S.; Nakatsuji, K.; Yoshinobu, J.; Komori, F.; Takahashi, R.; Lippmaa, M.; Mase, K.; Kudo, A. Epitaxial Rh-doped SrTiO3 thin film photocathode for water splitting under visible light irradiation. Appl. Phys. Lett. 2012, 101, 1–4. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Chen, T.; Xiong, J.Y.; Wang, T.; Lu, G.X.; Ye, J.H.; Bi, Y.P. Visible-light-driven photoelectrochemical and photocatalytic performances of Cr-doped SrTiO3/TiO2 heterostructured nanotube arrays. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Modak, B.; Ghosh, S.K. Enhancement of Visible Light Photocatalytic Activity of SrTiO3: A Hybrid Density Functional Study. J. Phys. Chem. C 2015, 119, 23503–23514. [Google Scholar] [CrossRef]
- Sangle, A.L.; Singh, S.; Jian, J.; Bajpe, S.R.; Wang, H.Y.; Khare, N.; MacManus-Driscoll, J.L. Very high surface area mesoporous thin films of SrTiO3 grown by pulsed laser deposition and application to efficient photoelectrochemical water splitting. Nano Lett. 2016, 16, 7338–7345. [Google Scholar] [CrossRef] [PubMed]
- Cen, J.J.; Wu, Q.Y.; Yan, D.H.; Tao, J.; Kisslinger, K.; Liu, M.Z.; Orlov, A. Photoelectrochemical water splitting with a SrTiO3:Nb/SrTiO3 n+-n homojunction structure. Phys. Chem. Chem. Phys. 2017, 19, 2760–2767. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, S.; Takahashi, R.; Yamamoto, T.; Kobayashi, M.; Kumigashira, H.; Yoshinobu, J.; Komori, F.; Kudo, A.; Lippmaa, M. Photoelectrochemical water splitting enhanced by self-assembled metal nanopillars embedded in an oxide semiconductor photoelectrode. Nat. Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yu, Y.H.; Yang, H.; German, L.N.; Li, Z.Q.; Chen, J.G.; Yang, W.G.; Huang, L.; Shi, W.M.; Wang, L.J.; et al. Simultaneous Enhancement of Charge Separation and Hole Transportation in a TiO2-SrTiO3 Core-Shell Nanowire Photoelectrochemical System. Adv. Mater. 2017, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Cao, D.W.; Wang, Z.J.; Nasori Wen, L.Y.; Mi, Y.; Lei, Y. Switchable Charge-Transfer in the Photoelectrochemical Energy-Conversion Process of Ferroelectric BiFeO3 Photoelectrodes. Angew. Chem. Int. Ed. 2014, 126, 11027–11031. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Yao, K.; Liang, Y.C. Bulk Photovoltaic Effect at Visible Wavelength in Epitaxial Ferroelectric BiFeO3 Thin Films. Adv. Mater. 2010, 22, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Chaudhuri, A.R.; Kim, Y.H.; Hesse, D.; Alexe, M. Role of domain walls in the abnormal photovoltaic effect in BiFeO3. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Jun, H.; Im, B.; Kim, J.Y.; Im, Y.O.; Jang, J.W.; Kim, E.S.; Kim, J.Y.; Kang, H.J.; Hong, S.J.; Lee, J.S. Photoelectrochemical water splitting over ordered honeycomb hematite electrodes stabilized by alumina shielding. Energy Environ. Sci. 2012, 5, 6375–6382. [Google Scholar] [CrossRef]
- Kim, J.Y.; Magesh, G.; Youn, D.H.; Jang, J.W.; Kubota, J.; Domen, K.; Lee, J.S. Single-crystalline, wormlike hematite photoanodes for efficient solar water splitting. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Ma, Y.H.; Oleynikov, P.; Domen, K.; Delaunay, J.J. A conductive ZnO–ZnGaON nanowire-array-on-a-film photoanode for stable and efficient sunlight water splitting. Energy Environ. Sci. 2014, 7, 1693–1699. [Google Scholar] [CrossRef]
- Hou, Y.; Zuo, F.; Dagg, A.; Feng, P.Y. A Three-Dimensional Branched Cobalt-Doped α-Fe2O3 Nanorod/MgFe2O4 Heterojunction Array as a Flexible Photoanode for Efficient Photoelectrochemical Water Oxidation. Angew. Chem. Int. Ed. 2013, 52, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Rao, K.S.R.K. Physics and chemistry of CdTe/CdS thin film heterojunction photovoltaic devices: Fundamental and critical aspects. Energy Environ. Sci. 2014, 7, 45–102. [Google Scholar] [CrossRef]
- Wang, M.Y.; Sun, L.; Lin, Z.Q.; Cai, J.H.; Xie, K.P.; Lin, C.J. p–n Heterojunction photoelectrodes composed of Cu2O-loaded TiO2 nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities. Energy Environ. Sci. 2013, 6, 1211–1220. [Google Scholar] [CrossRef]
- Jang, J.S.; Hwang, D.W.; Lee, J.S. CdS-AgGaS2 photocatalytic diodes for hydrogen production from aqueous Na2S/Na2SO3 electrolyte solution under visible light (lambda ≥ 420 nm). Catal. Today 2007, 120, 174–181. [Google Scholar] [CrossRef]
- Jang, J.S.; Ahn, C.W.; Won, S.S.; Kim, J.H.; Choi, W.; Lee, B.S.; Yoon, J.H.; Kim, H.G.; Lee, J.S. Vertically Aligned Core–Shell PbTiO3@TiO2 Heterojunction Nanotube Array for Photoelectrochemical and Photocatalytic Applications. J. Phys. Chem. C 2017, 121, 15063–15070. [Google Scholar] [CrossRef]
- Ahn, C.W.; Borse, P.H.; Kim, J.H.; Kim, J.Y.; Jang, J.S.; Cho, C.R.; Yoon, J.H.; Lee, B.S.; Bae, J.S.; Kim, H.G.; et al. Effective charge separation in site-isolated Pt-nanodot deposited PbTiO3 nanotube arrays for enhanced photoelectrochemical water splitting. Appl. Catal. B 2018, 224, 804–809. [Google Scholar] [CrossRef]
- Li, C.M.; Ahmed, T.; Ma, M.G.; Edvinsson, T.; Zhu, J.F. A facile approach to Zno/Cds nanoarrays and their photocatalytic and photoelectrochemical properties. Appl. Catal. B 2013, 138, 175–183. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Wang, B.; Li, Y.B.; Liu, Z.C.; Han, J.H.; Guo, K.Y.; Li, Y.J.; Cui, T.; Han, L.; et al. PEC electrode of ZnO nanorods sensitized by CdS with different size and its photoelectric properties. Int. J. Hydrogen Energy 2013, 38, 10226–10234. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, W.W.; Xu, R.; Shi, Y.M.; Zhang, B. Synthesis of ultrathin CdS nanosheets as efficient visible-light-driven water splitting photocatalysts for hydrogen evolution. Chem. Commun. 2013, 49, 9803–9805. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Purbia, R.; Paik, P.; Hebalkar, N.Y.; Kim, H.G.; Borse, P.H. Stabilizing effect in nano-titania functionalized CdS photoanode for sustained hydrogen generation. Int. J. Hydrogen Energy 2014, 39, 4170–4180. [Google Scholar] [CrossRef]
- Pareek, A.; Paik, P.; Borse, P.H. Nanoniobia Modification of CdS Photoanode for an Efficient and Stable Photoelectrochemical Cell. Langmuir 2014, 30, 15540–15549. [Google Scholar] [CrossRef] [PubMed]
- Han, S.C.; Pu, Y.C.; Zheng, L.X.; Hu, L.F.; Zhang, J.Z.; Fang, X.S. Uniform carbon-coated CdS core–shell nanostructures: Synthesis, ultrafast charge carrier dynamics, and photoelectrochemical water splitting. J. Mater. Chem. A 2016, 4, 1078–1086. [Google Scholar] [CrossRef]
- Zhao, K.; Yan, X.Q.; Gu, Y.S.; Kang, Z.; Bai, Z.M.; Cao, S.Y.; Liu, Y.C.; Zhang, X.H.; Zhang, Y. Self-Powered Photoelectrochemical Biosensor Based on CdS/RGO/ZnO Nanowire Array Heterostructure. Small 2016, 12, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.M.; Yang, X.Y.; Qian, F.; Zhang, J.Z.; Li, Y. Double-Sided CdS and CdSe Quantum Dot Co-Sensitized ZnO Nanowire Arrays for Photoelectrochemical Hydrogen Generation. Nano Lett. 2010, 10, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.Y.; Chen, Z.Y.; Li, W.B.; Yu, J.Q. High-efficiency photoelectrochemical properties by a highly crystalline CdS-sensitized ZnO nanorod array. ACS Appl. Mater. Interfaces 2013, 5, 5097–5104. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.B.; Xiao, Z.G.; Yang, B.; Huang, J.S. Arising applications of ferroelectric materials in photovoltaic devices. J. Mater. Chem. A 2014, 2, 6027–6041. [Google Scholar] [CrossRef]
- Wei, R.B.; Kuang, P.Y.; Cheng, H.; Chen, Y.B.; Long, J.Y.; Zhang, M.Y.; Liu, Z.Q. Plasmon-Enhanced Photoelectrochemical Water Splitting on Gold Nanoparticle Decorated ZnO/CdS Nanotube Arrays. ACS Sustain. Chem. Eng. 2017, 5, 4249–4257. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, J.J.; Wei, H.X.; Lee, S.T.; Li, Y.Q.; Tang, J.X. Facile approaching hierarchical CdS films as electrode toward photoelectrochemical water splitting. Nanotechnology 2015, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Gao, C.T.; Wu, Z.M.; Han, W.H.; Wang, Y.L.; Fu, W.B.; Li, X.D.; Xie, E.Q. Toward efficient photoelectrochemical water-splitting by using screw-like SnO2 nanostructures as photoanode after being decorated with CdS quantum dots. Nano Energy 2016, 19, 318–327. [Google Scholar] [CrossRef]
- Tran, T.K.; Park, W.; Tong, W.; Kyi, M.M.; Wagner, B.K.; Summers, C.J. Photoluminescence properties of ZnS epilayers. J. Appl. Phys. 1997, 81, 2803–2809. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J.G.; Jaroniec, M.; Gong, J.R. Noble Metal-Free Reduced Graphene Oxide-ZnxCd1−xS Nanocomposite with Enhanced Solar Photocatalytic H2-Production Performance. Nano Lett. 2012, 12, 4584–4589. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.P.; Zhang, J.; Wang, X.; Wang, Y.J.; Lin, Z.; Yu, J.G.; Huang, F. Influence of lattice integrity and phase composition on the photocatalytic hydrogen production efficiency of ZnS nanomaterials. Nanoscale 2012, 4, 2859–2862. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.H.; Meier, K. Photochemical production of hydrogen with Zinc Sulfide suspensions. J. Phys. Chem. 1984, 88, 5903–5913. [Google Scholar]
- Hu, J.S.; Ren, L.L.; Guo, Y.G.; Liang, H.P.; Cao, A.M.; Wan, L.J.; Bai, C.L. Mass Production and High Photocatalytic Activity of ZnS Nanoporous Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.C.; Barral, S.; Fendler, J.H. Dihexadecyl Phosphate, Vesicle-Stabilized and in Situ Generated Mixed CdS and ZnS Semiconductor Particles. Preparation and Utilization for Photosensitized Charge Separation and Hydrogen Generation. J. Phys. Chem. 1988, 92, 6320–6327. [Google Scholar] [CrossRef]
- Kudo, A.; Sekizawa, M. Photocatalytic H2 evolution under visible light irradiation on Zn1−xCuxS solid solution. Catal. Lett. 1999, 58, 241–243. [Google Scholar] [CrossRef]
- Kudo, A.; Sekizawa, M. Photocatalytic H2 evolution under visible light irradiation on Ni-doped ZnS photocatalyst. Chem. Commun. 2000, 0, 1371–1372. [Google Scholar] [CrossRef]
- Hassan, M.A.; Kang, J.H.; Johar, M.A.; Ha, J.S.; Ryu, S.W. High-performance ZnS/GaN heterostructure photoanode for photoelectrochemical water splitting applications. Acta Mater. 2018, 146, 171–175. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Disdier, J.; Pichat, P. Effect of chromium doping on the electrical and catalytic properties of powder titania under UV and visible illumination. Chem. Phys. Lett. 1984, 108, 618–622. [Google Scholar] [CrossRef]
- Kaneva, N.V.; Dimitrov, D.T.; Dushkin, C.D. Effect of nickel doping on the photocatalytic activity of ZnO thin films under UV and visible light. Appl. Surf. Sci. 2011, 257, 8113–8120. [Google Scholar] [CrossRef]
- Bellam, J.B.; Ruiz-Preciado, M.A.; Edely, M.; Szade, J.; Jouanneaux, A.; Kassiba, A.H. Visible-light photocatalytic activity of nitrogen-doped NiTiO3 thin films prepared by a co-sputtering process. RSC Adv. 2015, 5, 10551–10559. [Google Scholar] [CrossRef]
- Hajjaji, A.; Trabelsi, K.; Atyaoui, A.; Gaidi, M.; Bousselmi, L.; Bessais, B.; El Khakani, M.A. Photocatalytic activity of Cr-doped TiO2 nanoparticles deposited on porous multicrystalline silicon films. Nanoscale Res. Lett. 2014, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kurnia, F.; Ng, Y.H.; Amal, R.; Valanoor, N.; Hart, J.N. Defect engineering of ZnS thin films for photoelectrochemical water-splitting under visible light. Sol. Energy Mater. Sol. Cells C 2016, 153, 179–185. [Google Scholar] [CrossRef]
- Rai, S.C.; Wang, K.; Ding, Y.; Marmon, J.K.; Bhatt, M.; Zhang, Y.; Zhou, W.L.; Wang, Z.L. Piezo-phototronic Effect Enhanced UV/Visible Photodetector Based on Fully Wide Band Gap Type-II ZnO/ZnS Core/Shell Nanowire Array. ACS Nano 2015, 9, 6419–6427. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Qiu, Y.Y.; Wang, F.; Li, L.Z.; Liang, Q.; Chen, Z.D. Electrodeposition of ZnO nanoflake-based photoanode sensitized by carbon quantum dots for photoelectrochemical water oxidation. Ceram. Int. 2017, 43, 5329–5333. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, C.; Zhai, T.; Li, S.L.; Wang, X.; Liu, J.W.; Jie, X.; Liu, D.Q.; Liao, M.Y.; Koide, Y.S.; et al. Flexible Ultraviolet Photodetectors with Broad Photoresponse Based on Branched ZnS-ZnO Heterostructure Nanofilms. Adv. Mater. 2014, 26, 3088–3093. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Chen, C.X.; Qin, H.Y.; Zhang, L.; Fang, Y.T.; Liu, J.B.; Meng, L. Construction of FeS2-Sensitized ZnO@ZnS Nanorod Arrays with Enhanced Optical and Photoresponse Performances. Adv. Mater. Interfaces 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Tian, W.; Zhai, T.Y.; Zhang, C.; Li, S.L.; Wang, X.; Liu, F.; Liu, D.Q.; Cai, X.K.; Tsukagoshi, K.; Golberg, D.; et al. Low-Cost Fully Transparent Ultraviolet Photodetectors Based on Electrospun ZnO-SnO2 Heterojunction Nanofibers. Adv. Mater. 2013, 25, 4625–4630. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Aslam, M. Zns shielded ZnO nanowire photoanodes for efficient water splitting. Electrochim. Acta 2014, 130, 222–231. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, P.; Ding, Y.; Cai, W.; Xie, S.; Wang, Z.L. Mismatch strain induced formation of ZnO/ZnS heterostructured rings. Adv. Mater. 2007, 19, 2319–2323. [Google Scholar] [CrossRef]
- Sánchez-Tovar, R.; Fernández-Domene, R.M.; Montañés, M.T.; Sanz-Marco, A.; Garcia-Antón, J. ZnO/ZnS heterostructures for hydrogen production by photoelectrochemical water splitting. RSC Adv. 2016, 6, 30425–30435. [Google Scholar] [CrossRef]
- Liu, Y.C.; Gu, Y.S.; Yan, X.Q.; Kang, Z.; Lu, S.N.; Sun, Y.H.; Zhang, Y. Design of sandwich-structured ZnO/ZnS/Au photoanode for enhanced efficiency of photoelectrochemical water splitting. Nano Res. 2015, 8, 2891–2900. [Google Scholar] [CrossRef]
- Liu, C.H.; Qiu, Y.Y.; Wang, F.; Wang, K.; Liang, Q.; Chen, Z.D. Design of Core–Shell-Structured ZnO/ZnS Hybridized with Graphite-Like C3N4 for Highly Efficient Photoelectrochemical Water Splitting. Adv. Mater. Interfaces 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Huang, J.D.; Liu, J.Y.; Han, K.L. Hybrid functionals studies of structural and electronic properties of ZnxCd((1−x))S and (ZnxCd1−x)(SexS1−x) solid solution photocatalysts. Int. J. Hydrogen Energy 2012, 37, 17870–17881. [Google Scholar] [CrossRef]
- Jang, G.G.; Jacobs, C.B.; Ivanov, I.N.; Joshi, P.C.; Iii, H.M.M.; Kidder, M.; Armstrong, B.L.; Datskos, P.G.; Graham, D.E.; Moon, J.W. In situ capping for size control of monochalcogenide (ZnS, CdS and SnS) nanocrystals produced by anaerobic metal-reducing bacteria. Nanotechnology 2015, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Qutub, N.; Pirzada, B.M.; Umar, K.; Mehraj, O.; Muneer, M.; Sabir, S. Synthesis, characterization and visible-light driven photocatalysis by differently structured CdS/ZnS sandwich and core-shell nanocomposites. Physica E 2015, 74, 74–86. [Google Scholar] [CrossRef]
- Xu, J.Y. Preparation of ZnS-CdS nanocomposite for photoelectrochemical hydrogen production. Int. J. Electrochem. Sci. 2017, 12, 2253–2261. [Google Scholar] [CrossRef]
- Zhang, P.; Guan, B.Y.; Yu, L.; Lou, X.W. Facile Synthesis of Multi-shelled ZnS-CdS Cages with Enhanced Photoelectrochemical Performance for Solar Energy Conversion. CHEM 2018, 4, 162–173. [Google Scholar] [CrossRef]
- Im, J.H.; Jang, I.H.; Pellet, N.; Gratzel, M.; Park, N.G. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat. Nanotechnol. 2014, 9, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Mei, A.; Li, X.; Liu, L.F.; Ku, Z.L.; Liu, T.F.; Rong, Y.G.; Xu, M.; Hu, M.; Chen, J.Z.; Yang, Y.; et al. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, C.W.; Deng, X.L.; Wang, Y.Q.; Ni, L.L. High-crystallinity and large-grain CH3NH3PbI3 thin films for efficient TiO2 nanorod. IET Micro Nano Lett. 2018, 13, 131–134. [Google Scholar] [CrossRef]
- Dong, Q.F.; Fang, Y.J.; Shao, Y.C.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J.S. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef] [PubMed]
- NREL. Available online: https://www.nrel.gov/pv/assets/images/efficiency-chart.png 2016 (accessed on 4 July 2017).
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; van Schilfgaarde, M.; Walsh, A. Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef] [PubMed]
- Baikie, T.; Fang, Y.N.; Kadro, J.M.; Schreyer, M.; Wei, F.X.; Mhaisalkar, S.G.; Graetzel, M.; White, T.J. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J. Mater. Chem. A 2013, 1, 5628–5641. [Google Scholar] [CrossRef]
- Luo, J.S.; Im, J.H.; Mayer, M.T.; Schreier, M.; Nazeeruddin, M.K.; Park, N.G.; Tilley, S.D.; Fan, H.J.; Gratzel, M. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 2014, 345, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Da, P.M.; Cha, M.Y.; Sun, L.; Wu, Y.Z.; Wang, Z.S.; Zheng, G.F. High-performance perovskite photoanode enabled by Ni passivation and catalysis. Nano Lett. 2015, 15, 3452–3457. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Nguyen, N.T.; Bark, C.W. Ferroelectric Materials: A Novel Pathway for Efficient Solar Water Splitting. Appl. Sci. 2018, 8, 1526. https://doi.org/10.3390/app8091526
Kim S, Nguyen NT, Bark CW. Ferroelectric Materials: A Novel Pathway for Efficient Solar Water Splitting. Applied Sciences. 2018; 8(9):1526. https://doi.org/10.3390/app8091526
Chicago/Turabian StyleKim, Sangmo, Nguyen Thi Nguyen, and Chung Wung Bark. 2018. "Ferroelectric Materials: A Novel Pathway for Efficient Solar Water Splitting" Applied Sciences 8, no. 9: 1526. https://doi.org/10.3390/app8091526
APA StyleKim, S., Nguyen, N. T., & Bark, C. W. (2018). Ferroelectric Materials: A Novel Pathway for Efficient Solar Water Splitting. Applied Sciences, 8(9), 1526. https://doi.org/10.3390/app8091526





