The Effect of Functional Group Polarity in Palladium Immobilized Multiwalled Carbon Nanotube Catalysis: Application in Carbon–Carbon Coupling Reaction
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of CNT-Metal Nanohybrids
2.3. Catalytic C–C Coupling
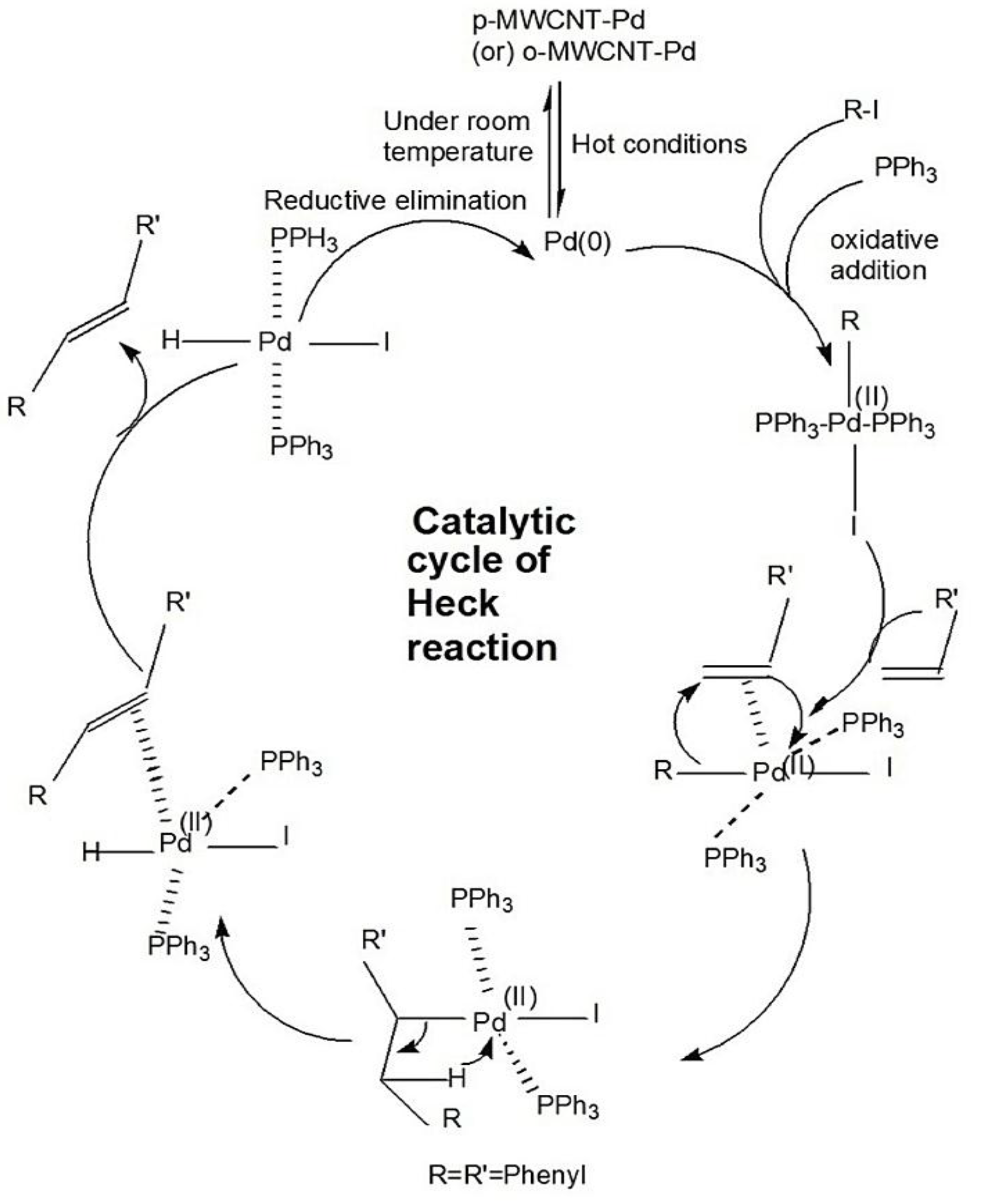
3. Results and Discussion
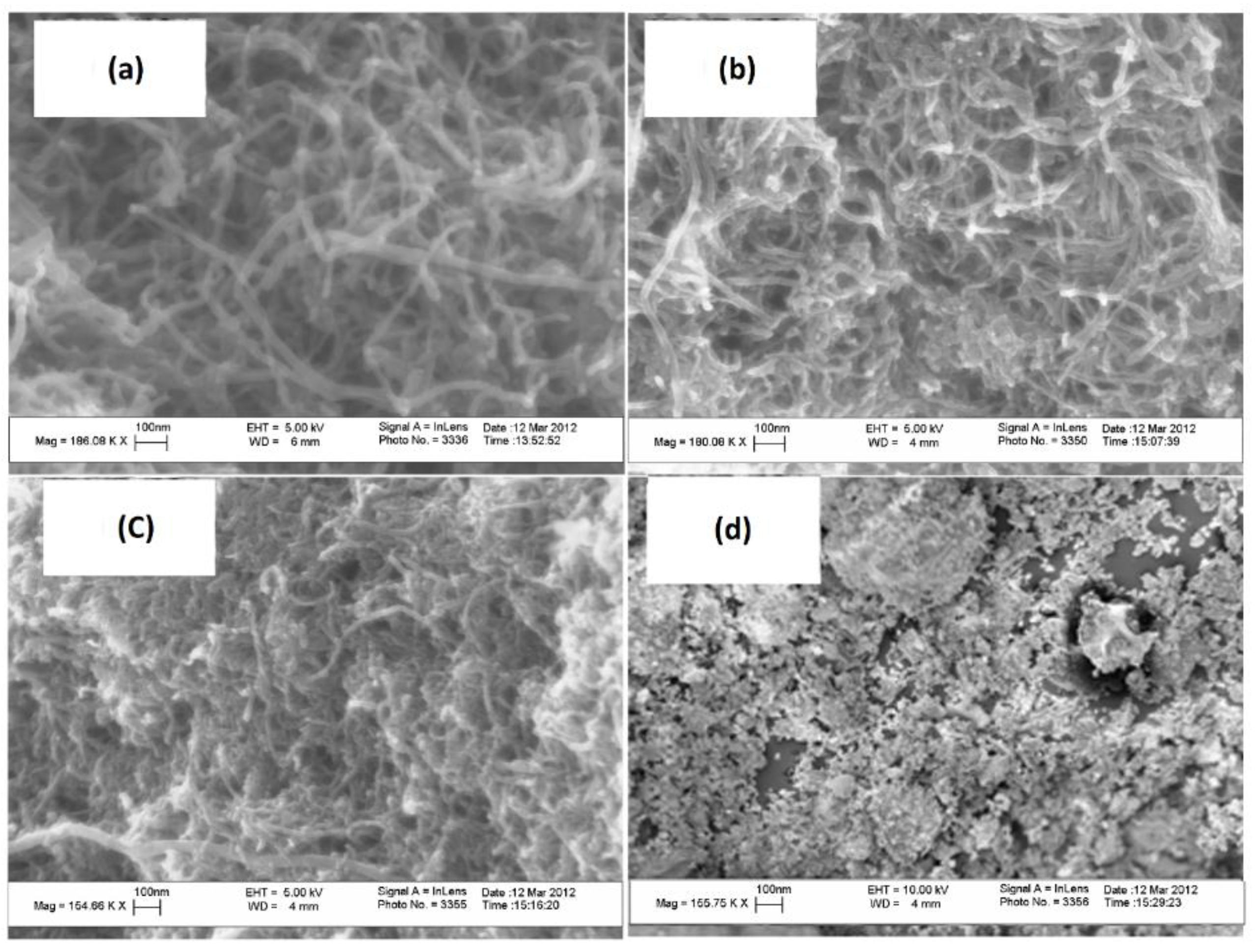
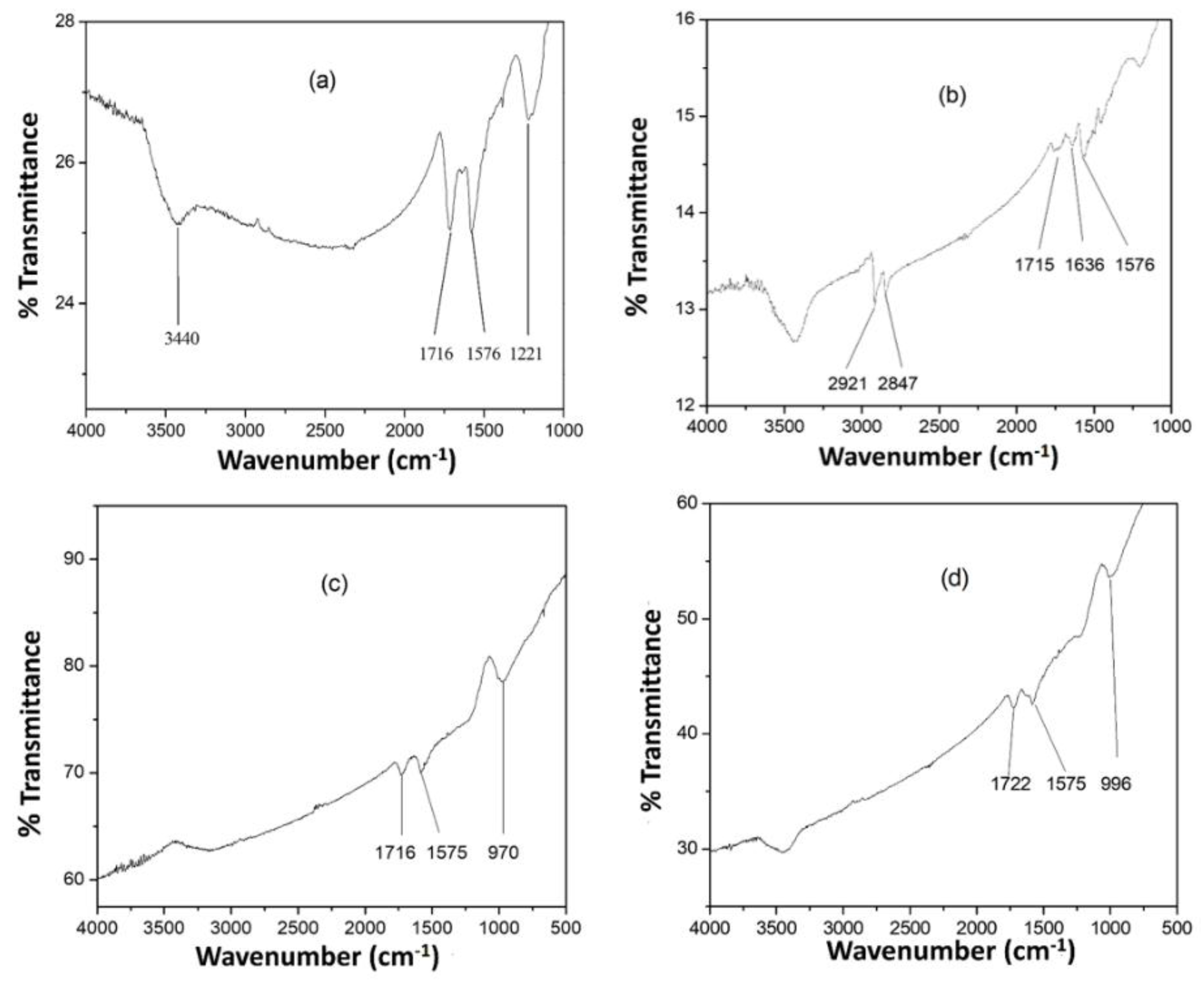
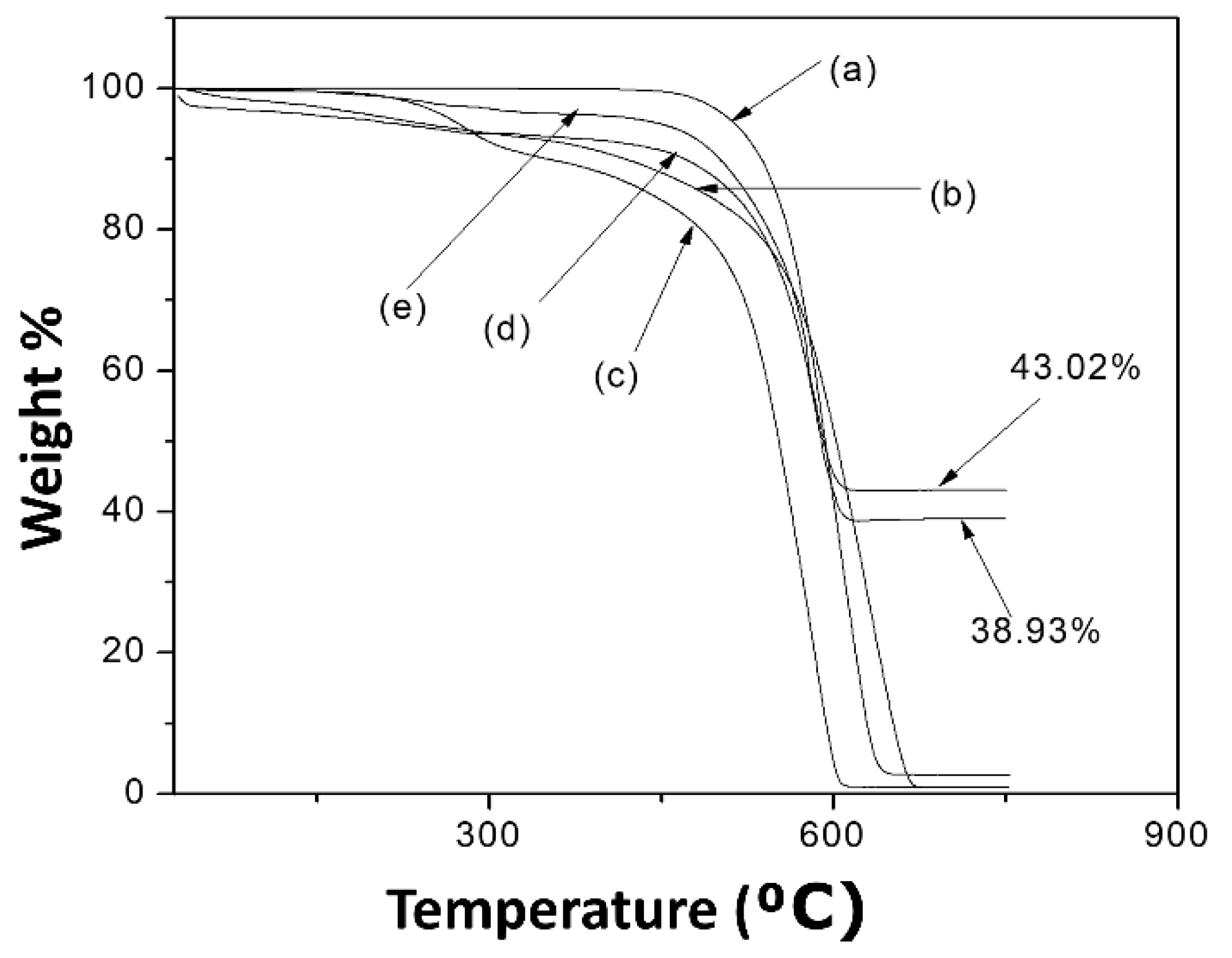
3.1. Nanohybrid Characterization
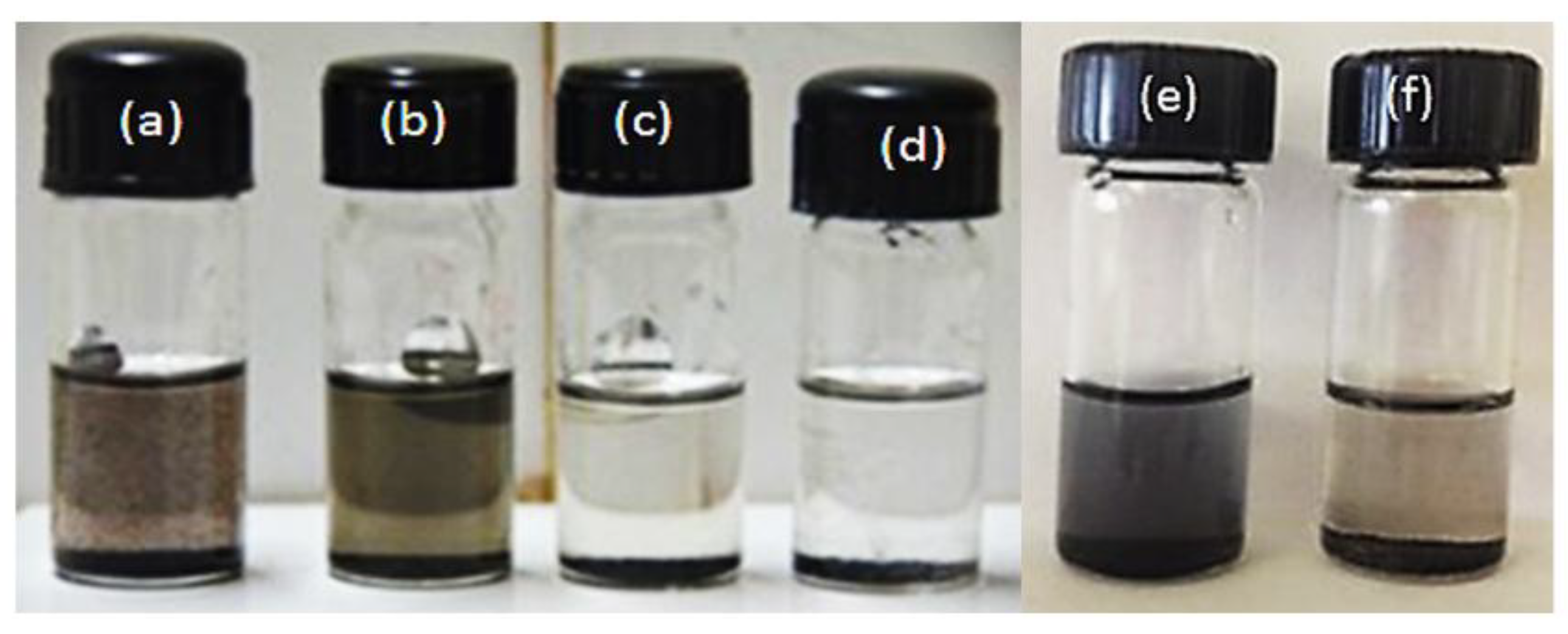
3.2. Dispersibility
3.3. Effect of CNT Functionalization
3.4. Active Metal Requirement and Reusability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, W.; Tu, W.; Liu, H. Synthesis of nanoscale platinum colloids by microwave dielectric heating. Langmuir 1999, 15, 6–9. [Google Scholar] [CrossRef]
- Gong, K.; Chakrabarti, S.; Dai, L. Electrochemistry at carbon nanotube electrodes: Is the nanotube tip more active than the sidewall? Angew. Chem. Int. Ed. 2008, 47, 5446–5450. [Google Scholar] [CrossRef] [PubMed]
- Ovejero, G.; Sotelo, J.; Rodríguez, A.; Díaz, C.; Sanz, R.; García, J. Platinum catalyst on multiwalled carbon nanotubes for the catalytic wet air oxidation of phenol. Ind. Eng. Chem. Res. 2007, 46, 6449–6455. [Google Scholar] [CrossRef]
- Yang, X.; Cao, Y.; Yu, H.; Huang, H.; Wang, H.; Peng, F. Unravelling the radical transition during the carbon-catalyzed oxidation of cyclohexane by in situ electron paramagnetic resonance in the liquid phase. Catal. Sci. Technol. 2017, 7, 4431–4436. [Google Scholar] [CrossRef]
- Iglesias, D.; Senokos, E.; Alemán, B.; Cabana, L.; Navío, C.; Marcilla, R.; Prato, M.; Vilatela, J.J.; Marchesan, S. Gas-phase functionalization of macroscopic carbon nanotube fiber assemblies: Reaction control, electrochemical properties, and use for flexible supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 5760–5770. [Google Scholar] [CrossRef] [PubMed]
- Su, D.S.; Wen, G.; Wu, S.; Peng, F.; Schlögl, R. Carbocatalysis in Liquid-Phase Reactions. Angew. Chem. Int. Ed. 2017, 56, 936–964. [Google Scholar] [CrossRef] [PubMed]
- Lamme, W.S.; Zečević, J.; de Jong, K.P. Influence of Metal Deposition and Activation Method on the Structure and Performance of Carbon Nanotube Supported Palladium Catalysts. ChemCatChem 2018, 10, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.-S.; Jung, D.-H.; Oh, S.-K.; Han, C.-S.; Jung, H.-T. Single-walled carbon nanotube gold nanohybrids: Application in highly effective transparent and conductive films. J. Phys. Chem. C 2007, 111, 8377–8382. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Star, A. Chemically induced potential barriers at the carbon nanotube-metal nanoparticle interface. Nano Lett. 2007, 7, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, T.; Qu, X.; Dong, S. In situ synthesis and characterization of multiwalled carbon nanotube/Au nanoparticle composite materials. J. Phys. Chem. B 2006, 110, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, R.; Yuet, P.K. Easy decoration of carbon nanotubes with well dispersed gold nanoparticles and the use of the material as an electrocatalyst. Carbon 2009, 47, 1146–1151. [Google Scholar] [CrossRef]
- Hou, X.; Wang, L.; Zhou, F.; Wang, F. High-density attachment of gold nanoparticles on functionalized multiwalled carbon nanotubes using ion exchange. Carbon 2009, 47, 1209–1213. [Google Scholar] [CrossRef]
- Tzitzios, V.; Georgakilas, V.; Oikonomou, E.; Karakassides, M.; Petridis, D. Synthesis and characterization of carbon nanotube/metal nanoparticle composites well dispersed in organic media. Carbon 2006, 44, 848–853. [Google Scholar] [CrossRef]
- Zhang, M.; Su, L.; Mao, L. Surfactant functionalization of carbon nanotubes (CNTs) for layer-by-layer assembling of CNT multi-layer films and fabrication of gold nanoparticle/CNT nanohybrid. Carbon 2006, 44, 276–283. [Google Scholar] [CrossRef]
- Yoon, B.; Wai, C.M. Microemulsion-Templated Synthesis of Carbon Nanotube-Supported Pd and Rh Nanoparticles for Catalytic Applications. J. Am. Chem. Soc. 2005, 127, 17174–17175. [Google Scholar] [CrossRef] [PubMed]
- Planeix, J.; Coustel, N.; Coq, B.; Brotons, V.; Kumbhar, P.; Dutartre, R.; Geneste, P.; Bernier, P.; Ajayan, P. Application of carbon nanotubes as supports in heterogeneous catalysis. J. Am. Chem. Soc. 1994, 116, 7935–7936. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H.; Leyva, A. Catalytic activity of palladium supported on single wall carbon nanotubes compared to palladium supported on activated carbon: Study of the Heck and Suzuki couplings, aerobic alcohol oxidation and selective hydrogenation. J. Mol. Catal. A Chem. 2005, 230, 97–105. [Google Scholar] [CrossRef]
- Guo, D.-J.; Li, H.-L. High dispersion and electrocatalytic properties of palladium nanoparticles on single-walled carbon nanotubes. J. Colloid Interface Sci. 2005, 286, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.; Mitra, S. Solvent dispersible nanoplatinum-carbon nanotube hybrids for application in homogeneous catalysis. Chem. Commun. 2010, 46, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Liang, H.; Hu, J.; Jiang, L.; Wan, L. Controllable Pt nanoparticle deposition on carbon nanotubes as an anode catalyst for direct methanol fuel cells. J. Phys. Chem. B 2005, 109, 22212–22216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, F.J.; Chen, M.L.; Oh, W.C. Comparison of catalytic activities for photocatalytic and sonocatalytic degradation of methylene blue in present of anatase TiO2–CNT catalysts. Ultrason. Sonochem. 2011, 18, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, P. CNT Applications in Microelectronics, Nanoelectronics, and Nanobioelectronics. In Conducting Polymers, Fundamentals and Applications; Springer: Berlin, Germany, 2018; pp. 65–72. [Google Scholar]
- Karakoti, M. Surface Modification of Carbon-Based Nanomaterials for Polymer Nanocomposites. In Carbon-Based Polymer Nanocomposites for Environmental and Energy Applications; Ismail, A.F., Goh, P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 27–56. [Google Scholar]
- Lordi, V.; Yao, N.; Wei, J. Method for supporting platinum on single-walled carbon nanotubes for a selective hydrogenation catalyst. Chem. Mater. 2001, 13, 733–737. [Google Scholar] [CrossRef]
- Ellis, A.V.; Vijayamohanan, K.; Goswami, R.; Chakrapani, N.; Ramanathan, L.; Ajayan, P.M.; Ramanath, G. Hydrophobic anchoring of monolayer-protected gold nanoclusters to carbon nanotubes. Nano Lett. 2003, 3, 279–282. [Google Scholar] [CrossRef]
- Giordano, R.; Serp, P.; Kalck, P.; Kihn, Y.; Schreiber, J.; Marhic, C.; Duvail, J.L. Preparation of rhodium catalysts supported on carbon nanotubes by a surface mediated organometallic reaction. Eur. J. Inorg. Chem. 2003, 2003, 610–617. [Google Scholar] [CrossRef]
- Haremza, J.M.; Hahn, M.A.; Krauss, T.D.; Chen, S.; Calcines, J. Attachment of single CdSe nanocrystals to individual single-walled carbon nanotubes. Nano Lett. 2002, 2, 1253–1258. [Google Scholar] [CrossRef]
- Azamian, B.R.; Coleman, K.S.; Davis, J.J.; Hanson, N.; Green, M.L. Directly observed covalent coupling of quantum dots to single-wall carbon nanotubes. Chem. Commun. 2002, 366–367. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, X.; Lee, J.Y.; Zhang, W.; Han, M.; Gan, L.M. Preparation and characterization of platinum-based electrocatalysts on multiwalled carbon nanotubes for proton exchange membrane fuel cells. Langmuir 2002, 18, 4054–4060. [Google Scholar] [CrossRef]
- Cano, M.; Benito, A.; Maser, W.K.; Urriolabeitia, E.P. One-step microwave synthesis of palladium–carbon nanotube hybrids with improved catalytic performance. Carbon 2011, 49, 652–658. [Google Scholar] [CrossRef]
- Trusova, M.E.; Rodriguez-Zubiri, M.; Kutonova, K.V.; Jung, N.; Bräse, S.; Felpin, F.X.; Postnikov, P.S. Ultra-fast Suzuki and Heck reactions for the synthesis of styrenes and stilbenes using arenediazonium salts as super-electrophiles. Org. Chem. Front. 2018, 5, 41–45. [Google Scholar] [CrossRef]
- Ichikawa, T.; Mizuno, M.; Ueda, S.; Ohneda, N.; Odajima, H.; Sawama, Y.; Monguchi, Y.; Sajiki, H. A practical method for heterogeneously-catalyzed Mizoroki–Heck reaction: Flow system with adjustment of microwave resonance as an energy source. Tetrahedron 2018, 74, 1810–1816. [Google Scholar] [CrossRef]
- Díaz-Ortiz, Á.; Prieto, P.; Vázquez, E. Heck reactions under microwave irradiation in solvent-free conditions. Synlett 1997, 3, 269–270. [Google Scholar] [CrossRef]
- Christoffel, F.; Ward, T.R. Palladium-catalyzed Heck cross-coupling reactions in water: A comprehensive review. Catal. Lett. 2018, 148, 489–511. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, X.; Qiu, J. Aerobic oxidation of alcohols over carbon nanotube-supported Ru catalysts assembled at the interfaces of emulsion droplets. Appl. Catal. A 2010, 382, 131–137. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Manas, M.; Pleixats, R. Formation of carbon-carbon bonds under catalysis by transition-metal nanoparticles. Acc. Chem. Res. 2003, 36, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Corain, B.; Kralik, M. Generating palladium nanoclusters inside functional cross-linked polymer frameworks. J. Mol. Catal. A Chem. 2001, 173, 99–115. [Google Scholar] [CrossRef]
- Li, W.; Liang, C.; Qiu, J.; Zhou, W.; Han, H.; Wei, Z.; Sun, G.; Xin, Q. Carbon nanotubes as support for cathode catalyst of a direct methanol fuel cell. Carbon 2002, 40, 791–794. [Google Scholar] [CrossRef]
- Li, W.; Liang, C.; Zhou, W.; Qiu, J.; Zhou, Z.; Sun, G.; Xin, Q. Preparation and characterization of multiwalled carbon nanotube-supported platinum for cathode catalysts of direct methanol fuel cells. J. Phys. Chem. B 2003, 107, 6292–6299. [Google Scholar] [CrossRef]
- Chen, Y.; Mitra, S. Fast Microwave-Assisted Purification, Functionalization and Dispersion of Multi-Walled Carbon Nanotubes. J. Nanosci. Nanotechnol. 2008, 8, 5770–5775. [Google Scholar] [CrossRef] [PubMed]
- Desai, C.; Addo Ntim, S.; Mitra, S. Antisolvent precipitation of hydrophobic functionalized multiwall carbon nanotubes in an aqueous environment. J. Colloid Interface Sci. 2012, 368, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, O.H.; Pabon, H.N.B. Synthesis of Stilbenes. A Comparative Study1. J. Org. Chem. 1965, 30, 1473–1477. [Google Scholar] [CrossRef]
- Sermon, P.; Bond, G. Hydrogen spillover. Catal. Rev. 1974, 8, 211–239. [Google Scholar] [CrossRef]
- Sun, W.; Liu, Z.; Jiang, C.; Xue, Y.; Chu, W.; Zhao, X. Experimental and theoretical investigation on the interaction between palladium nanoparticles and functionalized carbon nanotubes for Heck synthesis. Catal. Today 2013, 212, 206–214. [Google Scholar] [CrossRef]
- Bond, G.C. A Short History of Hydrogen Spillover. In Studies in Surface Science and Catalysis; Pajonk, G.M.S.J.T., Germain, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 1983; pp. 1–16. [Google Scholar]

| Samples/Solvents | p-MWCNT-Pd | np-MWCNT-Pd |
|---|---|---|
| Toluene | 240.3 nm | 234.2 nm |
| Dimethylformamide (DMF) | 168.2 nm | 126.4 nm |
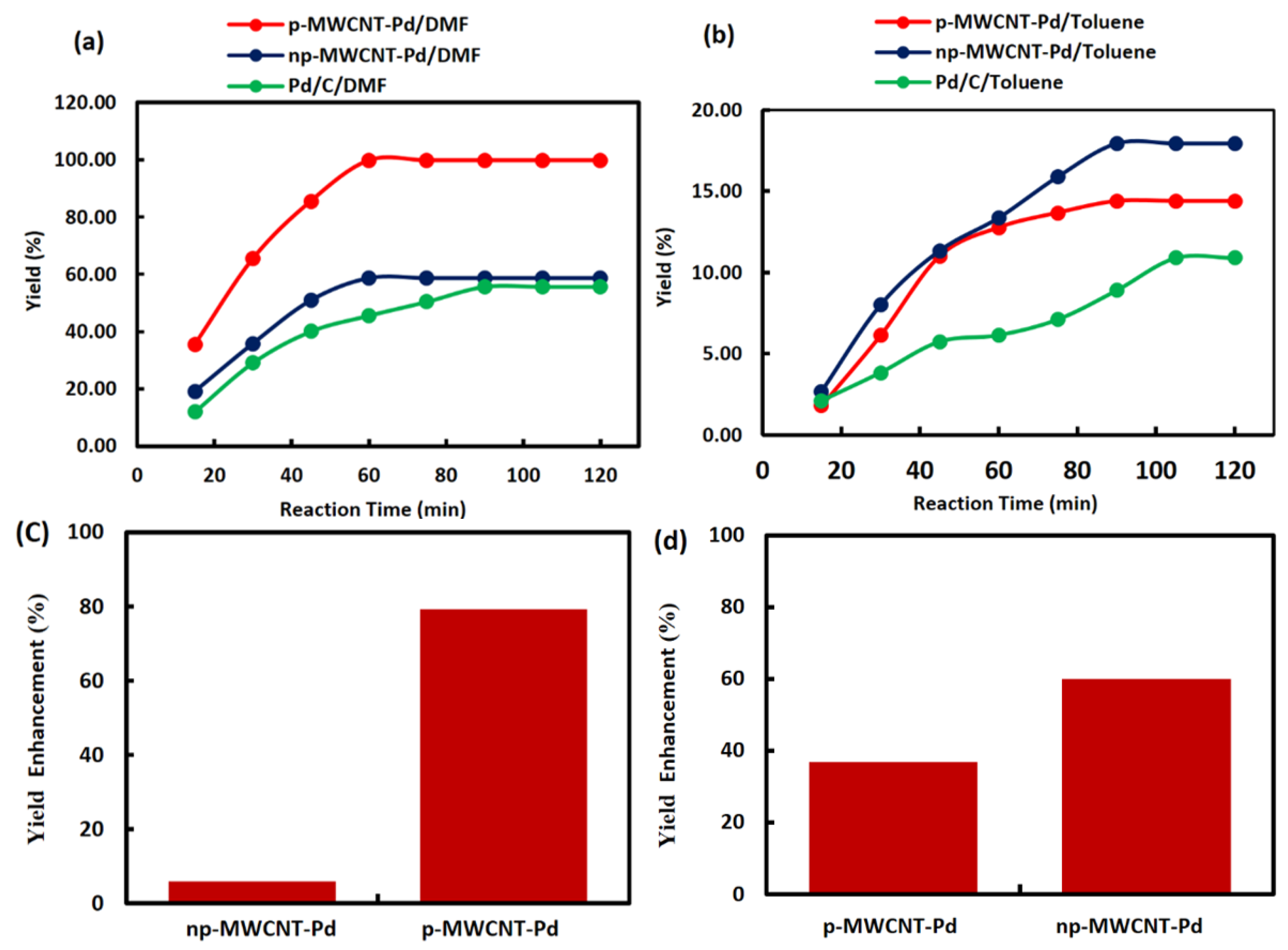
| S.No. | Catalyst | Reaction Completion Time (min) | % Yield | Yield Enhancement |
|---|---|---|---|---|
| Dimethylformamide (DMF) as solvent | ||||
| 1 | Pd/C | 90 | 55.70 | - |
| 2 | np-MWCNT-Pd | 60 | 58.70 | 5% |
| 3 | p-MWCNT-Pd | 60 | 99.78 | 79% |
| 4 | PdCl2 | 120 | 45.67 | - |
| Toluene as solvent | ||||
| 5 | Pd/C | 105 | 10.90 | - |
| 6 | p-MWCNT-Pd | 90 | 14.39 | 32% |
| 7 | np-MWCNT-Pd | 90 | 17.44 | 60% |
| Microwave (solvent free) | ||||
| 8 | p-MWCNT-Pd | 17 | 90.01 | - |
| 9 | np-MWCNT-Pd | 17 | 66.92 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra, B.P.; Wu, Z.; Ntim, S.A.; Rao, G.N.; Mitra, S. The Effect of Functional Group Polarity in Palladium Immobilized Multiwalled Carbon Nanotube Catalysis: Application in Carbon–Carbon Coupling Reaction. Appl. Sci. 2018, 8, 1511. https://doi.org/10.3390/app8091511
Chandra BP, Wu Z, Ntim SA, Rao GN, Mitra S. The Effect of Functional Group Polarity in Palladium Immobilized Multiwalled Carbon Nanotube Catalysis: Application in Carbon–Carbon Coupling Reaction. Applied Sciences. 2018; 8(9):1511. https://doi.org/10.3390/app8091511
Chicago/Turabian StyleChandra, Boggarapu Praphulla, Zheqiong Wu, Susana Addo Ntim, Golakoti Nageswara Rao, and Somenath Mitra. 2018. "The Effect of Functional Group Polarity in Palladium Immobilized Multiwalled Carbon Nanotube Catalysis: Application in Carbon–Carbon Coupling Reaction" Applied Sciences 8, no. 9: 1511. https://doi.org/10.3390/app8091511
APA StyleChandra, B. P., Wu, Z., Ntim, S. A., Rao, G. N., & Mitra, S. (2018). The Effect of Functional Group Polarity in Palladium Immobilized Multiwalled Carbon Nanotube Catalysis: Application in Carbon–Carbon Coupling Reaction. Applied Sciences, 8(9), 1511. https://doi.org/10.3390/app8091511





