Effects and Mechanisms of Microbial Remediation of Heavy Metals in Soil: A Critical Review
Abstract
1. Introduction
2. Sources and Hazards of Heavy Metal Pollution in Soil
3. Remediation of Heavy Metal Contaminated Soil
4. Microbial Remediation of Heavy Metal-Contaminated Soil
4.1. Remediation Mechanisms
4.1.1. Biosorption
4.1.2. Bioleaching
4.1.3. Plant–Microbial Remediation
4.2. Comparison of Microbial Removal Ability
4.2.1. Microbial Remediation Potential
4.2.2. Adsorption Equilibria
4.2.3. Kinetics of Adsorption
4.2.4. Methods for Microbial Remediation
5. Microbial Remediation of Heavy Metal Pollution in Soil and Water
5.1. Microbial Living Environments
5.1.1. pH
5.1.2. Ambient Temperature
5.1.3. Substrate Species
5.1.4. Substrate Concentration
5.2. Composite Reclamation System
6. Conclusions
- (1)
- The mechanisms of microbial degradation of heavy metals are mainly biosorption, biomineralization, and co-metabolism, and biosorption is the main degradation mechanism. Both biosorption and biomineralization can be divided into a variety of physiological processes.
- (2)
- Microbes have different abilities to degrade heavy metal, and the degradation ability mainly depends on degradative plasmids and spores. Usually, Escherichia coli K–12 adsorb the majority of heavy metal ions, and the adsorption capacity of Pseudomonas and Bacillus are strong.
- (3)
- The optimum pH ranges of microorganisms are various. Most microorganisms have suitable pH values in 5.5–6.5, except for Bacillus jeotgali. Ambient temperature affects the ability of microorganisms to adsorb heavy metals. Although the optimum temperature is related with heavy metal and microbial species, the optimum temperature for most microorganisms is generally between 25 °C and 35 °C.
- (4)
- The difference in concentrations of six heavy metal ions, and the presence or absence of competitive ions will affect the adsorption capacity of heavy metals for organisms.
- (5)
- Composite repair systems, such as microbial plant joint repair systems and chemical microbial joint repair systems, can often improve repair efficiency.
Author Contributions
Funding
Conflicts of Interest
References
- Abd-Alla, M.H.; Morsy, F.M.; El-Enany, A.W.E.; Ohyama, T. Isolation and characterization of a heavy-metal-resistant isolate of Rhizobium leguminosarum bv. viciae potentially applicable for biosorption of Cd2+ and Co2+. Int. Biodeter. Biodegr. 2012, 67, 48–55. [Google Scholar] [CrossRef]
- Li, J.; Yu, H.; Luan, Y. Meta-Analysis of the Copper, Zinc, and Cadmium Absorption Capacities of Aquatic Plants in Heavy Metal-Polluted Water. Int. J. Environ. Res. Public Health 2015, 12, 14958–14973. [Google Scholar] [CrossRef] [PubMed]
- Malidareh, H.B.; Mahvi, A.H.; Yunesian, M.; Alimohammadi, M.; Nazmara, S. Effect of fertilizer application on paddy soil heavy metals concentration and groundwater in North of Iran. Middle-East. J. Sci. Res. 2014, 20, 1721–1727. [Google Scholar]
- Lloyd, J.R. Bioremediation of metals; the application of micro-organisms that make and break minerals. Microbiol. Today 2002, 29, 67–69. [Google Scholar]
- Race, M. Applicability of alkaline precipitation for the recovery of EDDS spent solution. J. Environ. Manag. 2017, 203, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Gazso, G.L. The key microbial processes in the removal of toxic metals and radionuclides from the environment. Cent. Eur. J. Occup. Environ. Med. Hung. 2001, 7, 178–185. [Google Scholar]
- Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Remediation of heavy metal contaminated soils: Phytoremediation as a potentially promising clean-up technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Ranieri, E.; Fratino, U.; Petrella, A.; Torretta, V.; Rada, E.C. Ailanthus Altissima and Phragmites Australis for chromium removal from a contaminated soil. Environ. Sci. Pollut. Res. Int. 2016, 23, 15983–15989. [Google Scholar] [CrossRef] [PubMed]
- Mattuschka, B.; Straube, G. Biosorption of metals by a waste biomass. J. Chem. Technol. Biotechnol. 1993, 58, 57–63. [Google Scholar] [CrossRef]
- Mullen, M.D.; Wolf, D.C.; Ferris, F.G.; Beveridge, T.J.; Flemming, C.A.; Bailey, G.W. Bacterial sorption of heavy metals. Appl. Environ. Microbiol. 1989, 55, 3143–3149. [Google Scholar] [PubMed]
- Jã Rup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Blagodatskaya, E.V.; Pampura, T.V.; Myakshina, T.N.; Dem Yanova, E.G. The influence of lead on the respiration and biomass of microorganisms in gray forest soil in a long-term field experiment. Eurasian Soil Sci. 2006, 39, 498–506. [Google Scholar] [CrossRef]
- Bahadir, T.; Bakan, G.; Altas, L.; Buyukgungor, H. The investigation of lead removal by biosorption: An application at storage battery industry wastewaters. Enzym. Microb. Technol. 2007, 41, 98–102. [Google Scholar] [CrossRef]
- Singh, A.; Prasad, S.M. Remediation of heavy metal contaminated ecosystem: an overview on technology advancement. Int. J. Environ. Sci. Technol. 2015, 12, 353–366. [Google Scholar] [CrossRef]
- Race, M.; Ferraro, A.; Fabbricino, M.; La, A.M.; Panico, A.; Spasiano, D.; Tognacchini, A.; Pirozzi, F. Ethylenediamine-N,N’-Disuccinic Acid (EDDS)-Enhanced Flushing Optimization for Contaminated Agricultural Soil Remediation and Assessment of Prospective Cu and Zn Transport. Int. J. Environ. Res. Public Health 2018, 15, 543. [Google Scholar] [CrossRef] [PubMed]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richerlaflèche, M. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Trellu, C.; Mousset, E.; Pechaud, Y.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Removal of hydrophobic organic pollutants from soil washing/flushing solutions: A critical review. J. Hazard. Mater. 2016, 306, 149–174. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Heavy Metals in Soils, 2nd ed.; Springer: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Jovancicevic, V.; Bockris, J.O.; Carbajal, J.L.; Zelenay, P.; Mizuno, T. ChemInform Abstract: Adsorption and Absorption of Chloride Ions on Passive Iron Systems. ChemInform 1987, 18, 2219–2226. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Danis, U.; Nuhoglu, A.; Demirbas, A. Ferrous ion-oxidizing in Thiobacillus ferrooxidans batch cultures: Influence of pH, temperature and initial concentration of Fe2+. Fresenius Environ. Bull. 2008, 17, 371–377. [Google Scholar]
- Brady, D.; Duncan, J.R. Cation loss during accumulation of heavy metal cations by Saccharomyces cerevisiae. Biotechnol. Lett. 1994, 16, 543–548. [Google Scholar] [CrossRef]
- Sarret, G.; Manceau, A.; Spadini, L.; Roux, J.C.; Hazemann, J.L.; Soldo, Y.; Eybert-Bérard, L.; Menthonnex, J. Structural Determination of Zn and Pb Binding Sites in Penicillium chrysogenum Cell Walls by EXAFS Spectroscopy. Environ. Sci. Technol. 1998, 32, 1648–1655. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Liu, R. Biosorption of heavy metals by bacteria isolated from activated sludge. Appl. Biochem. Biotechnol. 2001, 91–93, 171–184. [Google Scholar]
- He, L.M.; Tebo, B.M. Surface charge properties of and Cu(II) Adsorption by Spores of the Marine Bacillus sp. Strain SG-1. Appl. Environ. Microbiol. 1998, 64, 1123–1129. [Google Scholar] [PubMed]
- Gan, W.J.; Yue, H.E.; Zhang, X.F.; Shan, Y.H.; Zheng, L.P.; Lin, Y.S. Speciation Analysis of Heavy Metals in Soils Polluted by Electroplating and Effect of Washing to the Removal of the Pollutants. J. Ecol. Rural Environ. 2012, 28, 82–87. [Google Scholar]
- Di, P.L.; Ferrantelli, P.; Merli, C.; Biancifiori, F. Recovery of EDTA and metal precipitation from soil flushing solutions. J. Hazard. Mater. 2003, 103, 153–168. [Google Scholar]
- Chen, G. Research progress on chemical restorer of heavy metal contaminated soil. Appl. Chem. Ind. 2017, 16, 722–729. [Google Scholar]
- Long, X.; Yang, X.; Ni, W. Current situation and prospect on the remediation of soils contaminated by heavy metals. Chin. J. Appl. Ecol. 2002, 13, 757–762. [Google Scholar]
- Bautistahernández, D.A.; Ramírezburgos, L.I.; Duranpáramo, E.; Fernándezlinares, L. Zinc and Lead Biosorption by Delftia tsuruhatensis: A Bacterial Strain Resistant to Metals Isolated from Mine Tailings. J. Water Resour. Prot. 2012, 4, 207–216. [Google Scholar] [CrossRef]
- Jiang, X.J.; Luo, Y.M.; Zhao, Q.G.; Wu, S.C.; Wu, L.H.; Qiao, X.L.; Song, J. Phytoremediation of heavy metal-contaminated soils. I. Response of metal accumulator plant Brassica juncea to soil contamination of copper, zinc, cadmium and lead. Soils 2000, 32, 71–74. [Google Scholar]
- Wei, S.; Zhou, Q. Discussion on basic principles and strengthening measures for phytoremediation of soils contaminated by heavy metals. Chin. J. Ecol. 2004, 65–72. [Google Scholar]
- Wang, Q.; Cui, Y.; Dong, Y. Phytoremediation—An effective approach of heavy metal cleanup from contaminated soil. Acta Ecol. Sin. 2001, 21, 326–331. [Google Scholar]
- Bojórquez, C.; Voltolina, D. Removal of cadmium and lead by adapted strains of Pseudomonas aeruginosa and Enterobacter cloacae. Rev. Int. Contam. Ambie. 2016, 32, 407–412. [Google Scholar] [CrossRef]
- Illera, V.; Garrido, F.; Serrano, S.; Garciagonzalez, M.T. Immobilization of the heavy metals Cd, Cu and Pb in an acid soil amended with gypsum- and lime-rich industrial by-products. Eur. J. Soil Sci. 2004, 55, 135–145. [Google Scholar] [CrossRef]
- Cao, R.X.; Ma, L.Q.; Chen, M.; Singh, S.P.; Harris, W.G. Phosphate-induced metal immobilization in a contaminated site. Environ. Pollut. 2003, 122, 19–28. [Google Scholar] [CrossRef]
- Zhou, N. Experimental Study on the Adsorption and Resistance of Heavy Metal Ions by Active Fungi. Master’s Thesis, Hunan University, Changsha, China, 2008. [Google Scholar]
- Wang, F. Hotspots in arbuscular mycorrhiza-assisted phytoremediation of heavy metal-contaminated soils. Ecol. Environ. 2006, 15, 1086–1090. [Google Scholar]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Freitas, O.; Rui, B.; Delerue-Matos, C. Adsorption study of lead by Ascophyllum nodosum using a factorial experimental design. In Combined and Hybrid Adsorbents; Springer: Dordrecht, The Netherlands, 2006; pp. 269–274. [Google Scholar]
- Brunetti, G.; Farrag, K.; Soler-Rovira, P.; Ferrara, M.; Nigro, F.; Senesi, N. The effect of compost and Bacillus licheniformis on the phytoextraction of Cr, Cu, Pb and Zn by three Brassicaceae species from contaminated soils in the Apulia region, Southern Italy. Geoderma 2012, 170, 322–330. [Google Scholar] [CrossRef]
- Chen, X. Studies on bioremediation of arbuscular mycorrhizal fungi in heavy metal and rare earth element conctaminated soil. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2007. [Google Scholar]
- Volesky, B.; Holan, Z.R. Biosorption of heavy metals. Biotechnol. Prog. 1995, 11, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Sathasivam, K.V. Heavy Metal Adsorption onto Kappaphycus sp. from Aqueous Solutions: The Use of Error Functions for Validation of Isotherm and Kinetics Models. Biomed. Res. Int. 2015, 2015, 126298. [Google Scholar] [CrossRef] [PubMed]
- Chanmugathas, P.; Bollag, J.M. A column study of the biological mobilization and speciation of cadmium in soil. Arch. Environ. Contam. Toxicol. 1988, 17, 229–237. [Google Scholar] [CrossRef]
- Marchenko, A.M.; Pshinko, G.N.; Demchenko, V.Y.; Goncharuk, V.V. Leaching heavy metal from deposits of heavy metals with bacteria oxidizing elemental sulphur. J. Water Chem. Technol. 2015, 37, 311–316. [Google Scholar] [CrossRef]
- Gavrilescu, M. Removal of Heavy Metals from the Environment by Biosorption. Eng. Life Sci. 2004, 4, 219–232. [Google Scholar] [CrossRef]
- Goyal, N.; Jain, S.C.; Banerjee, U.C. Comparative studies on the microbial adsorption of heavy metals. Adv. Environ. Res. 2003, 7, 311–319. [Google Scholar] [CrossRef]
- Joner, E.J.; Leyval, C. Uptake of 109Cd by roots and hyphae of a Glomus mosseae/Trifolium subterraneum mycorrhiza from soil amended with high and low concentrations of cadmium. New Phytol. 1997, 135, 353–360. [Google Scholar] [CrossRef]
- Bissonnette, L.; St-Arnaud, M.; Labrecque, M. Phytoextraction of heavy metals by two Salicaceae clones in symbiosis with arbuscular mycorrhizal fungi during the second year of a field trial. Plant Soil 2010, 332, 55–67. [Google Scholar] [CrossRef]
- Chen, T.B.; Wong, J.W.C.; Zhou, H.Y.; Wong, M.H. Assessment of trace metal distribution and contamination in surface soils of Hong Kong. Environ. Pollut. 1997, 96, 61–68. [Google Scholar] [CrossRef]
- Niu, Z.X.; Sun, L.N.; Sun, T.H. The Bioadsorption of Cadmium and Lead by Bacteria in Root Exudates Culture. J. Soil Contam. 2011, 20, 877–891. [Google Scholar] [CrossRef]
- Cervantes, C.; Campos-García, J.; Devars, S.; Gutiérrez-Corona, F.; Loza-Tavera, H.; Torres-Guzmán, J.C.; Moreno-Sánchez, R. Interactions of chromium with microorganisms and plants. FEMS Microbiol. Rev. 2001, 25, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Wierzba, S. Biosorption of lead(II), zinc(II) and nickel(II) from industrial wastewater by Stenotrophomonas maltophilia and Bacillus subtilis. Pol. J. Chem. Technol. 2015, 17, 79–87. [Google Scholar] [CrossRef]
- Vullo, D.; Ceretti, H.; Alejandra Daniel, M.; Ramírez, S.; Zalts, A. Cadmium, Zinc and Copper Biosorption Mediated by Pseudomonas Veronii 2E. Bioresour. Technol. 2008, 99, 5574–5581. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.L. Microbes and metals. Appl. Microbiol. Biotechnol. 1997, 48, 687–692. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T. Heavy metal biosorption sites in Aspergillus niger. Bioresour. Technol. 1997, 61, 221–227. [Google Scholar] [CrossRef]
- Taştan, B.E.; Ertuğrul, S.; Dönmez, G. Effective bioremoval of reactive dye and heavy metals by Aspergillus versicolor. Bioresour. Technol. 2010, 101, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Dasola, A.M.; Adeyemi, A.L.; Tunbosun, L.A.; Abidemi, O.O.; Razaq, S.O. Kinetic and equilibrium studies of the heavy metal remediation potential of Helix pomentia. Afr. J. Pure Appl. Chem. 2014, 8, 123–133. [Google Scholar]
- Leusch, A.; Holan, Z.R.; Volesky, B. Biosorption of heavy metals (Cd, Cu, Ni, Pb, Zn) by chemically-reinforced biomass of marine algae. J. Chem. Technol. Biotechnol. 1995, 62, 279–288. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Schiewer, S.; Volesky, B. Modeling of the Proton-Metal Ion Exchange in Biosorption. Environ. Sci. Technol. 1995, 29, 3049–3058. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.A.H.; Ghadiri, M.; Chrzanowski, W.; Gan, Y. Scalable Surface Area Characterization by Electrokinetic Analysis of Complex Anion Adsorption. Langmuir 2014, 30, 15143–15152. [Google Scholar] [CrossRef] [PubMed]
- Jaroniec, M. Adsorption on heterogeneous surfaces: The exponential equation for the overall adsorption isotherm. Surf. Sci. 1975, 50, 553–564. [Google Scholar] [CrossRef]
- Omorogie, M.O.; Babalola, J.O.; Unuabonah, E.I.; Gong, J.R. Kinetics and thermodynamics of heavy metal ions sequestration onto novel Nauclea diderrichii seed biomass. Bioresour. Technol. 2012, 118, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; Mahmoud, D.K.; Ahmad, A.L. Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: Coconut (Cocos nucifera) bunch waste. J. Hazard. Mater. 2008, 158, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhang, Y.; Qin, J.; Wang, X.; Zhu, X. Biosorption of Cr(VI) from aqueous solutions by nonliving green algae Cladophora albida. Miner. Eng. 2009, 22, 372–377. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Loukidou, M.X.; Matis, K.A. Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process. Biochem. 2004, 39, 909–916. [Google Scholar] [CrossRef]
- Gola, D.; Dey, P.; Bhattacharya, A.; Mishra, A.; Malik, A.; Namburath, M.; Ahammad, S.Z. Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Bioresour. Technol. 2016, 218, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, F.; Nasseri, S.; Mesdaghinia, A.; Nabizadeh, R.; Zafari, D.; Khodakaramian, G.; Chehregani, A. Phytoremediation of petroleum-polluted soils: Application of Polygonum aviculare and its root-associated (penetrated) fungal strains for bioremediation of petroleum-polluted soils. Ecotoxicol. Environ. Saf. 2010, 73, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Morto-Bermea, O.; Hernández, A.E.; Gaso, I.; Segovia, N. Heavy metal concentrations in surface soils from Mexico City. Bull. Environ. Contam. Toxicol. 2002, 68, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Galiulin, R.V.; Galiulina, R.A. Removing heavy metals from soil with plants. Her. Russ. Acad. Sci. 2008, 78, 141–143. [Google Scholar] [CrossRef]
- Rodríguez-Tirado, V.; Green-Ruiz, C.; Gómez-Gil, B. Cu and Pb biosorption on Bacillus thioparans strain U3 in aqueous solution: Kinetic and equilibrium studies. Chem. Eng. J. 2012, 181–182, 352–359. [Google Scholar] [CrossRef]
- Hu, N.; Luo, Y.; Song, J. Influence of soil organic matter, pH and temperature on cd sorption by four soils from Yangtze river delta. Acta Pedol. Sin. 2010, 44, 437–443. [Google Scholar]
- Fang, L.; Zhou, C.; Cai, P.; Chen, W.; Rong, X.; Dai, K.; Liang, W.; Gu, J.; Huang, Q. Binding characteristics of copper and cadmium by cyanobacterium Spirulina platensis. J. Hazard. Mater. 2011, 190, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Acar, F.N.; Malkoc, E. The removal of chromium (VI) from aqueous solutions by Fagus orientalis L. Bioresour. Technol. 2004, 94, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.J.; Lee, M.E.; Chung, J.W. Comparison of heavy metal immobilization in contaminated soils amended with peat moss and peat moss-derived biochar. Environ. Sci. Process. Impacts 2016, 18, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Kumar, V.; Singh, J.; Teotia, P.; Bisht, S.; Sharma, S. Bioremediation of Pulp and Paper mill Effluent by Dominant Aboriginal Microbes and Their Consortium. Int. J. Environ. Res. 2014, 8, 561–568. [Google Scholar]
- Laurenti, M.; Blanco, F.G.; Lopez-Cabarcos, E.; Rubio-Retama, J. Detection of heavy metal ions using a water-soluble conjugated polymer based on thiophene and meso-2,3-dimercaptosuccinic acid. Polym. Int. 2013, 62, 811–816. [Google Scholar] [CrossRef]
- Karci, A.; Arslan-Alaton, I.; Bekbolet, M.; Ozhan, G.; Alpertunga, B. H2O2/UV-C and Photo-Fenton treatment of a nonylphenol polyethoxylate in synthetic freshwater: Follow-up of degradation products, acute toxicity and genotoxicity. Chem. Eng. J. 2014, 241, 43–51. [Google Scholar] [CrossRef]
- Kandeler, F.; Kampichler, C.; Horak, O. Influence of heavy metals on the functional diversity of soil microbial communities. Biol. Fertil. Soil 1996, 23, 299–306. [Google Scholar] [CrossRef]
- Günther, T.; Dornberger, U.; Fritsche, W. Effects of ryegrass on biodegradation of hydrocarbons in soil. Chemosphere 1996, 33, 203–215. [Google Scholar] [CrossRef]
- Rajendran, P.; Muthukrishnan, J.; Gunasekaran, P. Microbes in heavy metal remediation. Indian J. Exp. Biol. 2003, 41, 935–944. [Google Scholar] [PubMed]
- Soleimani, M.; Afyuni, M.; Hajabbasi, M.A.; Nourbakhsh, F.; Sabzalian, M.R.; Christensen, J.H. Phytoremediation of an aged petroleum contaminated soil using endophyte infected and non-infected grasses. Chemosphere 2010, 81, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Sitaula, B.K.; Almas, A.; Bakken, L.R.; Singh, B.R. Assessment of heavy metals associated with bacteria in soil. Soil Biol. Biochem. 1999, 31, 315–316. [Google Scholar] [CrossRef]
- Shewry, P.R.; Peterson, P.J. Distribution of Chromium and Nickel in Plants and Soil from Serpentine and Other Sites. J. Ecol. 1976, 64, 195–212. [Google Scholar] [CrossRef]
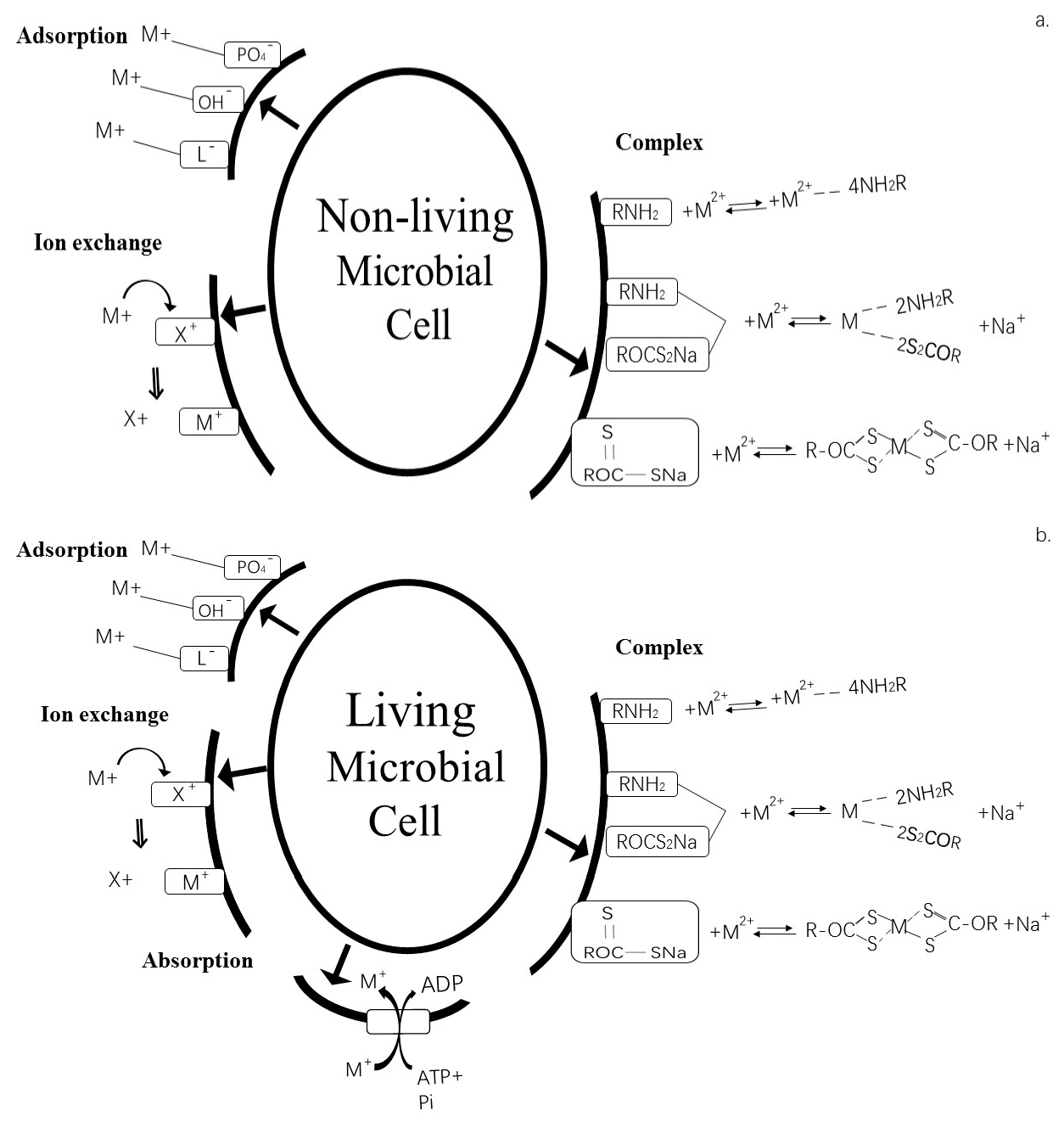
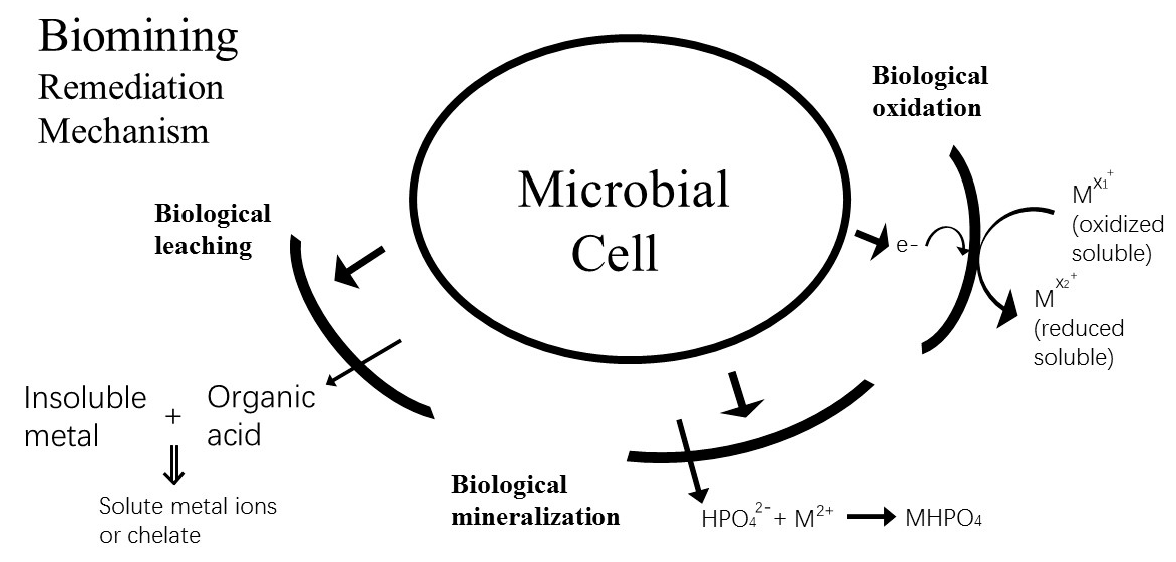
| Type | Method | Application | Advantages (+) and Disadvantages (–) |
|---|---|---|---|
| Soil washing | Physical Separation Chemical Extraction Integrated process | Large area (a large volume of soil) | |
| Soil flushing | Direct injection of a leaching solution | Large area (a large volume of soil) | |
| Engineering management | Change soil | Small area (a small volume of soil) of heavily polluted soil | |
| Chemical repair | Add chemical modifier | Wide range of applications | |
| Phytoremediation | Introduce plant life | Wide range of applications (especially suitable for mining reclamation) | |
| Physical chemistry repair | Electro-chemical methods | Low permeability clay and silt soil | |
| Electro-thermal methods | Volatile heavy metals (e.g., Hg) | ||
| Soil leaching | Small area of severely polluted soil |
|
| Type | Condition | Mechanism | Example |
|---|---|---|---|
| Surface precipitation | Edge charges on adsorbent | Reagent residue enhances metal ion stability in soil solid phase components, reduces migration and bioavailability | |
| Ion exchange | Other metal ions existing | The metal ions bound by the cell material are combined by other metal ions with stronger binding ability. | |
| Ligand exchange | There are organic functional groups, such as R-COOH, R-SH | Metal ions and ligands are bonded to the surface of the adsorbent by covalent or ionic bonding | |
| Diffusion and chemical modification of adsorbent surfaces | Manganese oxide Iron oxide Zeolite | Reduction of toxicity of heavy metal ions by chemical transfer between heavy metal ions and chemical modification |
| Microbe | Metals Which Can Be Easily Removed |
|---|---|
| Escherichia coli K-12 | Hg, Cd, Pb, Cu, Ni, Zn etc. |
| Rhizopus arrhizus | Zn, Cu, Cd, Th |
| Aspergillus niger | Zn, Cu, Cr, Pb, Th, Co, Mn, Ni |
| Saccharomyces cerevisiae | Cu, Cd, Pb, Ag |
| Thiobacillus thiooxidans | Cu, Pb, Zn, Cd |
| Microbial | Metal | Best-Fitted Model | Source |
|---|---|---|---|
| Bacillus Subtilius | Hg2+ | Second-order | [31] |
| Nauclea diderrichii | Cd2+ Hg2+ | Second-order | [65] |
| Rice husk | Pb2+ | Second-order | [66] |
| Kappaphycus sp. | Pb2+ Cu2+ Fe2+ Zn2+ | Second-order | [44] |
| Helix pomentia | Fe2+ Cr3+ | First-order | [59] |
| Helix pomentia | Cd2+ Pb2+ | Second-order | [59] |
| Microbe | Qmax | |||
|---|---|---|---|---|
| Cd | Cr | Zn | Pb | |
| Bacillus subtilius | 101 | 137 | ||
| Pseudomonas aeruginosa | 57.37 | 13.7 | 79.5 | |
| Streptomyces noursei | 3.4 | 1.6 | 99 | |
| Bacillus licheniformis | 142.7 | 62 | ||
| Bacillus laterosporus | 159.2 | 72.6 | ||
| Rhizopus arrhizus | 26.8 | 4.5 | 55.6 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Luan, Y.; Ning, Y.; Wang, L. Effects and Mechanisms of Microbial Remediation of Heavy Metals in Soil: A Critical Review. Appl. Sci. 2018, 8, 1336. https://doi.org/10.3390/app8081336
Jin Y, Luan Y, Ning Y, Wang L. Effects and Mechanisms of Microbial Remediation of Heavy Metals in Soil: A Critical Review. Applied Sciences. 2018; 8(8):1336. https://doi.org/10.3390/app8081336
Chicago/Turabian StyleJin, Yuyao, Yaning Luan, Yangcui Ning, and Lingyan Wang. 2018. "Effects and Mechanisms of Microbial Remediation of Heavy Metals in Soil: A Critical Review" Applied Sciences 8, no. 8: 1336. https://doi.org/10.3390/app8081336
APA StyleJin, Y., Luan, Y., Ning, Y., & Wang, L. (2018). Effects and Mechanisms of Microbial Remediation of Heavy Metals in Soil: A Critical Review. Applied Sciences, 8(8), 1336. https://doi.org/10.3390/app8081336






