Effects of Different Variables on the Formation of Mesopores in Y Zeolite by the Action of CTA+ Surfactant
Abstract
:1. Introduction
2. Materials and Methods
2.1. USY Zeolite Modification
2.2. Influence of Surfactant
2.3. Characterization
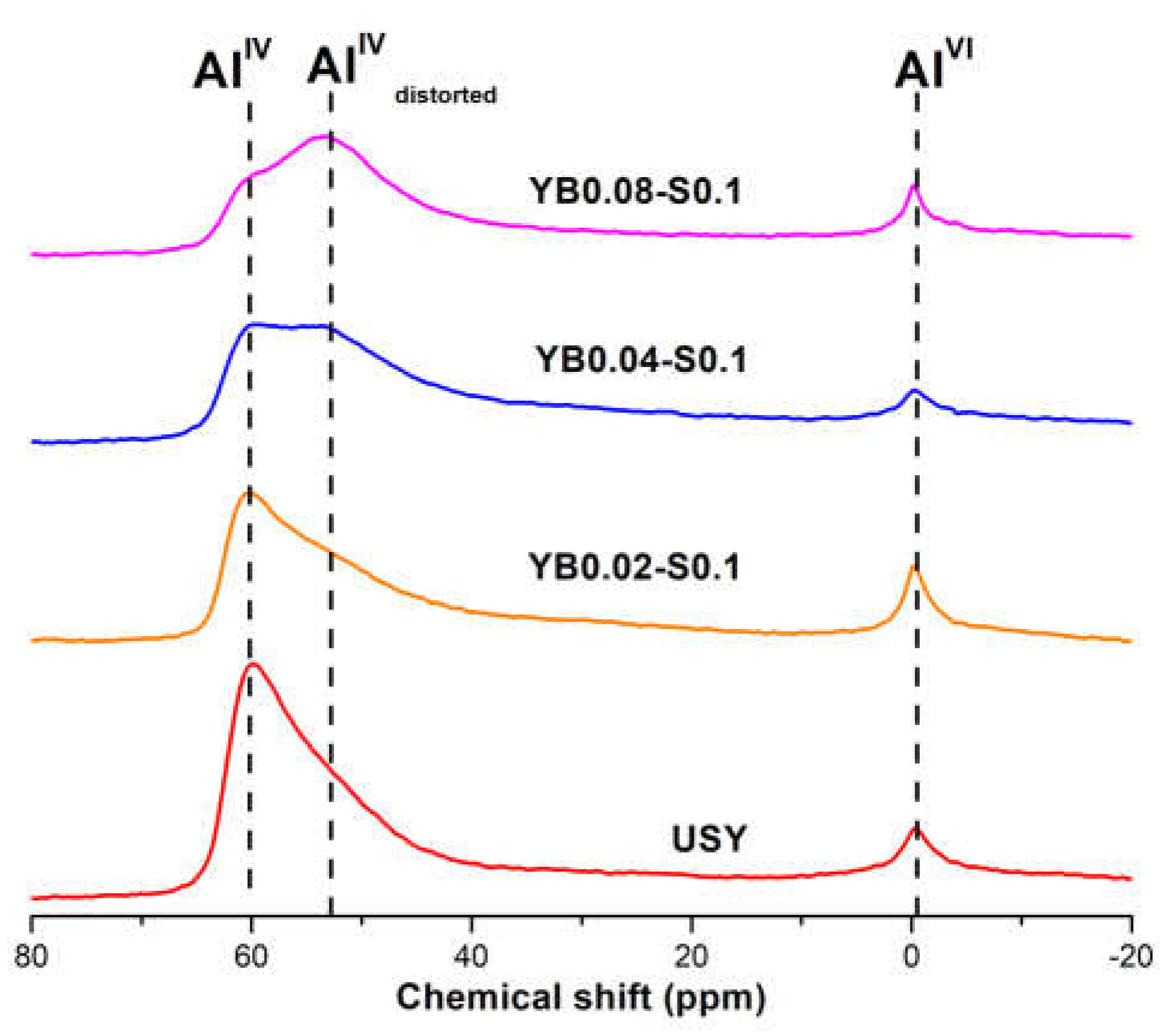
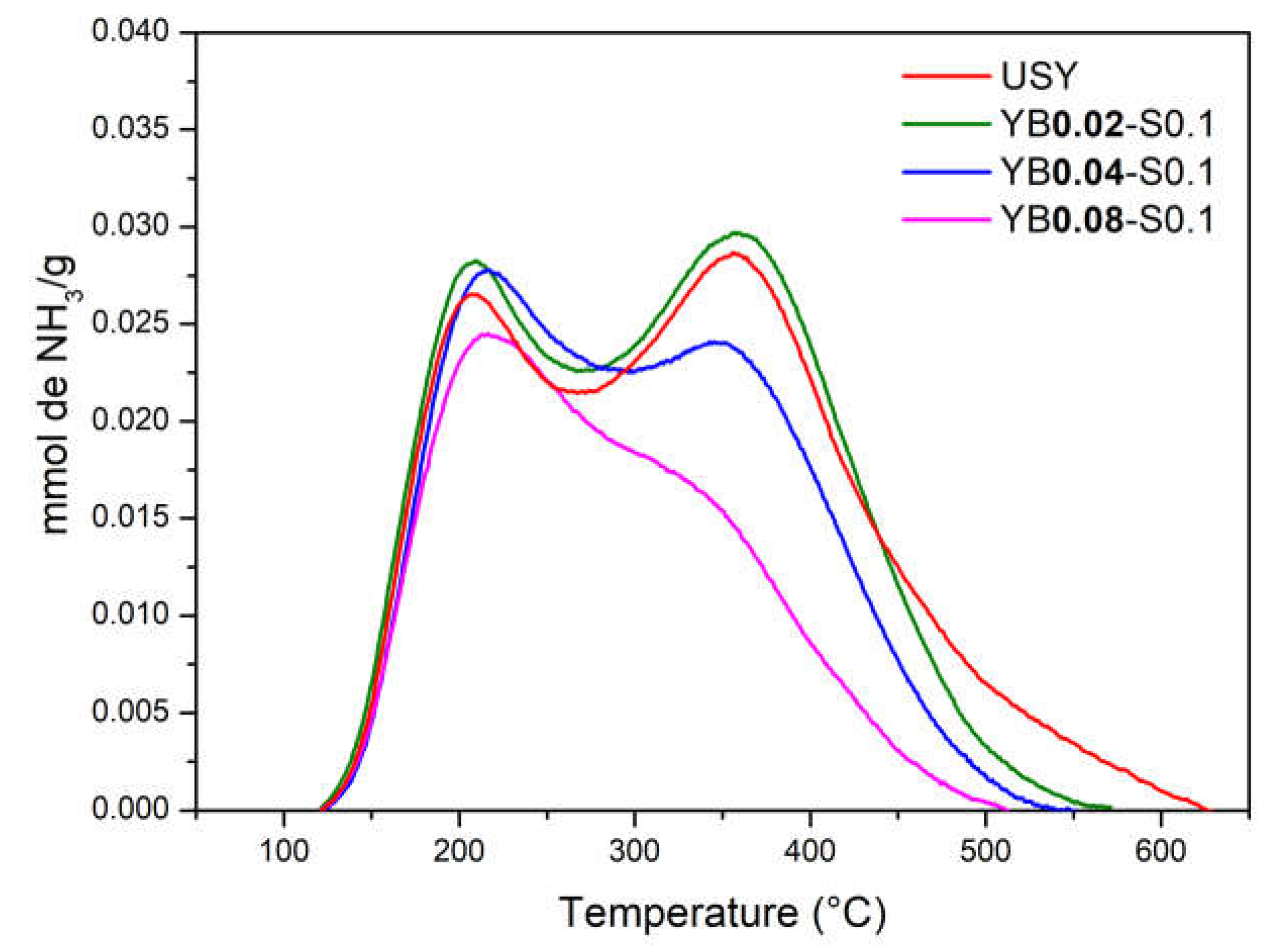
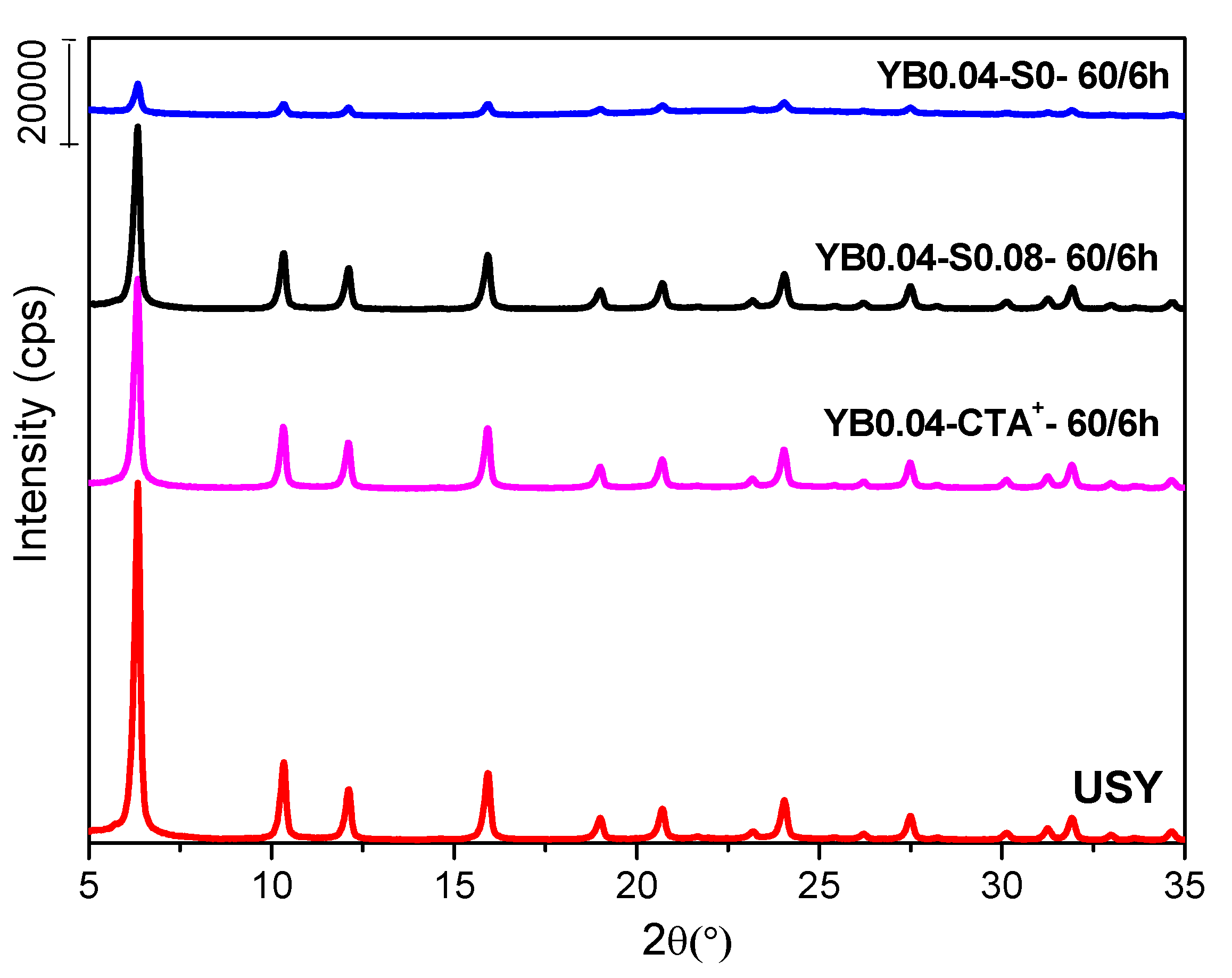
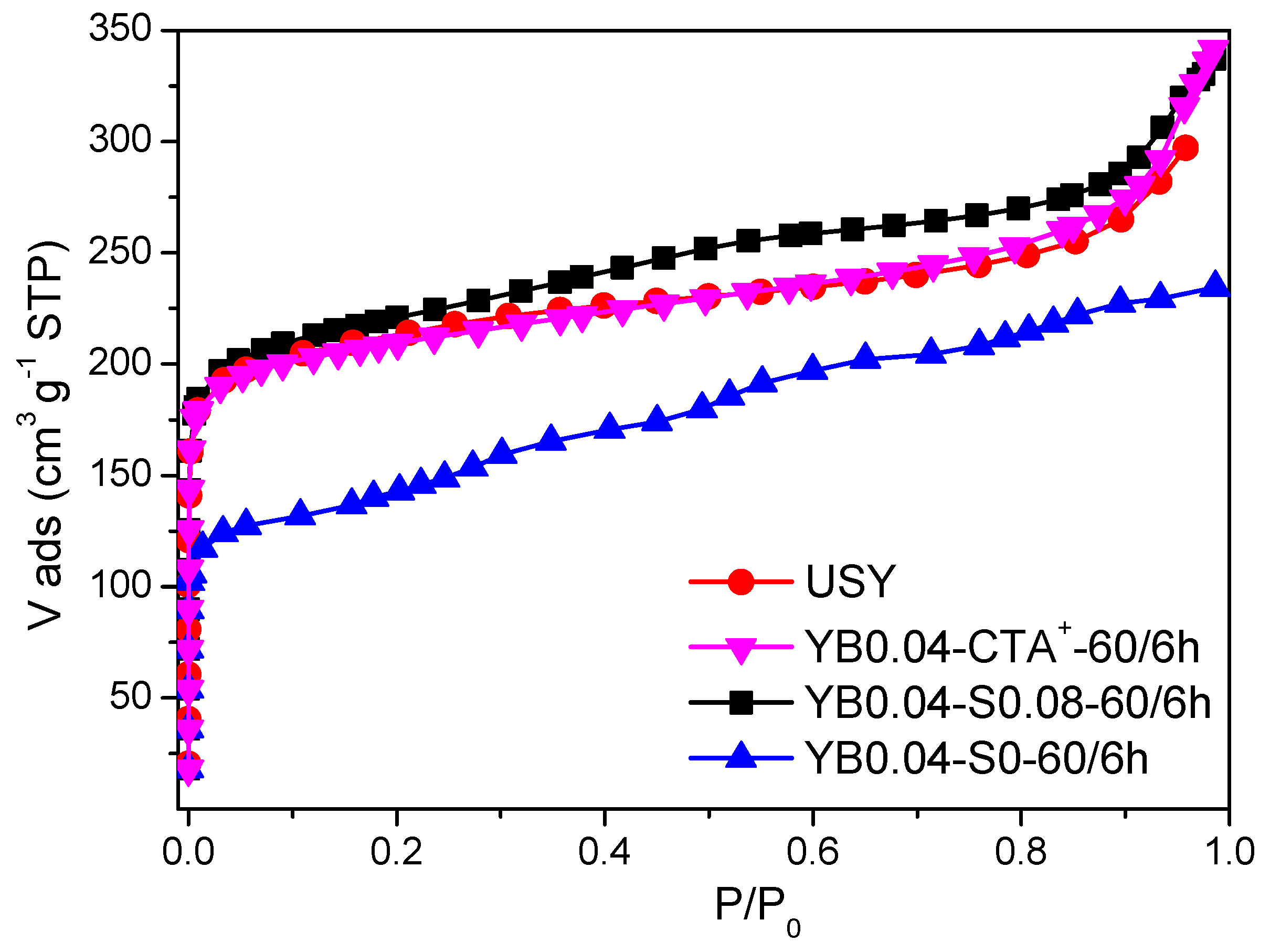
3. Results
3.1. Influence of Surfactant Concentration
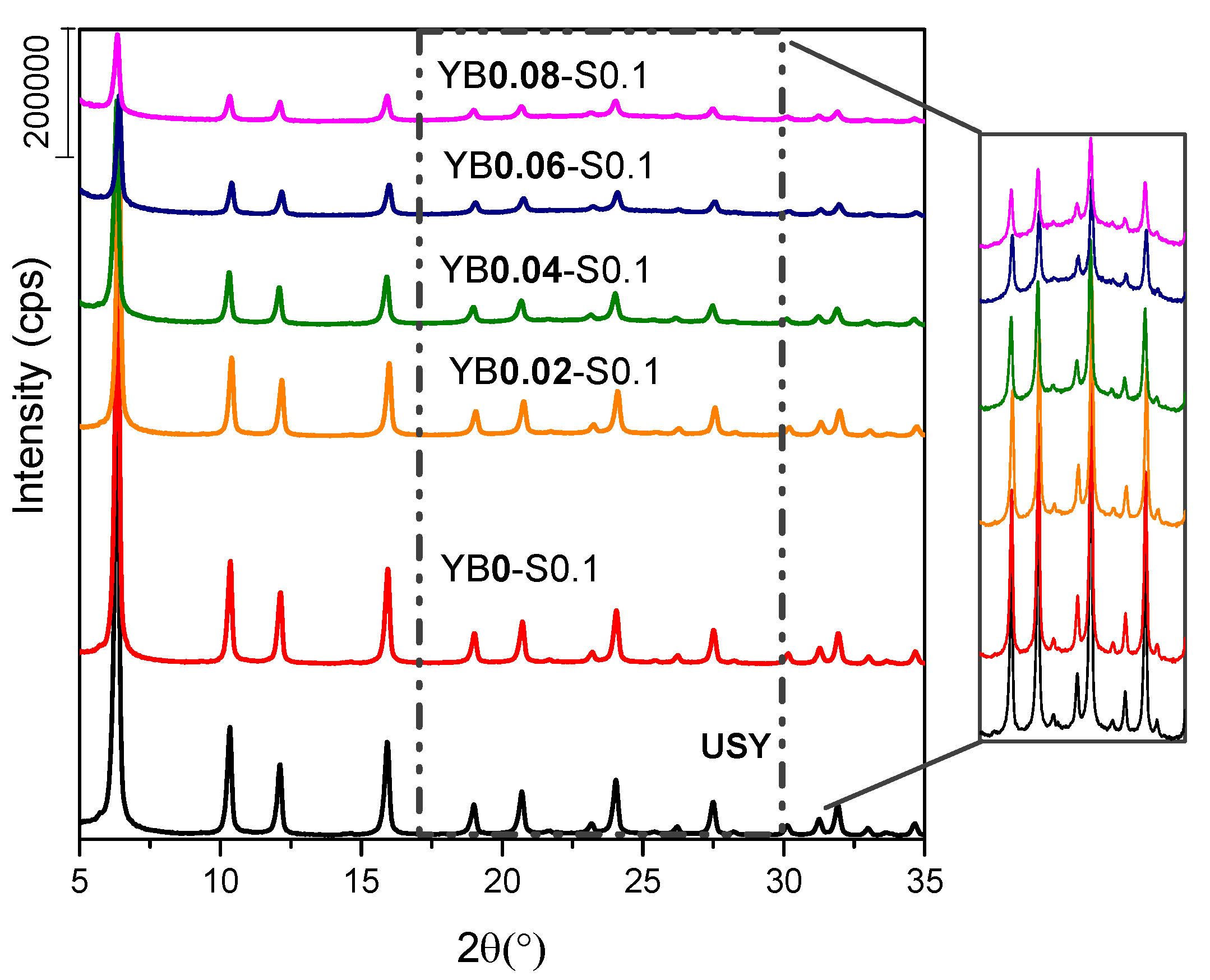
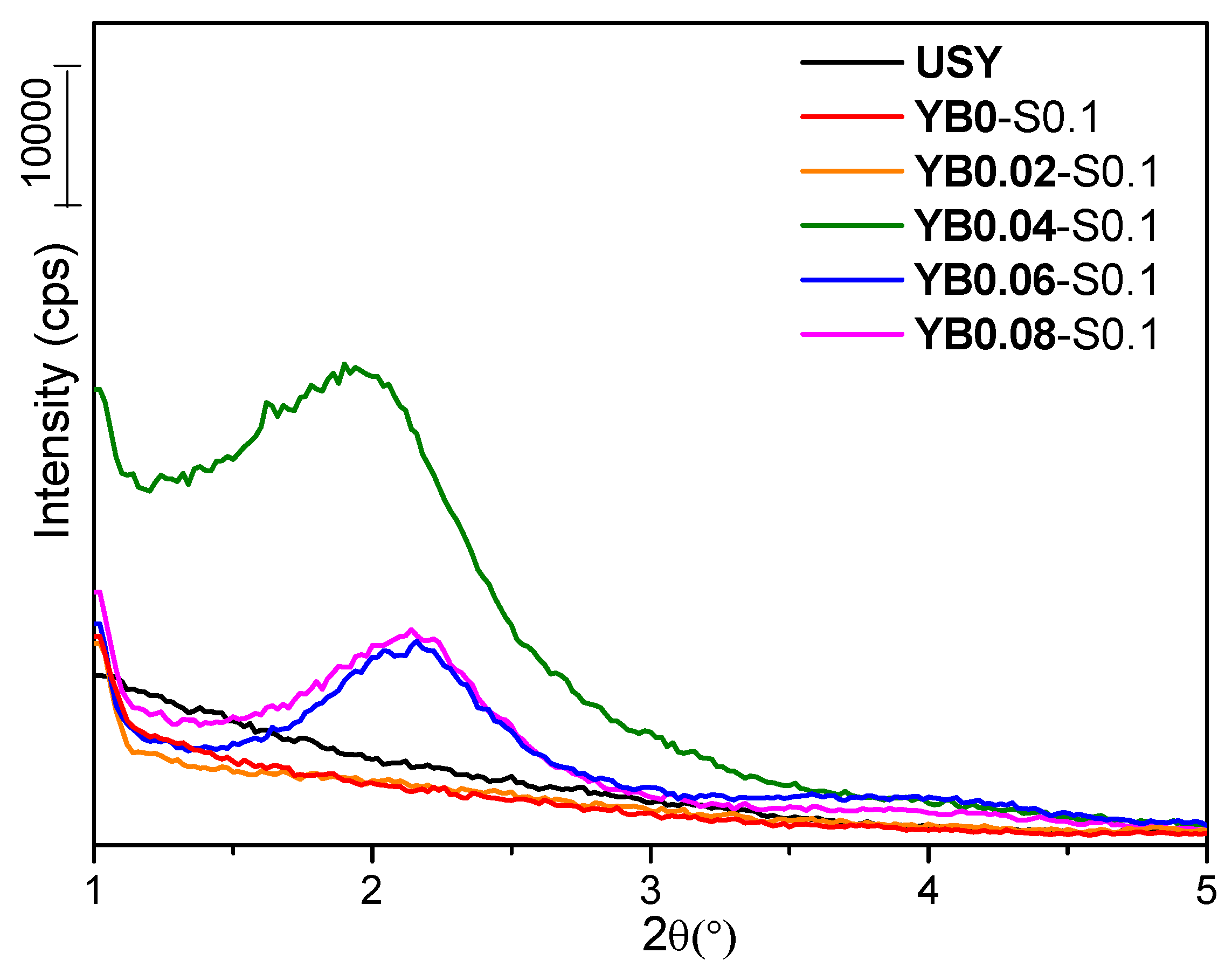
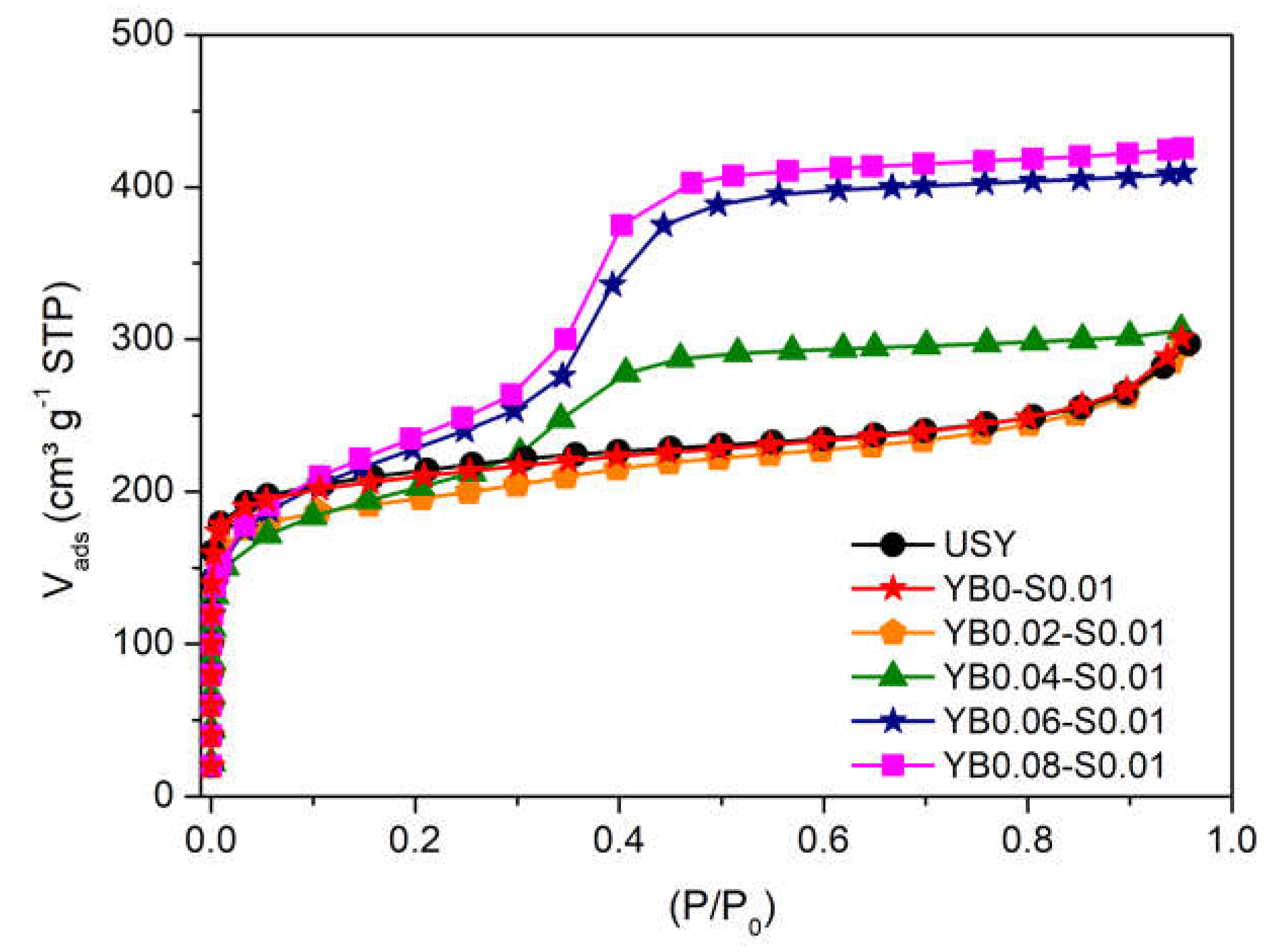
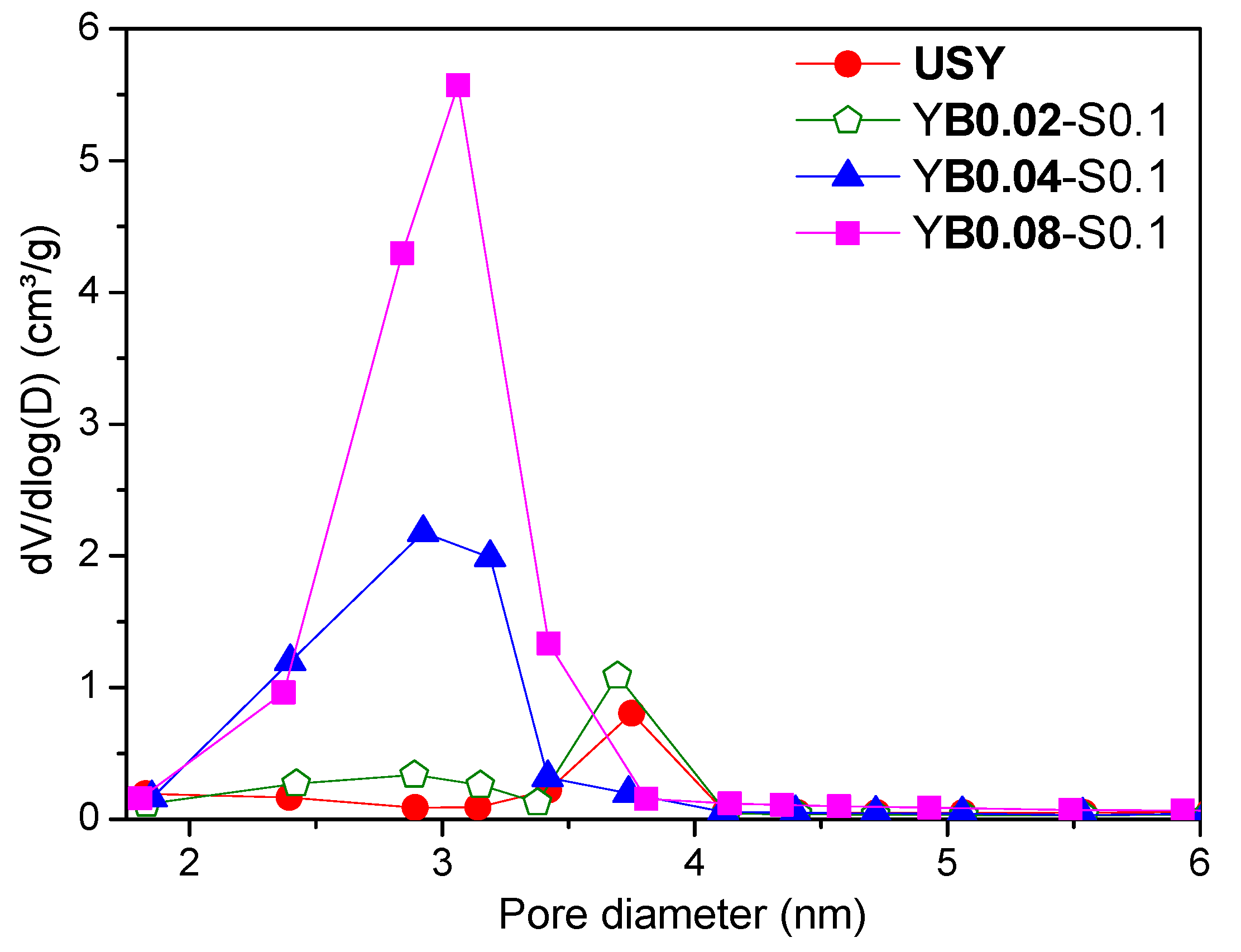
3.2. Influence of Base Concentration
3.3. Effect of the Presence of the Surfactant
4. Conclusions
Supplementary Materials
Author contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giannetto, G. Zeolitas: Características, Propiedades y Aplicaciones Industriales, 1st ed.; Editorial Innovación Tecnológica: Caracas, Venezuela, 1990; p. 170. [Google Scholar]
- Chal, R.; Cacciaguerra, T.; van Donk, S.; Gérardin, C. Pseudomorphic synthesis of mesoporous zeolite Y. crystals. Chem. Commun. 2010, 46, 7840–7842. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Verboekend, D.; Nuttens, N.; Locus, R.; Van Aelst, J.; Verolme, P.; Groen, J.C.; Pérez-Ramírez, J.; Sels, B.F. Synthesis, characterisation, and catalytic evaluation of hierarchical faujasite zeolites: Milestones, challenges, and future directions. Chem. Soc. Rev. 2016, 45, 3331–3352. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Valla, J.; Garcia-Martinez, J. Realizing the commercial potential of hierarchical zeolites: New opportunities in catalytic cracking. Chem. Cat. Chem. 2014, 6, 46–66. [Google Scholar]
- Koster, A.J.; Ziese, U.; Verkleij, A.J.; Janssen, A.H.; de Jong, K.P. Three-dimensional transmission electron microscopy: A novel imaging and characterization technique with nanometer scale resolution for Materials Science. J. Phys. Chem. B 2000, 104, 9368–9370. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Kasyanov, I.A.; Maerle, A.A.; Zaikovskii, V.I. Mechanistic study of zeolites recrystallization into micro-mesoporous materials. Microporous Mesoporous Mater. 2014, 189, 163–172. [Google Scholar] [CrossRef]
- García-Martínez, J.; Johnson, M.; Valla, J.; Li, K.; Ying, J.Y. Mesostructured zeolite Y—high hydrothermal stability and superior FCC catalytic performance. Catal. Sci. Technol. 2012, 2, 987–994. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Kuznetsov, A.S.; Ponomareva, O.A.; Yuschenko, V.V.; Knyazeva, E.E. Micro/mesoporous catalysts obtained by recrystallization of mordenite. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2005; pp. 121–128. [Google Scholar]
- Sachse, A.; Grau-Atienza, A.; Jardim, E.O.; Linares, N.; Thommes, M.; García-Martínez, J. Development of intracrystalline mesoporosity in zeolites through surfactant-templating. Cryst. Growth Des. 2017, 17, 4289–4305. [Google Scholar] [CrossRef]
- Sachse, A.; Wuttke, C.; Díaz, U.; de Souza, M.O. Mesoporous Y zeolite through ionic liquid based surfactant templating. Microporous Mesoporous Mater. 2015, 217, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Ying, Y.; García-Martínez, J. Mesostructured Zeolitic Materials, and Methods of Making and Using the Same. U.S. Patent 20050239634A1, 27 October 2005. [Google Scholar]
- Verboekend, D.; Vilé, G.; Pérez-Ramírez, J. Mesopore formation in USY and beta zeolites by base leaching: Selection criteria and optimization of pore-directing agents. Cryst. Growth Des. 2012, 12, 3123–3132. [Google Scholar] [CrossRef]
- Shutkina, O.V.; Knyazeva, E.E.; Ivanova, I.I. Preparation and physicochemical and catalytic properties of micro-mesoporous catalysts based on faujasite. Pet. Chem. 2016, 56, 138–145. [Google Scholar] [CrossRef]
- Thomas, J.M.; Leary, R.K. A Major Advance in Characterizing Nanoporous Solids Using a Complementary Triad of Existing Techniques Angew. Chemistry 2014, 53, 12020–12021. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Van Aelst, J.; Verboekend, D.; Philippaerts, A.; Nuttens, N.; Kurttepeli, M.; Gobechiya, E.; Haouas, M.; Sree, S.P.; Denayer, J.F.M.; Martens, J.A.; et al. Catalyst design by NH4OH treatment of USY zeolite. Adv. Funct. Mater. 2015, 25, 7130–7144. [Google Scholar] [CrossRef]
- Peters, A.W.; Wu, C.C. Selectivity effects of a new aluminum species in strongly dealuminated USY containing FCC catalysts. Catal. Lett. 1995, 30, 171–179. [Google Scholar] [CrossRef]
- Katada, N.; Igi, H.; Kim, J.-H. Determination of the acidic properties of zeolite by theoretical analysis of temperature-programmed desorption of ammonia based on adsorption equilibrium. J. Phys. Chem. B 1997, 101, 5969–5977. [Google Scholar] [CrossRef]
- Niwa, M.; Katada, N. New method for the temperature-programmed desorption (TPD) of ammonia experiment for characterization of zeolite acidity: A review: TPD of ammonia for characterization of zeolite acidity. Chem. Rec. 2013, 13, 432–455. [Google Scholar] [CrossRef] [PubMed]

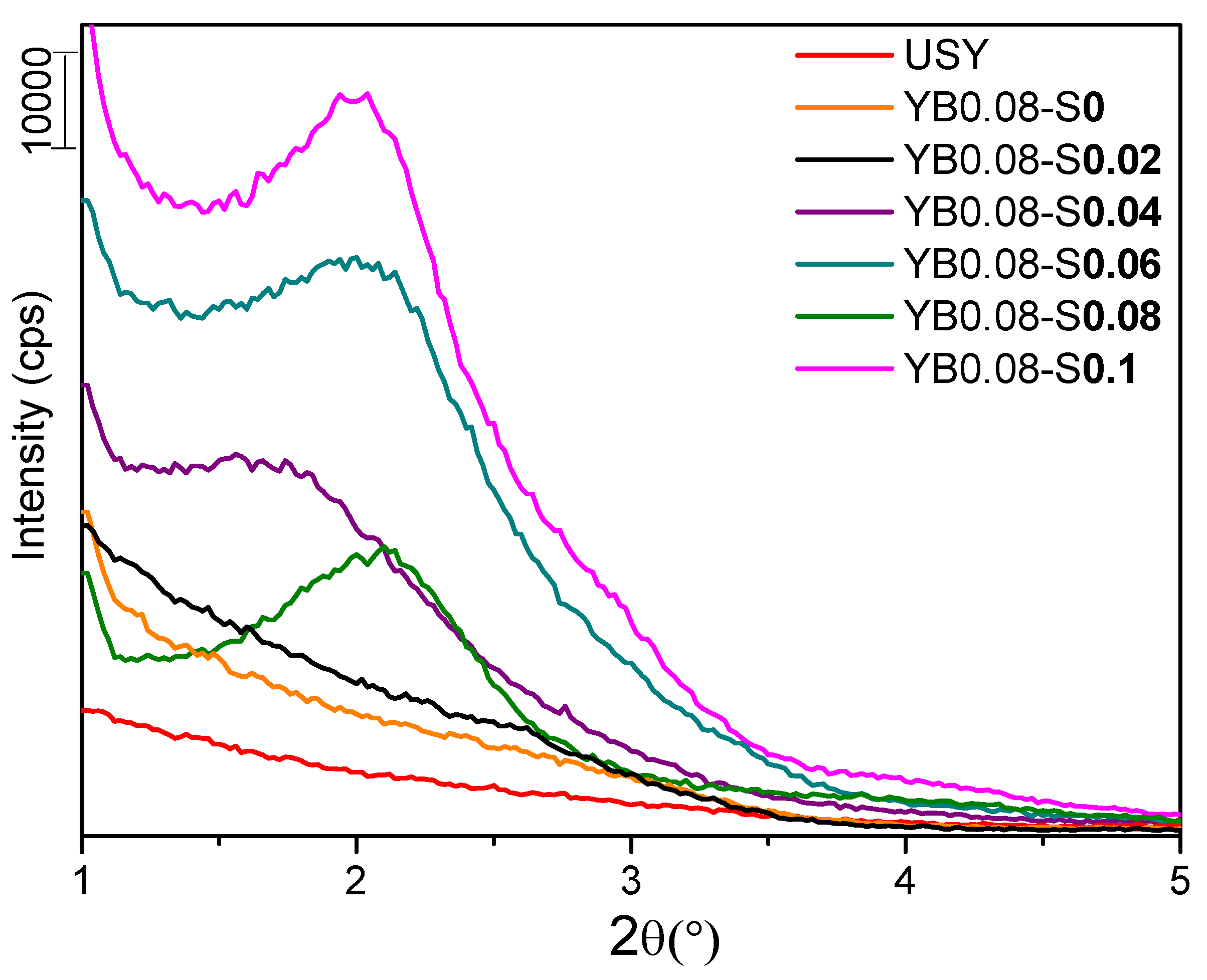
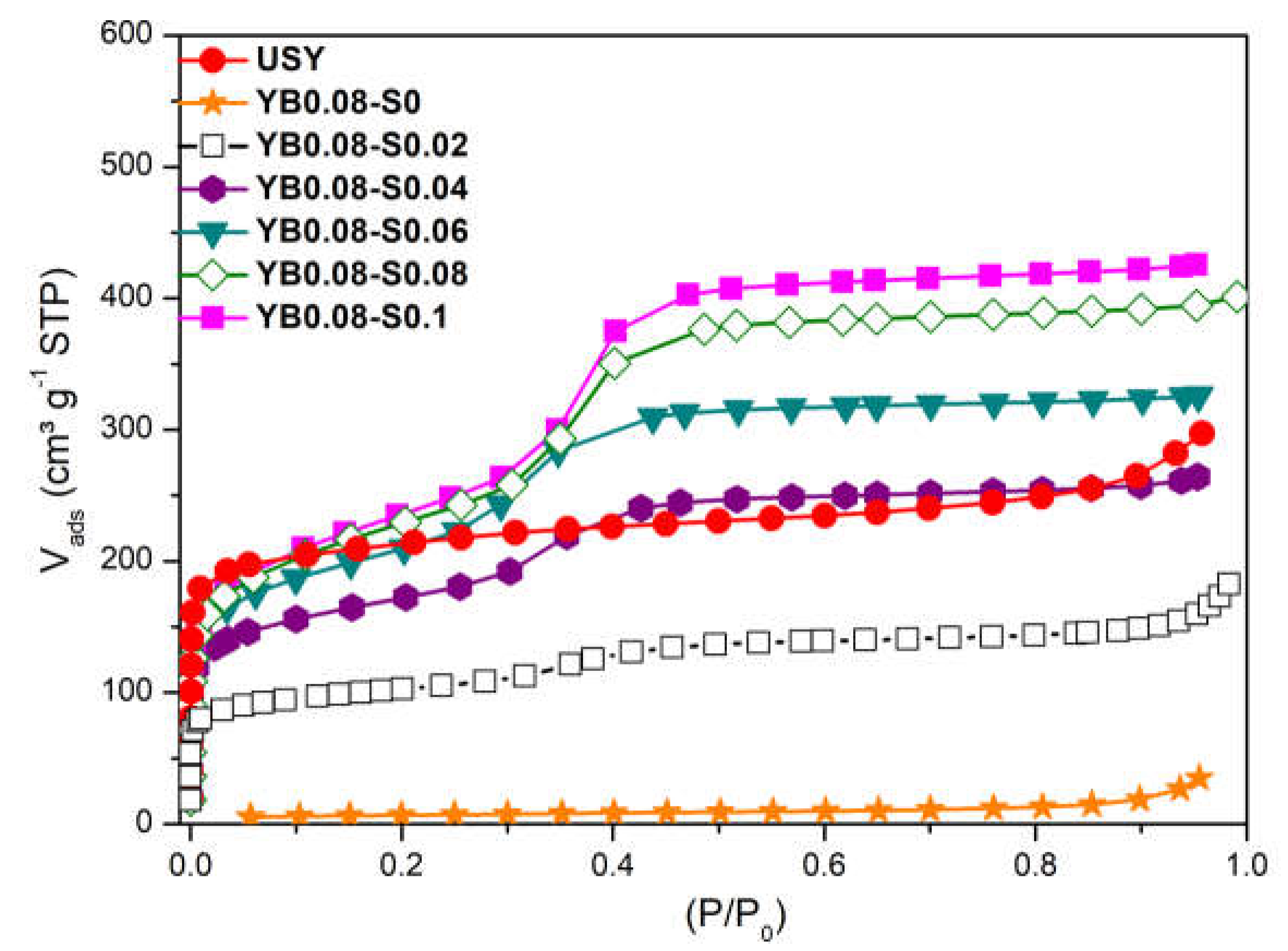
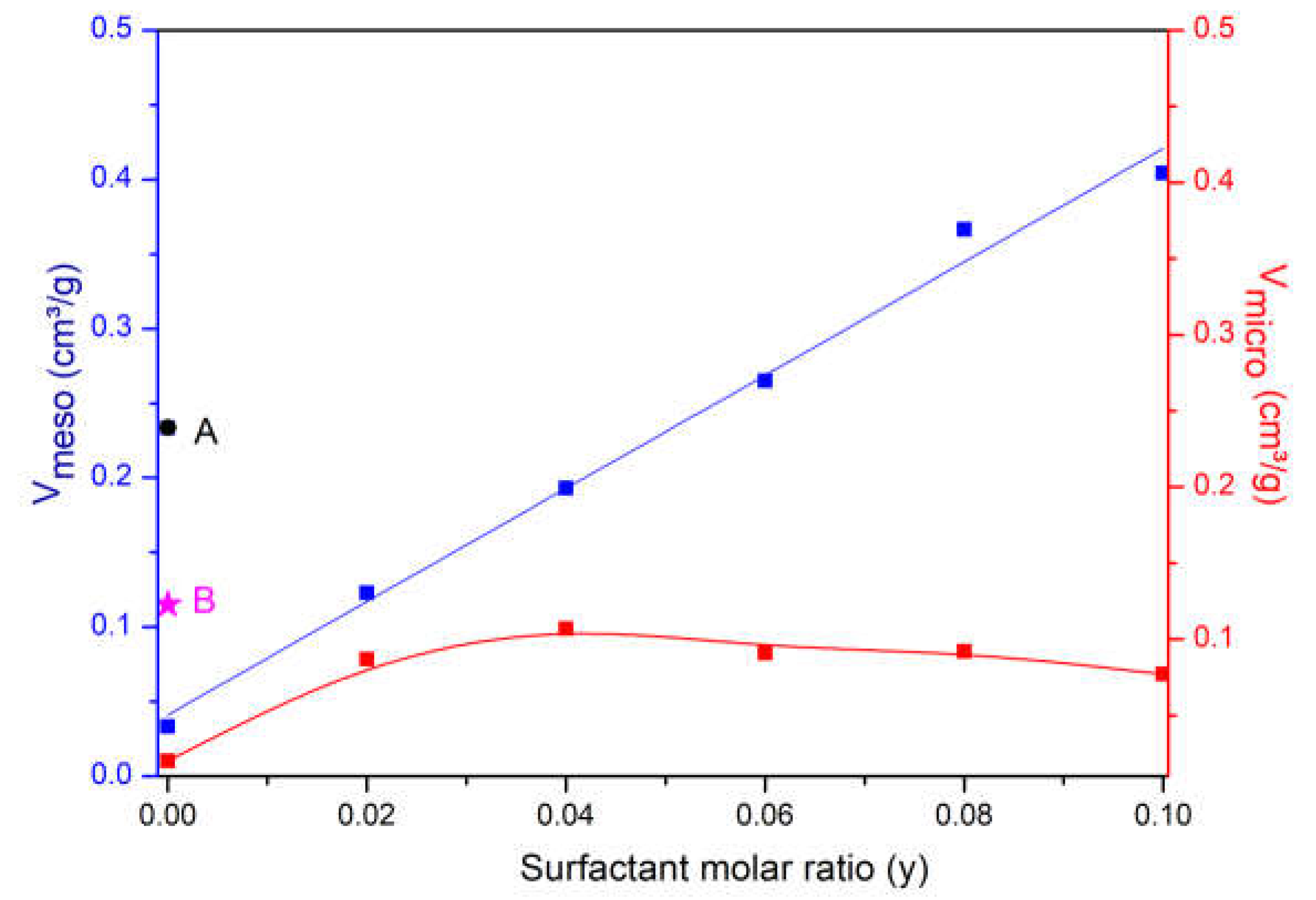
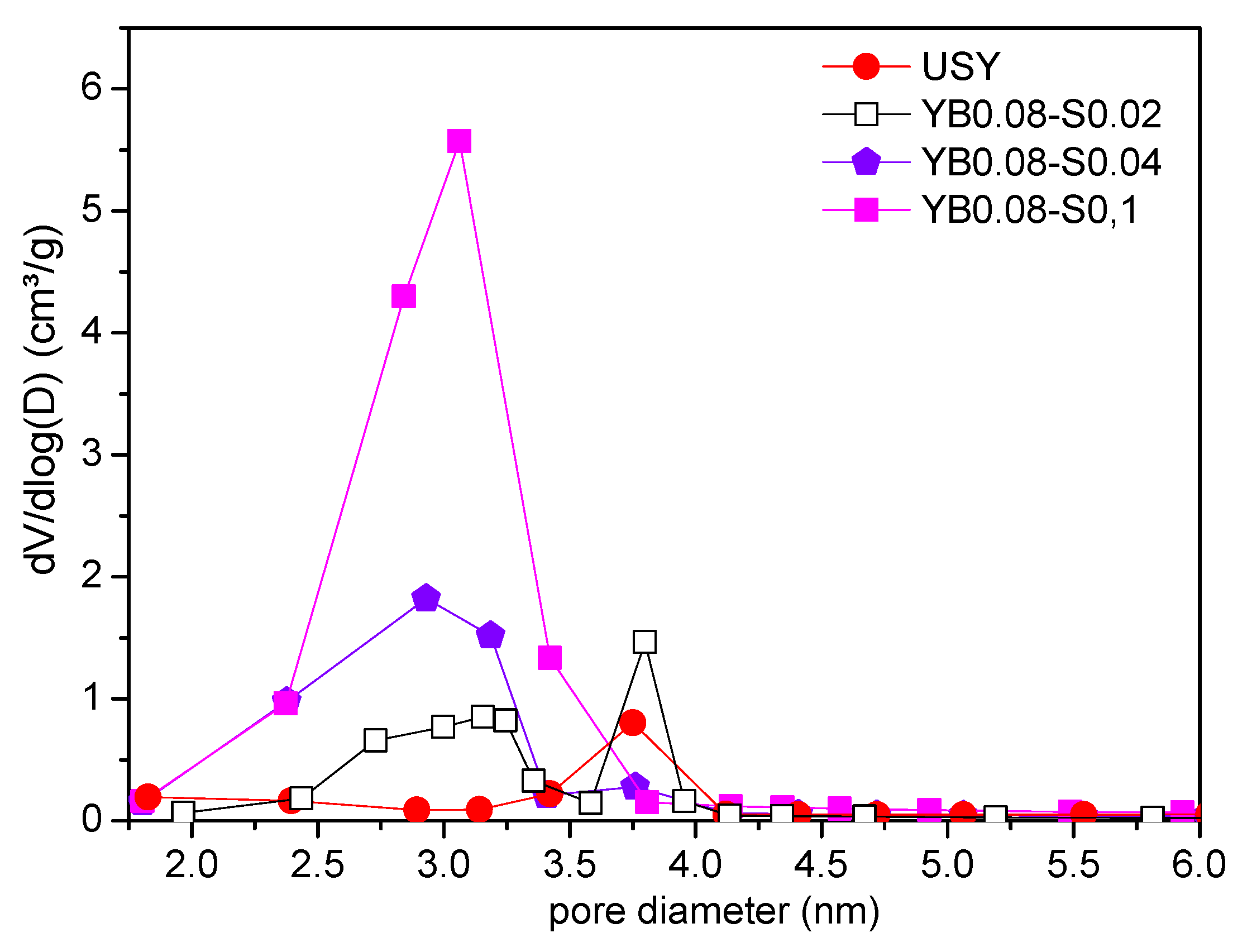

| Sample | Base Molar Ratio (x) | Surfactant Molar Ratio (y) |
|---|---|---|
| YB0-S0.1 | 0 | 0.1 |
| YB0.02-S0.1 | 0.02 | 0.1 |
| YB0.04-S0.1 | 0.04 | 0.1 |
| YB0.06-S0.1 | 0.06 | 0.1 |
| YB0.08-S0.1 | 0.08 | 0.1 |
| YB0.08-S0 | 0.08 | 0 |
| YB0.08-S0.02 | 0.08 | 0.02 |
| YB0.08-S0.04 | 0.08 | 0.04 |
| YB0.08-S0.06 | 0.08 | 0.06 |
| YB0.08-S0.08 | 0.08 | 0.08 |
| Sample | RC (%) | Vtotal 1 (cm³/g) | Vmicro 2 (cm³/g) | Vmeso 3 (cm³/g) | Sext 2 (cm³/g) |
|---|---|---|---|---|---|
| USY | 100 | 0.395 | 0.239 | 0.115 | 208 |
| YB0.08-S0 | 0 | 0.023 | 0.002 | 0.033 | 20 |
| YB0.08-S0.02 | 32 | 0.225 | 0.087 | 0.123 | 165 |
| YB0.08-S0.04 | 39 | 0.395 | 0.107 | 0.193 | 364 |
| YB0.08-S0.06 | 38 | 0.468 | 0.091 | 0.265 | 536 |
| YB0.08-S0.08 | 38 | 0.603 | 0.092 | 0.363 | 597 |
| YB0.08-S0.1 | 33 | 0.649 | 0.077 | 0.404 | 661 |
| Sample | RC (%) | Vtotal 1 (cm³/g) | Vmicro 2 (cm³/g) | Vmeso 3 (cm³/g) | Sext 2 (cm³/g) | pH 4 |
|---|---|---|---|---|---|---|
| USY | 100 | 0.395 | 0.239 | 0.115 | 208 | - |
| YB0-S0.1 | 96 | 0.396 | 0.242 | 0.142 | 186 | 3 |
| YB0.02-S0.1 | 80 | 0.367 | 0.199 | 0.170 | 209 | 9 |
| YB0.04-S0.1 | 52 | 0.464 | 0.129 | 0.229 | 420 | 10 |
| YB0.06-S0.1 | 38 | 0.627 | 0.095 | 0.379 | 591 | 11 |
| YB0.08-S0.1 | 33 | 0.649 | 0.077 | 0.404 | 661 | 11 |
| Sample | T (°C) | NH3 | NH3(total) | Si/Al |
|---|---|---|---|---|
| USY | 209 | 101 | 547 | 14 |
| 358 | 446 | |||
| YB0.02-S0.1 | 208 | 144 | 535 | 15 |
| 358 | 391 | |||
| YB0.04-S0.1 | 216 | 115 | 462 | 14 |
| 350 | 347 | |||
| YB0.08-S0.1 | 216 | 99 | 344 | 14 |
| 325 | 245 |
| Sample | RC(%) | Vtotal 1 (cm³/g) | Vmicro 2 (cm³/g) | Vmeso 3 (cm³/g) | Sext 2 (cm³/g) |
|---|---|---|---|---|---|
| USY | 100 | 0.395 | 0.239 | 0.156 | 208 |
| YB0.04-CTA+-60/6h | 98 | 0.405 | 0.245 | 0.160 | 179 |
| YB0.04-S0.08-60/6h | 94 | 0.426 | 0.240 | 0.186 | 233 |
| YB0.04-S0.-60/6h | 23 | 0.355 | 0.084 | 0.271 | 334 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.F.; Ferracine, E.D.; Cardoso, D. Effects of Different Variables on the Formation of Mesopores in Y Zeolite by the Action of CTA+ Surfactant. Appl. Sci. 2018, 8, 1299. https://doi.org/10.3390/app8081299
Silva JF, Ferracine ED, Cardoso D. Effects of Different Variables on the Formation of Mesopores in Y Zeolite by the Action of CTA+ Surfactant. Applied Sciences. 2018; 8(8):1299. https://doi.org/10.3390/app8081299
Chicago/Turabian StyleSilva, Juliana F., Edilene Deise Ferracine, and Dilson Cardoso. 2018. "Effects of Different Variables on the Formation of Mesopores in Y Zeolite by the Action of CTA+ Surfactant" Applied Sciences 8, no. 8: 1299. https://doi.org/10.3390/app8081299






