Hyperthermia Efficiency of Magnetic Nanoparticles in Dense Aggregates of Cerium Oxide/Iron Oxide Nanoparticles
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
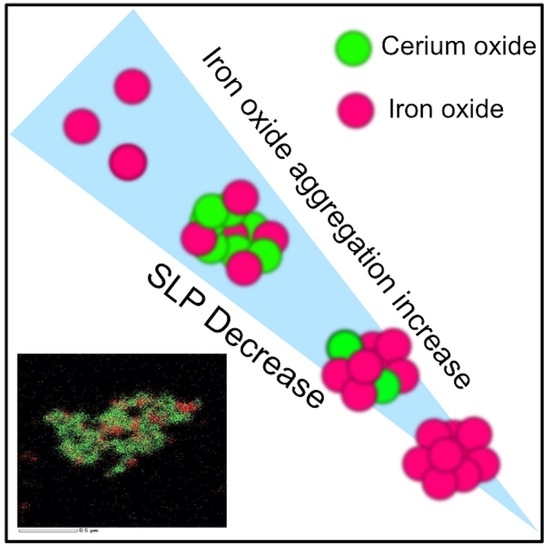
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Di Corato, R.; Espinosa, A.; Lartigue, L.; Tharaud, M.; Chat, S.; Pellegrino, T.; Ménager, C.; Gazeau, F.; Wilhelm, C. Magnetic Hyperthermia Efficiency in the Cellular Environment for Different Nanoparticle Designs. Biomaterials 2014, 35, 6400–6411. [Google Scholar] [CrossRef] [PubMed]
- Petri-Fink, A.; Steitz, B.; Finka, A.; Salaklang, J.; Hofmann, H. Effect of Cell Media on Polymer Coated Superparamagnetic Iron Oxide Nanoparticles (SPIONs): Colloidal stability, cytotoxicity, and cellular uptake studies. Eur. J. Pharm. Biopharm. 2008, 68, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Javed, Y.; Lartigue, L.; Volatron, J.; Elgrabli, D.; Marangon, I.; Pugliese, G.; Caron, B.; Figuerola, A.; Luciani, N.; et al. The One Year Fate of Iron Oxide Coated Gold Nanoparticles in Mice. ACS Nano 2015, 9, 7925–7939. [Google Scholar] [CrossRef] [PubMed]
- Guibert, C.; Dupuis, V.; Peyre, V.; Fresnais, J. Hyperthermia of Magnetic Nanoparticles: An Experimental Study of the Role of Aggregation. J. Phys. Chem. C 2015, 119, 28148–28154. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating Magnetic Fluid with Alternating Magnetic Field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Fortin, J.-P.; Wilhelm, C.; Servais, J.; Ménager, C.; Bacri, J.-C.; Gazeau, F. Size-Sorted Anionic Iron Oxide Nanomagnets as Colloidal Mediators for Magnetic Hyperthermia. J. Am. Chem. Soc. 2007, 129, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Hugounenq, P.; Levy, M.; Alloyeau, D.; Lartigue, L.; Dubois, E.; Cabuil, V.; Ricolleau, C.; Roux, S.; Wilhelm, C.; Gazeau, F.; et al. Iron Oxide Monocrystalline Nanoflowers for Highly Efficient Magnetic Hyperthermia. J. Phys. Chem. C 2012, 116, 15702–15712. [Google Scholar] [CrossRef]
- Riedinger, A.; Guardia, P.; Curcio, A.; Garcia, M.A.; Cingolani, R.; Manna, L.; Pellegrino, T. Subnanometer Local Temperature Probing and Remotely Controlled Drug Release Based on Azo-Functionalized Iron Oxide Nanoparticles. Nano Lett. 2013, 13, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zink, J.I. Taking the Temperature of the Interiors of Magnetically Heated Nanoparticles. ACS Nano 2014, 8, 5199–5207. [Google Scholar] [CrossRef] [PubMed]
- Griffete, N.; Fresnais, J.; Espinosa, A.; Wilhelm, C.; Bee, A.; Menager, C. Design of Magnetic Molecularly Imprinted Polymer Nanoparticles for Controlled Release of Doxorubicin under an Alternative Magnetic Field in Athermal Conditions. Nanoscale 2015, 7, 18891–18896. [Google Scholar] [CrossRef] [PubMed]
- N’Guyen, T.T.T.; Duong, H.T.T.; Basuki, J.; Montembault, V.; Pascual, S.; Guibert, C.; Fresnais, J.; Boyer, C.; Whittaker, M.R.; Davis, T.P.; et al. Functional Iron Oxide Magnetic Nanoparticles with Hyperthermia-Induced Drug Release Ability by Using a Combination of Orthogonal Click Reactions. Angew. Chem. Int. Ed. 2013, 52, 14152–14156. [Google Scholar] [CrossRef]
- Jolivet, J.P.; Massart, R.; Fruchart, J.M. Synthesis and Physicochemical Study of Non-Surfactant Magnetic Colloids in an Aqueous-Medium. New J. Chem. 1983, 7, 325–331. [Google Scholar]
- Lefebure, S.; Dubois, E.; Cabuil, V.; Neveu, S.; Massart, R. Monodisperse Magnetic Nanoparticles: Preparation and Dispersion in Water and Oils. J. Mater. Res. 1998, 13, 2975–2981. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Samiee, S.; Nancarrow, P. Fabrication of Cerium Oxide Nanoparticles: Characterization and Optical Properties. J. Colloid Interface Sci. 2011, 356, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Fresnais, J.; Yan, M.; Courtois, J.; Bostelmann, T.; Bée, A.; Berret, J.-F. Poly(Acrylic Acid)-Coated Iron Oxide Nanoparticles: Quantitative Evaluation of the Coating Properties and Applications for the Removal of a Pollutant Dye. J. Colloid Interface Sci. 2013, 395, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, M.; Muller, P.; Lizarraga, G.; Bauer, C.; Cottet, H.; Théodoly, O. Characterization of Amphiphilic Diblock Copolymers Synthesized by MADIX Polymerization Process. Macromolecules 2007, 40, 2672–2682. [Google Scholar] [CrossRef]
- Fresnais, J.; Berret, J.F.; Frka-Petesic, B.; Sandre, O.; Perzynski, R. Electrostatic Co-Assembly of Iron Oxide Nanoparticles and Polymers: Towards the Generation of Highly Persistent Superparamagnetic Nanorods. Adv. Mater. 2008, 20, 3877–3881. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Fresnais, J.; Berret, J.F. Growth Mechanism of Nanostructured Superparamagnetic Rods Obtained by Electrostatic Co-Assembly. Soft Matter 2010, 6, 1997–2005. [Google Scholar] [CrossRef]
- Fresnais, J.; Lavelle, C.; Berret, J.F. Nanoparticle Aggregation Controlled by Desalting Kinetics. J. Phys. Chem. C 2009, 113, 16371–16379. [Google Scholar] [CrossRef] [Green Version]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the Reliable Measurement of Specific Absorption Rates and Intrinsic Loss Parameters in Magnetic Hyperthermia Materials. J. Phys. D Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Chanteau, B.; Fresnais, J.; Berret, J.F. Electrosteric Enhanced Stability of Functional Sub-10 nm Cerium and Iron Oxide Particles in Cell Culture Medium. Langmuir 2009, 25, 9064–9070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanzaki, R.; Guibert, C.; Fresnais, J.; Peyre, V. Dispersion Mechanism of Polyacrylic Acid-Coated Nanoparticle in Protic Ionic Liquid, N,N-Diethylethanolammonium Trifluoromethanesulfonate. J. Colloid Interface Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, D.; Camarero, J.; Ortega, D.; Teran, F.J. Influence of the Aggregation, Concentration, and Viscosity on the Nanomagnetism of Iron Oxide Nanoparticle Colloids for Magnetic Hyperthermia. J. Nanopart. Res. 2015, 17. [Google Scholar] [CrossRef]
- Landi, G.T. The Random Dipolar-Field Approximation for Systems of Interacting Magnetic Particles. J. Appl. Phys. 2013, 113, 163908. [Google Scholar] [CrossRef]
- Branquinho, L.C.; Carrião, M.S.; Costa, A.S.; Zufelato, N.; Sousa, M.H.; Miotto, R.; Ivkov, R.; Bakuzis, A.F. Effect of Magnetic Dipolar Interactions on Nanoparticle Heating Efficiency: Implications for cancer hyperthermia. Sci. Rep. 2013. [Google Scholar] [CrossRef] [PubMed]
- Guibert, C.; Fresnais, J.; Peyre, V.; Dupuis, V. Magnetic Fluid Hyperthermia Probed by both Calorimetric and Dynamic Hysteresis Measurements. J. Magn. Magn. Mater. 2017, 421, 384–392. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadel, C.; Michel, A.; Casale, S.; Fresnais, J. Hyperthermia Efficiency of Magnetic Nanoparticles in Dense Aggregates of Cerium Oxide/Iron Oxide Nanoparticles. Appl. Sci. 2018, 8, 1241. https://doi.org/10.3390/app8081241
Yadel C, Michel A, Casale S, Fresnais J. Hyperthermia Efficiency of Magnetic Nanoparticles in Dense Aggregates of Cerium Oxide/Iron Oxide Nanoparticles. Applied Sciences. 2018; 8(8):1241. https://doi.org/10.3390/app8081241
Chicago/Turabian StyleYadel, Cindy, Aude Michel, Sandra Casale, and Jerome Fresnais. 2018. "Hyperthermia Efficiency of Magnetic Nanoparticles in Dense Aggregates of Cerium Oxide/Iron Oxide Nanoparticles" Applied Sciences 8, no. 8: 1241. https://doi.org/10.3390/app8081241
APA StyleYadel, C., Michel, A., Casale, S., & Fresnais, J. (2018). Hyperthermia Efficiency of Magnetic Nanoparticles in Dense Aggregates of Cerium Oxide/Iron Oxide Nanoparticles. Applied Sciences, 8(8), 1241. https://doi.org/10.3390/app8081241