Evaluation of Near-Infrared Hyperspectral Imaging for Detection of Peanut and Walnut Powders in Whole Wheat Flour
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Hyperspectral Image Collection
2.2. Hyperspectral Image Calibration and Region of Interest (ROI) Extraction
2.3. Spectral Preprocessing
2.4. Model Development Based on Full Spectra
2.5. Optimal Wavelength Extraction and Multispectral Model Development
2.6. Visualization and Post-Processingof Predicted Results
2.7. Software
3. Results and Discussion
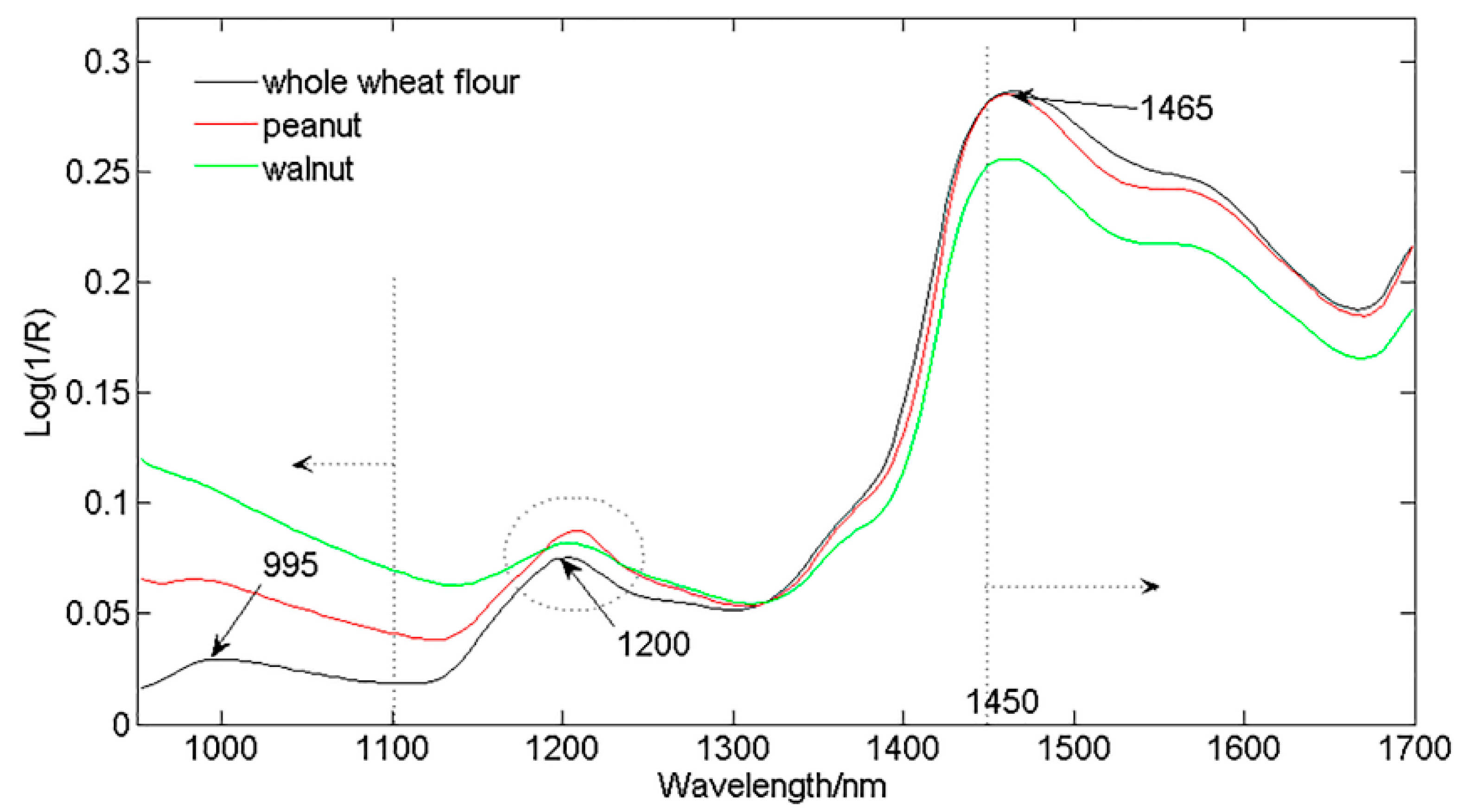
3.1. Spectral Features of Pure Whole Wheat Flour, Peanut Powder, and Walnut Powder
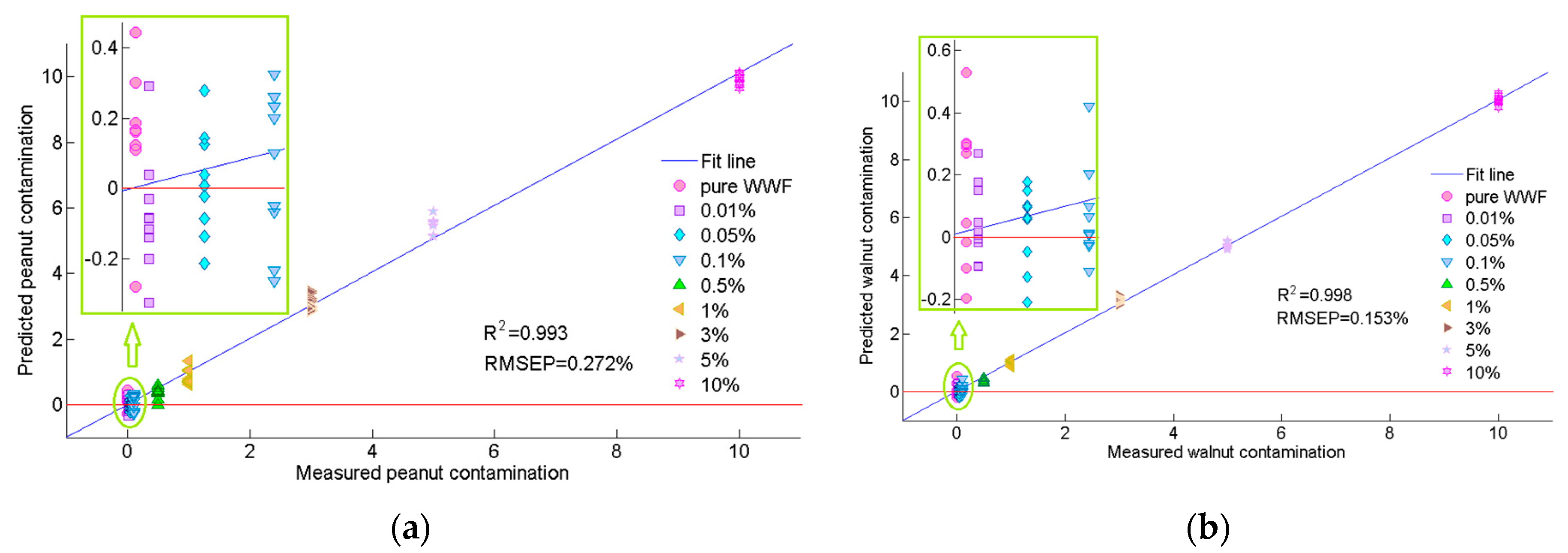
3.2. Comparison of Preprocessed Methods and Full Spectra Modeling
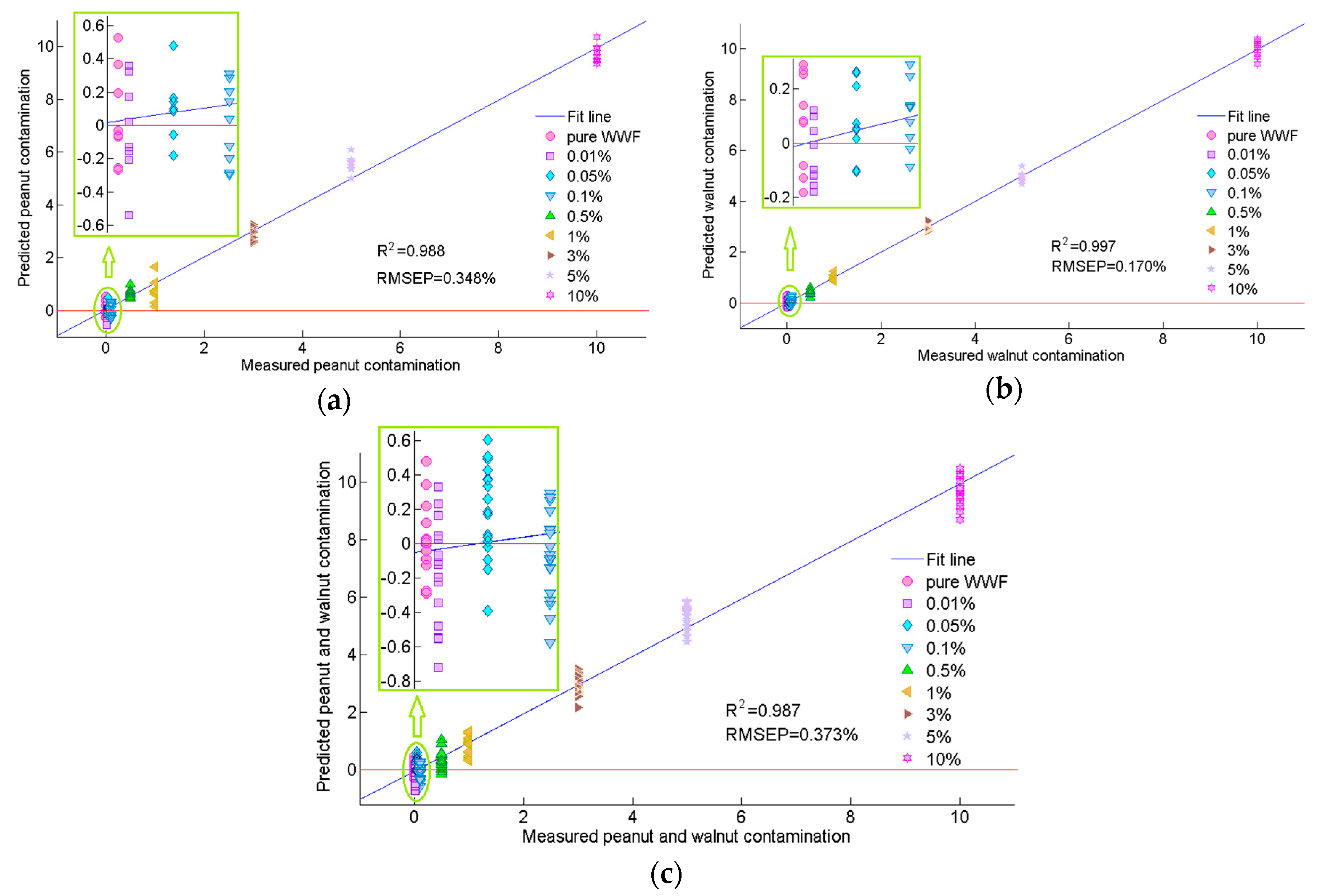
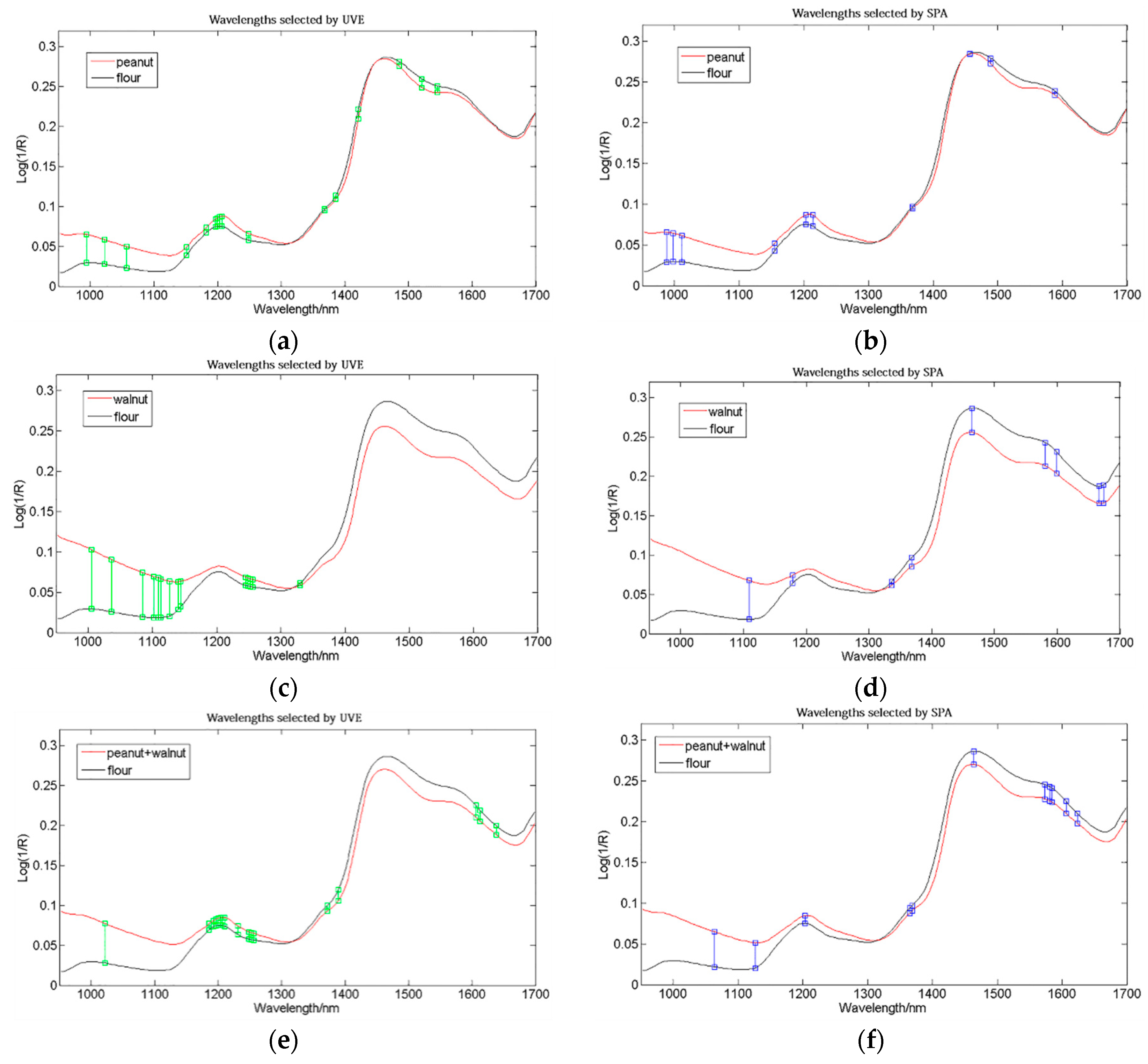
3.3. Selection of Optimal Wavelengths and Multispectral Model Development
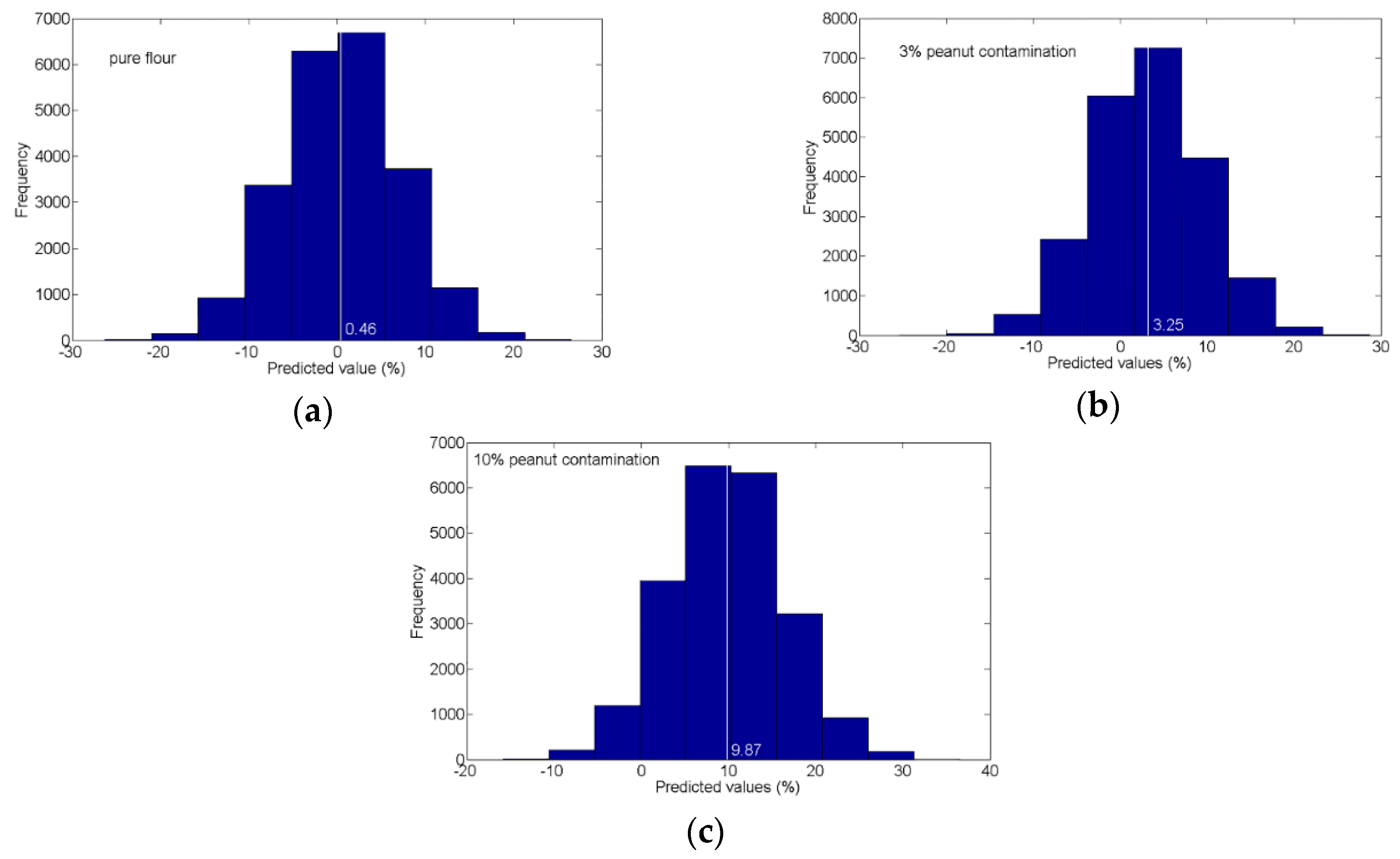
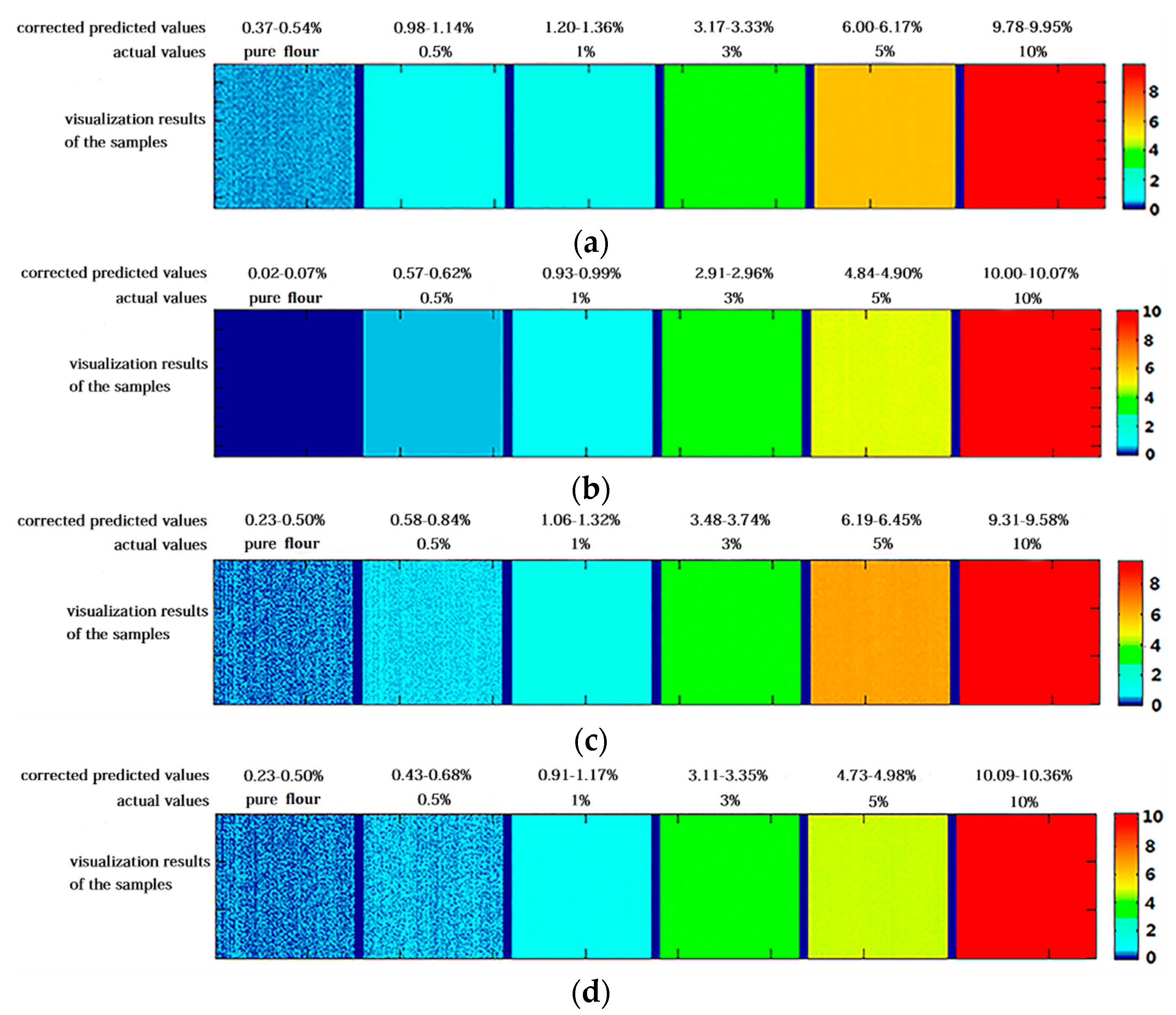
3.4. Post-Processing and Visualization of Prediction Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- López-Calleja, I.M.; de la Cruz, S.; Pegels, N.; González, I.; García, T.; Martín, R. Development of a real time PCR assay for detection of allergenic trace amounts of peanut (Arachishypogaea) in processed foods. Food Control. 2013, 30, 480–490. [Google Scholar] [CrossRef]
- Platteau, C.; De Loose, M.; De Meulenaer, B.; Taverniers, I. Detection of allergenic ingredients using real-time PCR: A case study on hazelnut (Corylus avellena) and soy (Glycine max). J. Agric. Food Chem. 2011, 59, 10803–10814. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Herrero-Langreo, A.; Barreiro, P.; Roger, J.M.; Diezma, B.; Gorretta, N.; Lleó, L. Detection and quantification of peanut traces in wheat flour by near infrared hyperspectral imaging spectroscopy using principal-component analysis. J. Near Infrared Spectrosc. 2015, 23, 15–22. [Google Scholar] [CrossRef]
- Pannico, A.; Schouten, R.E.; Basile, B.; Romano, R.; Woltering, E.J.; Cirillo, C. Non-destructive detection of flawed hazelnut kernels and lipid oxidation assessment using NIR spectroscopy. J. Food Eng. 2015, 160, 42–48. [Google Scholar] [CrossRef]
- Su, W.H.; He, H.J.; Sun, D.W. Non-destructive and rapid evaluation of staple foods quality by using spectroscopic techniques: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Domínguez, T.R.C.; Diezma, B.; Lleó, L.; Barreiro, P.; Lacarra, T.G.; Roger, J.M. VIS/NIR spectral signature for the identification of peanut contamination of powder foods. Agric. Eng. Int. CIGR J. 2015, 17, 310–329. [Google Scholar]
- Ghosh, S.; Mishra, P.; Mohamad, S.N.H.; de Santos, R.M.; Iglesias, B.D.; Elorza, P.B. Discrimination of peanuts from bulk cereals and nuts by near infrared reflectance spectroscopy. Biosyst. Eng. 2016, 151, 178–186. [Google Scholar] [CrossRef]
- Williams, P.J.; Kucheryavskiy, S. Classification of maize kernels using NIR hyperspectral imaging. Food Chem. 2016, 209, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Kim, G.; Kim, M.S.; Lim, J.; Lee, S.H.; Lee, H.S.; Cho, B.K. Discrimination methods for biological contaminants in fresh-cut lettuce based on VNIR and NIR hyperspectral imaging. Infrared Phys. Technol. 2017, 85, 1–12. [Google Scholar] [CrossRef]
- Munera, S.; Amigo, J.M.; Blasco, J.; Cubero, S.; Talens, P.; Aleixos, N. Ripeness monitoring of two cultivars of nectarine using VIS-NIR hyperspectral reflectance imaging. J. Food Eng. 2017, 214, 29–39. [Google Scholar] [CrossRef]
- Mollazade, K. Non-destructive Identifying Level of Browning Development in Button Mushroom (Agaricusbisporus) Using Hyperspectral Imaging Associated with Chemometrics. Food Anal. Methods 2017, 10, 2743–2754. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, D.W.; Cheng, W. Development of simplified models for nondestructive hyperspectral imaging monitoring of TVB-N contents in cured meat during drying process. J. Food Eng. 2017, 192, 53–60. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Fisk, I.D. Protein content prediction in single wheat kernels using hyperspectral imaging. Food Chem. 2018, 240, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.T.; Wilson, D.I.; Yu, W.; Young, B.R. An evaluation of hyperspectral imaging for characterising milk powders. J. Food Eng. 2018, 221, 1–10. [Google Scholar] [CrossRef]
- Lim, J.; Kim, G.; Mo, C.; Kim, M.S.; Chao, K.; Qin, J.; Fu, X.; Baek, I.; Cho, B.K. Detection of melamine in milk powders using near-infrared hyperspectral imaging combined with regression coefficient of partial least square regression model. Talanta 2016, 151, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Verdú, S.; Vásquez, F.; Grau, R.; Ivorra, E.; Sánchez, A.J.; Barat, J.M. Detection of adulterations with different grains in wheat products based on the hyperspectral image technique: The specific cases of flour and bread. Food Control. 2016, 62, 373–380. [Google Scholar] [CrossRef]
- Su, W.H.; Sun, D.W. Evaluation of spectral imaging for inspection of adulterants in terms of common wheat flour, cassava flour and corn flour in organic Avatar wheat (Triticum spp.) flour. J. Food Eng. 2017, 200, 59–69. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Makino, Y.; Oshita, S. Rapid and non-destructive detection of chicken adulteration in minced beef using visible near-infrared hyperspectral imaging and machine learning. J. Food Eng. 2016, 170, 8–15. [Google Scholar] [CrossRef]
- Jia, B.; Yoon, S.C.; Zhuang, H.; Wang, W.; Li, C. Prediction of pH of fresh chicken breast fillets by VNIR hyperspectral imaging. J. Food Eng. 2017, 208, 57–65. [Google Scholar] [CrossRef]
- Luypaert, J.; Zhang, M.H.; Massart, D.L. Feasibility study for the use of near infrared spectroscopy in the qualitative and quantitative analysis of green tea, Camellia sinensis (L.). Anal. Chim. Acta 2003, 478, 303–312. [Google Scholar] [CrossRef]
- Hailey, P.A.; Doherty, P.; Tapsell, P.; Oliver, T.; Aldridge, P.K. Automated system for the on-line monitoring of powder blending processes using near-infrared spectroscopy part I. system development and control. J. Pharm. Biomed. Anal. 1996, 14, 551–559. [Google Scholar] [CrossRef]
- Marchi, M.D.; Riovanto, R.; Penasa, M.; Cassandro, M. At-line prediction of fatty acid profile in chicken breast using near infrared reflectance spectroscopy. Meat Sci. 2012, 90, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, L.E.; Fry, F.S.; McLaughlin, M.A.; Calvey, E.M. Rapid analysis of sugars in fruit juices by FT-NIR spectroscopy. Carbohydr. Res. 2001, 336, 63–74. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Li, Y.-K. Determination of diesel cetane number by consensus modeling based on uninformative variable elimination. Anal. Methods 2012, 4, 254–258. [Google Scholar] [CrossRef]
- Ye, S.; Wang, D.; Min, S. Successive projections algorithm combined with uninformative variable elimination for spectral variable selection. Chemom. Intell. Lab. Syst. 2008, 91, 194–199. [Google Scholar] [CrossRef]
- Pontes, M.J.C.; Galvao, R.K.H.; Araújo, M.C.U.; Moreira, P.N.T.; Neto, O.D.P.; Jose, G.E.; Saldanha, T.C.B. The successive projections algorithm for spectral variable selection in classification problems. Chemom. Intell. Lab. Syst. 2005, 78, 11–18. [Google Scholar] [CrossRef]
- Chu, X.; Wang, W.; Yoon, S.C.; Ni, X.; Heitschmidt, G.W. Detection of aflatoxin B1, (AFB1) in individual maize kernels using short wave infrared (SWIR) hyperspectral imaging. Biosyst. Eng. 2017, 157, 13–23. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, B.; Wei, Y.; Zhang, B. Near infrared reflectance spectroscopy for determination of the geographical origin of wheat. Food Chem. 2013, 138, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Y.; Liu, C.; Wang, W.; Rao, P.; Fu, C.; Wang, S. SPA Combined with Swarm Intelligence Optimization Algorithms for Wavelength Variable Selection to Rapidly Discriminate the Adulteration of Apple Juice. Food Anal. Methods 2017, 10, 1965–1971. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Pu, H. Combining the genetic algorithm and successive projection algorithm for the selection of feature wavelengths to evaluate exudative characteristics in frozen–thawed fish muscle. Food Chem. 2016, 197, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Herrero-Langreo, A.; Barreiro, P.; Roger, J.M.; Diezma, B.; Gorretta, N.; Lleó, L. Hyperspectral to multispectral imaging for detection of tree nuts and peanut traces in wheat flour. J. Spectr. Imaging 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Mishra, P.; Cordella, C.B.Y.; Rutledge, D.N.; Barreiro, P.; Roger, J.M.; Diezma, B. Application of independent components analysis with the jade algorithm and nirhyperspectral imaging for revealing food adulteration. J. Food Eng. 2016, 168, 7–15. [Google Scholar] [CrossRef]
- Lammertyn, J.; Nicolai, B.; Ooms, K.; De Smedt, V.; De Baerdemaeker, J. Non-destructive measurement of acidity, soluble solids, and firmness of Jonagold apples using NIR-spectroscopy. Trans. ASAE 1998, 41, 1089–1094. [Google Scholar] [CrossRef]
- Downey, G.; Sheehan, E.; Delahunty, C.; O’Callaghan, D.; Guinee, T.; Howard, V. Prediction of maturity and sensory attributes of Cheddar cheese using near-infrared spectroscopy. Int. Dairy J. 2005, 15, 701–709. [Google Scholar] [CrossRef]
| Contaminant | Preprocessing Method | LVs | Calibration | Cross-Validation | Prediction | |||
|---|---|---|---|---|---|---|---|---|
| Rc2 | RMSEC (%) | Rcv2 | RMSECV (%) | Rp2 | RMSEP (%) | |||
| Peanut | None | 11 | 0.989 | 0.342 | 0.986 | 0.386 | 0.985 | 0.398 |
| SNV | 12 | 0.996 | 0.208 | 0.992 | 0.282 | 0.992 | 0.287 | |
| 1st Der | 10 | 0.994 | 0.259 | 0.991 | 0.298 | 0.991 | 0.307 | |
| Det | 13 | 0.995 | 0.225 | 0.992 | 0.293 | 0.993 | 0.267 | |
| SNV + 1st Der | 12 | 0.997 | 0.183 | 0.994 | 0.253 | 0.993 | 0.272 | |
| SNV + Det | 12 | 0.996 | 0.195 | 0.992 | 0.285 | 0.994 | 0.251 | |
| Walnut | None | 8 | 0.998 | 0.145 | 0.998 | 0.159 | 0.997 | 0.180 |
| SNV | 6 | 0.998 | 0.142 | 0.998 | 0.153 | 0.997 | 0.168 | |
| 1st Der | 8 | 0.998 | 0.159 | 0.997 | 0.175 | 0.998 | 0.161 | |
| Det | 9 | 0.998 | 0.154 | 0.997 | 0.184 | 0.996 | 0.198 | |
| SNV + 1st Der | 8 | 0.999 | 0.125 | 0.998 | 0.144 | 0.998 | 0.153 | |
| SNV + Det | 10 | 0.999 | 0.119 | 0.998 | 0.145 | 0.998 | 0.153 | |
| Contaminant | Selection Method | Number of Wavelengths | LVs | Calibration | Cross-Validation | Prediction | |||
|---|---|---|---|---|---|---|---|---|---|
| Rc2 | RMSEC (%) | Rcv2 | RMSECV (%) | Rp2 | RMSEP (%) | ||||
| Peanut | UVE | 16 | 12 | 0.991 | 0.312 | 0.989 | 0.340 | 0.988 | 0.348 |
| SPA | 10 | 9 | 0.983 | 0.422 | 0.981 | 0.447 | 0.981 | 0.465 | |
| Walnut | UVE | 14 | 6 | 0.998 | 0.147 | 0.998 | 0.157 | 0.997 | 0.170 |
| SPA | 9 | 8 | 0.992 | 0.295 | 0.991 | 0.312 | 0.990 | 0.324 | |
| Peanut + walnut | UVE | 17 | 10 | 0.988 | 0.345 | 0.987 | 0.360 | 0.987 | 0.373 |
| SPA | 11 | 8 | 0.964 | 0.608 | 0.961 | 0.635 | 0.960 | 0.645 | |
| Contaminant | Selection Method | Number of Wavelengths | Wavelengths |
|---|---|---|---|
| Peanut | UVE | 16 | 994, 1022, 1057, 1151, 1182, 1196, 1200, 1203, 1207, 1249, 1368, 1386, 1421, 1485, 1521, 1545 |
| SPA | 10 | 988, 998, 1012, 1154, 1203, 1214, 1368, 1457, 1489, 1588 | |
| Walnut | UVE | 14 | 1005, 1036, 1085, 1102, 1109, 1113, 1127, 1140, 1144, 1245, 1249, 1252, 1256, 1330 |
| SPA | 9 | 1109, 1179, 1337, 1368, 1464, 1581, 1599, 1667, 1674 | |
| Peanut + walnut | UVE | 17 | 1022, 1186, 1193, 1196, 1200, 1203, 1207, 1210, 1231, 1249, 1252, 1256 1372, 1390, 1606, 1613, 1638 |
| SPA | 11 | 1064, 1127, 1203, 1365, 1368, 1464, 1574, 1581, 1585, 1606, 1624 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Wang, W.; Ni, X.; Chu, X.; Li, Y.-F.; Sun, C. Evaluation of Near-Infrared Hyperspectral Imaging for Detection of Peanut and Walnut Powders in Whole Wheat Flour. Appl. Sci. 2018, 8, 1076. https://doi.org/10.3390/app8071076
Zhao X, Wang W, Ni X, Chu X, Li Y-F, Sun C. Evaluation of Near-Infrared Hyperspectral Imaging for Detection of Peanut and Walnut Powders in Whole Wheat Flour. Applied Sciences. 2018; 8(7):1076. https://doi.org/10.3390/app8071076
Chicago/Turabian StyleZhao, Xin, Wei Wang, Xinzhi Ni, Xuan Chu, Yu-Feng Li, and Changpo Sun. 2018. "Evaluation of Near-Infrared Hyperspectral Imaging for Detection of Peanut and Walnut Powders in Whole Wheat Flour" Applied Sciences 8, no. 7: 1076. https://doi.org/10.3390/app8071076
APA StyleZhao, X., Wang, W., Ni, X., Chu, X., Li, Y.-F., & Sun, C. (2018). Evaluation of Near-Infrared Hyperspectral Imaging for Detection of Peanut and Walnut Powders in Whole Wheat Flour. Applied Sciences, 8(7), 1076. https://doi.org/10.3390/app8071076






