Nonlinearity Analysis and Multi-Model Modeling of an MEA-Based Post-Combustion CO2 Capture Process for Advanced Control Design
Abstract
1. Introduction
- (1)
- Good prediction accuracy and concise model structure. In general, increasing the number of the local linear models will improve the model accuracy; however, it also intensifies the complexity of the model structure, which can lead to a significant growth in the computation time of the model predictions and thus make the multi-linear model lose its advantage. Therefore, the accuracy and complexity of the multi-linear model must be balanced by choosing appropriate local linear models. In order to determine the best local linear models for the construction of a multi-linear model, the nonlinearity distribution of the process must be investigated.
- (2)
- Proper mathematical expression. For multi-input multi-output (MIMO) nonlinear process, the model with an appropriate mathematical expression can greatly reduce the complexity of the advanced controller design and the computational cost of the control law.
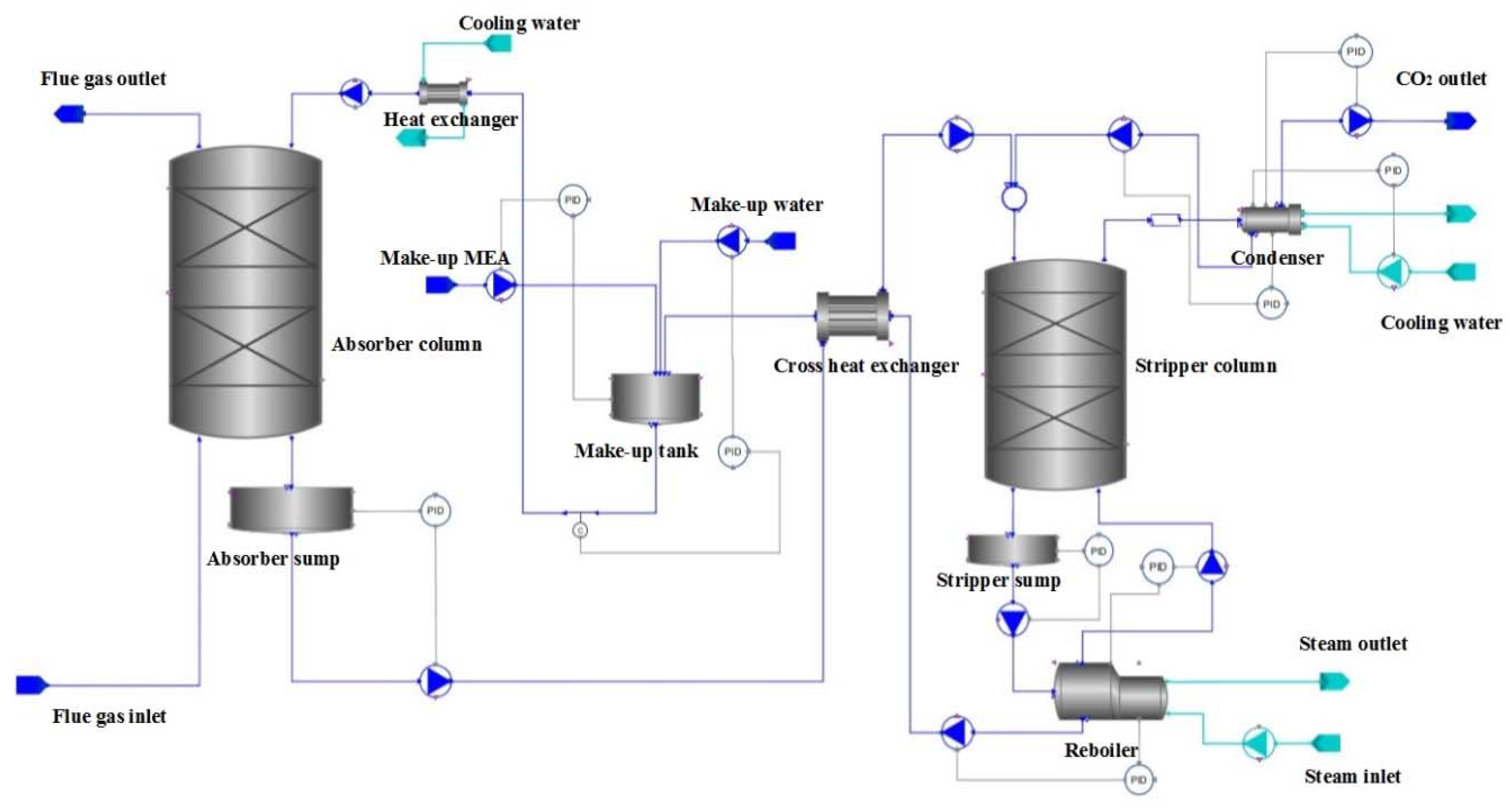
2. Dynamic Model Configuration for the CO2 Capture Process
- (1)
- All the chemical reactions are in equilibrium state.
- (2)
- The pressure drop along the column is linear.
- (3)
- The holdup in the vapor balk and the solvent degradation are neglected.
- (4)
- The phase at the interface between liquid and vapor films attains equilibrium.
3. Multi-Model Modeling of the CO2 Capture Process
3.1. Identification of the Local Linear Models
- (1)
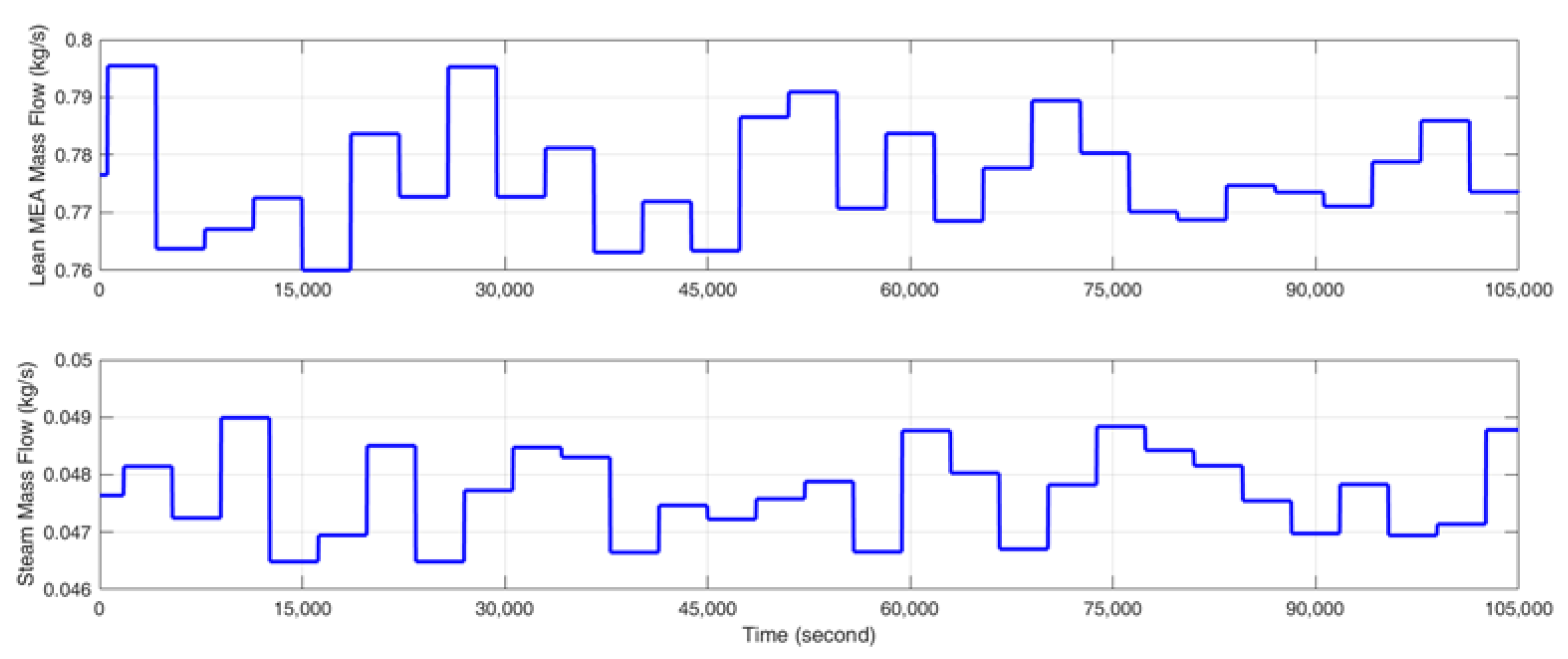
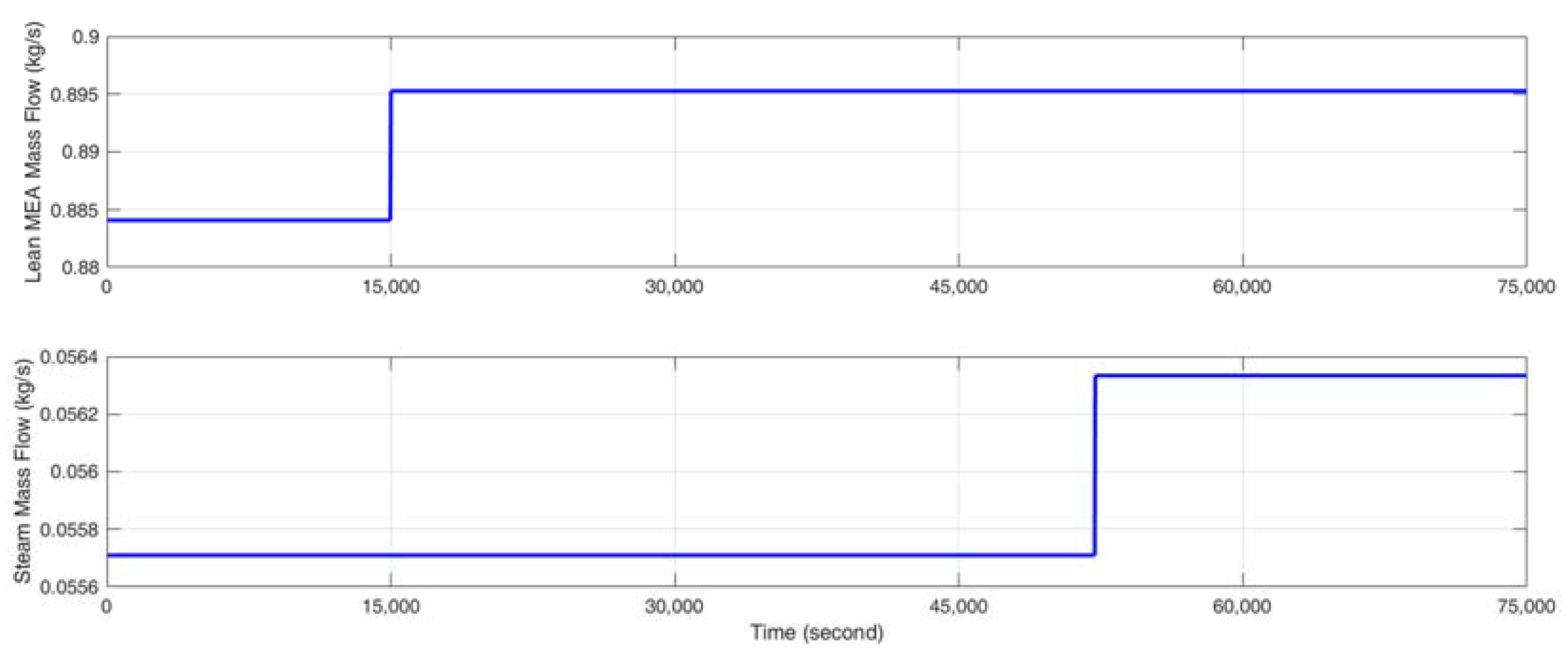
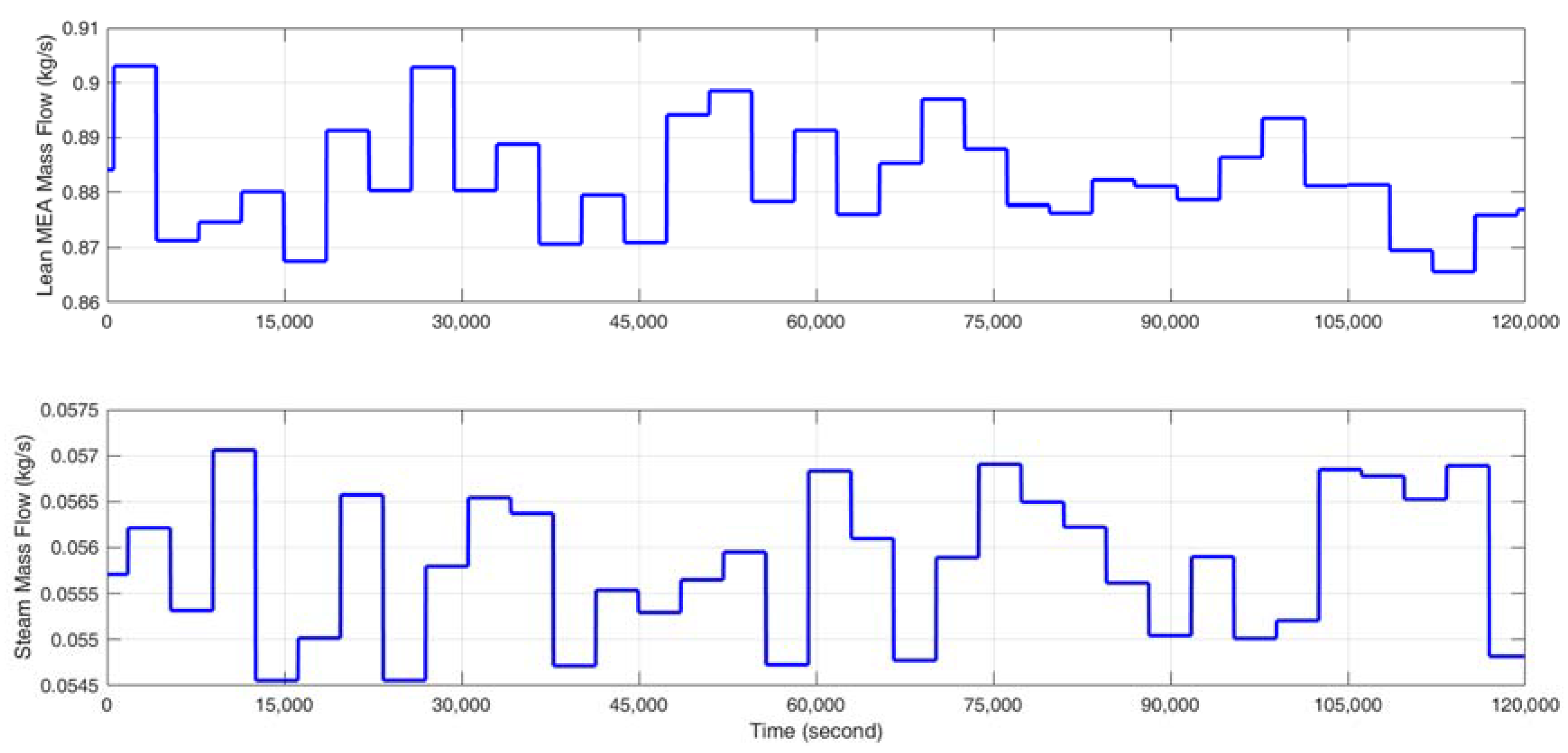
- Stimulate the CO2 capture process with a random identification signal, which fluctuates about ±2% of the steady-state input value around the selected operating point so that the CO2 capture rate varies about ±5% in absolute value and the reboiler temperature varies within ±0.5 K. The gCCS simulator can provide data at every second; however, considering the slow dynamics of the MEA-based post-combustion CO2 capture process, the sampling time is selected as 30 s. The input signal is designed to change every 3000 s since a fast identification signal change is not suitable for capturing the dynamics and the steady state of the CO2 capture process.
- (2)
- Construct the Hankel Matrixes using the collected input-output data, and then partition the Hankel Matrixes into past and the future block matrixes.
- (3)
- Combine the partitioned Hankel Matrixes into a new data matrix and perform QR factorization on them.
- (4)
- Calculate the subspace matrix from the factorization results of step (3) and perform singular-value decomposition (SVD) on the subspace matrix. Extract the system matrixes from the subspace matrices.
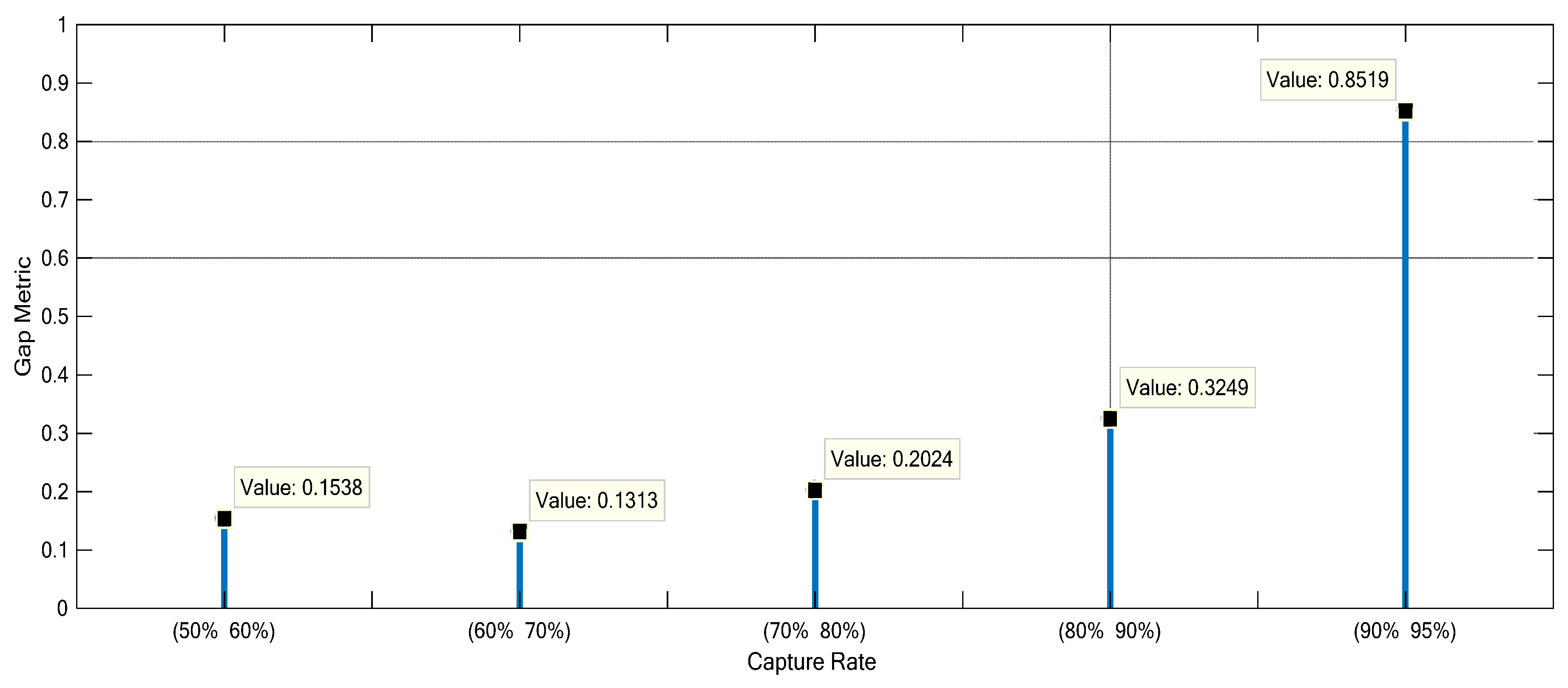
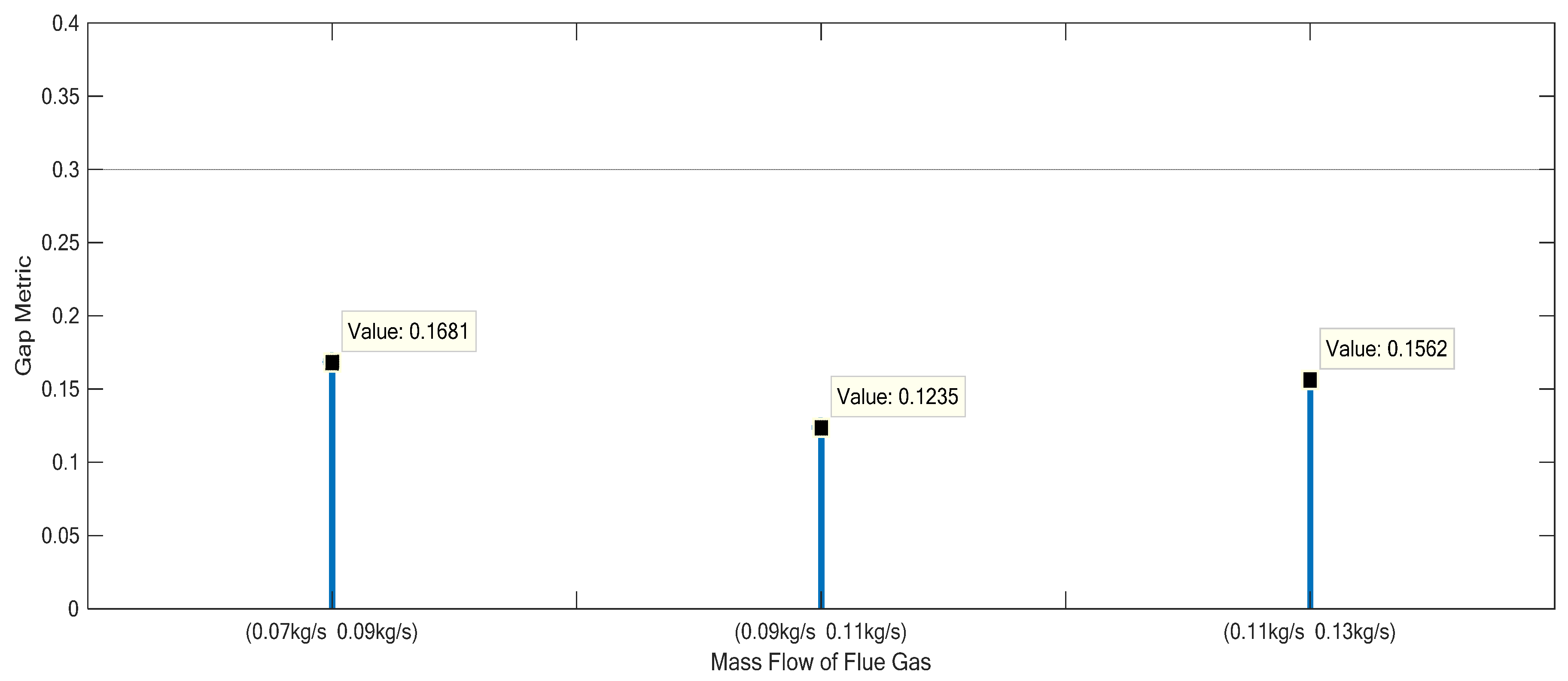
3.2. Nonlinearity Analysis of the Monoethanolamine-Based Post-Combustion CO2 Capture Process
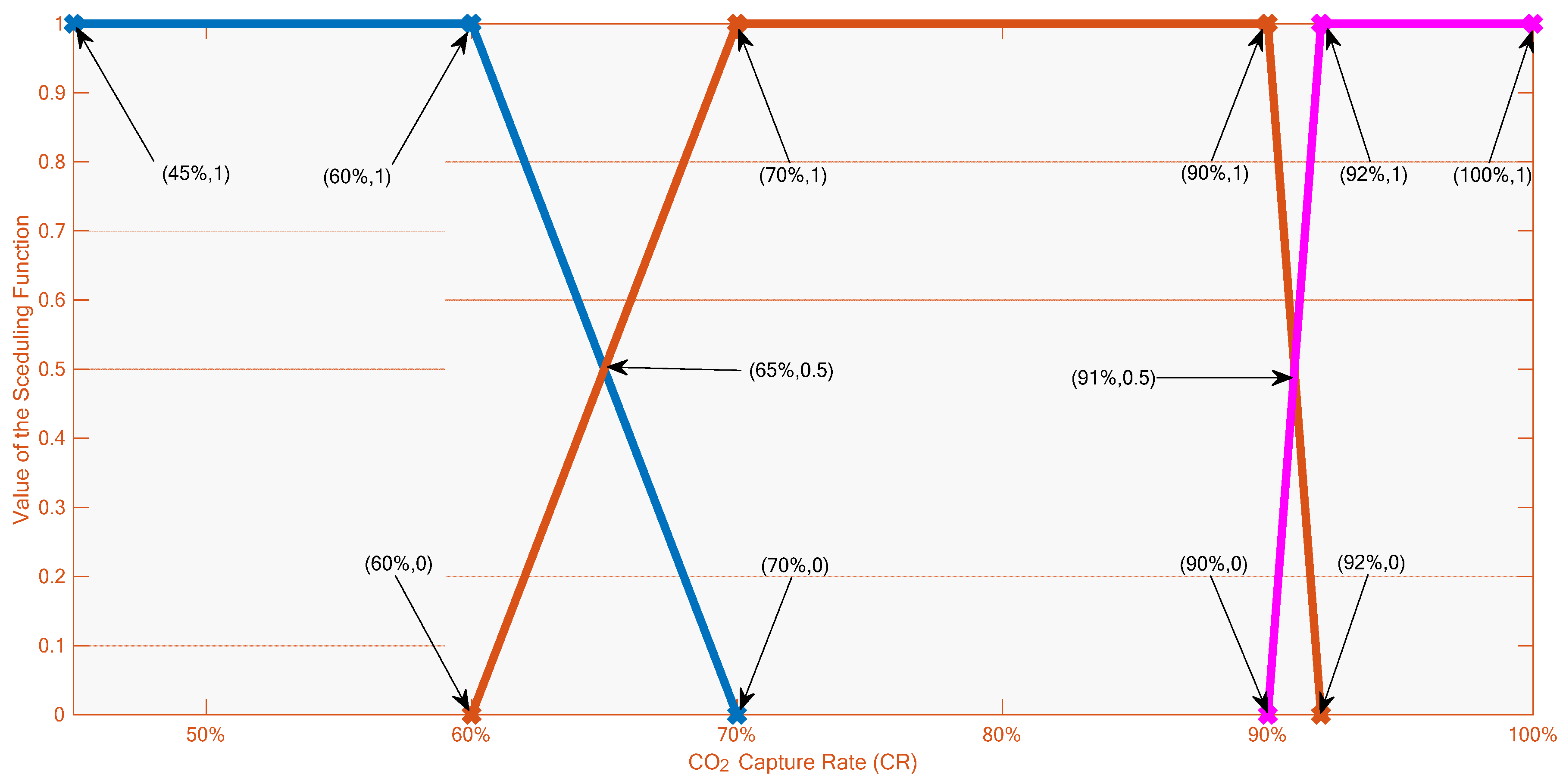
3.3. Derivation of the Multi-Linear Model
4. Simulation Results
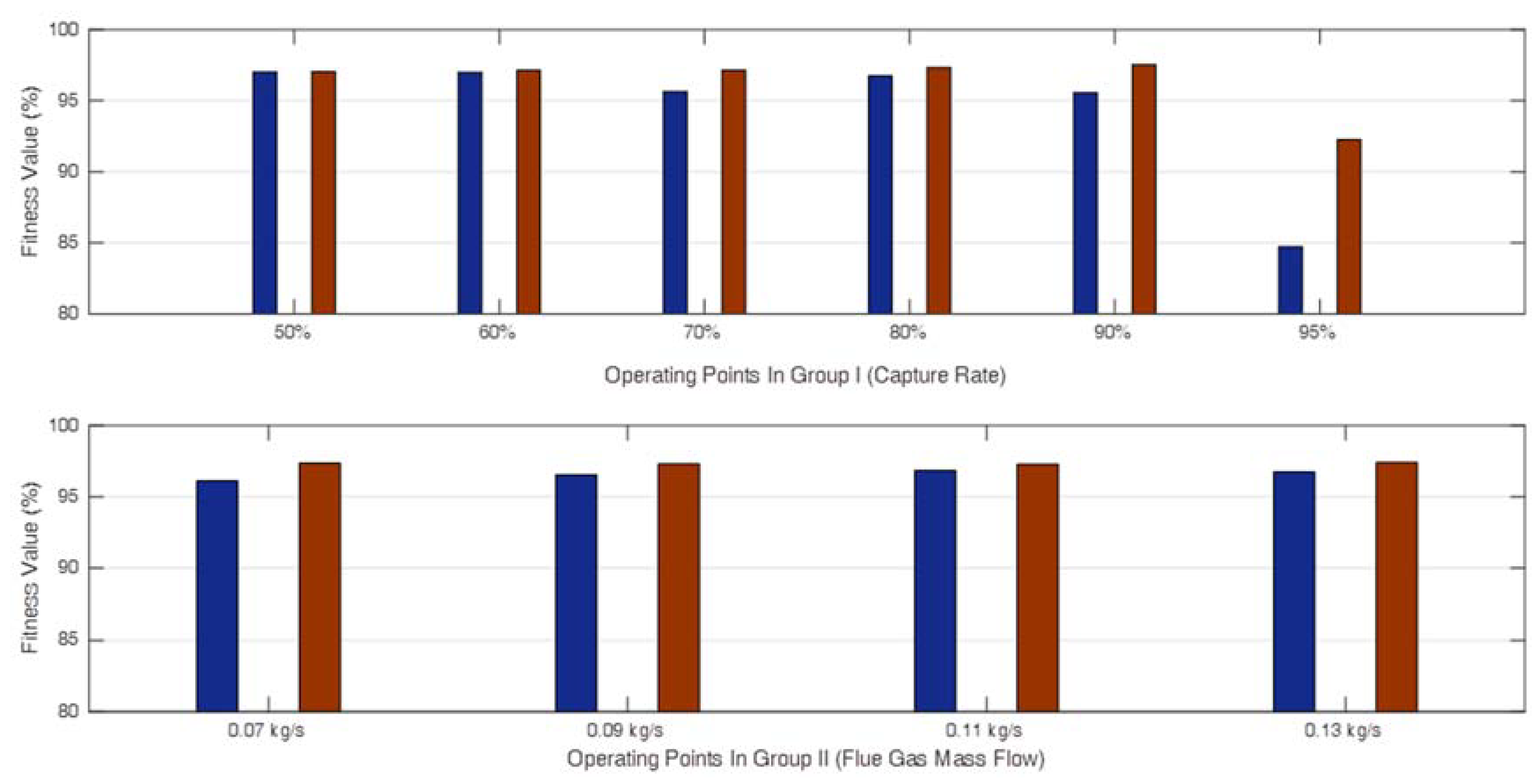
4.1. Validation of the Local Linear Models
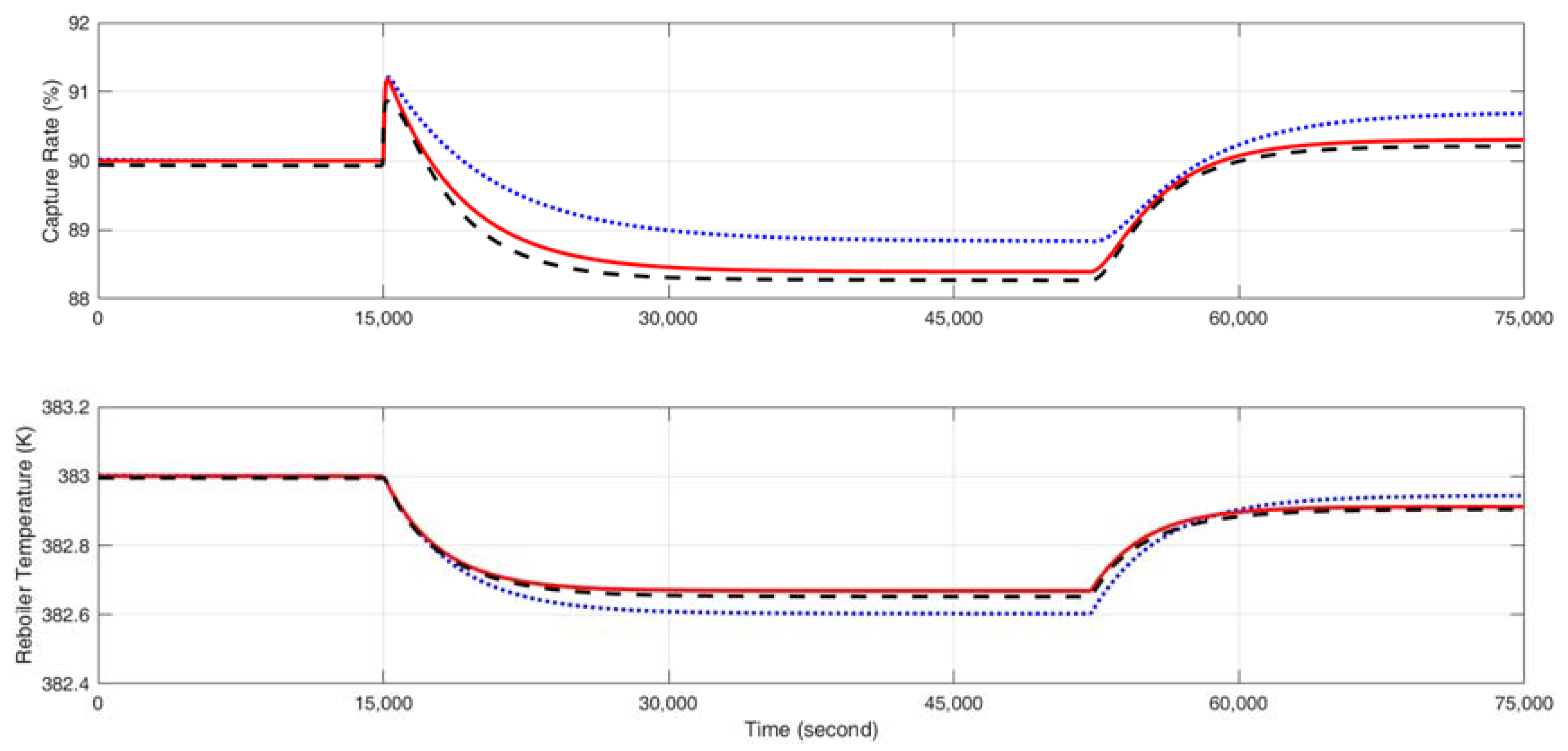
4.2. Validation of the Multi-Linear Model
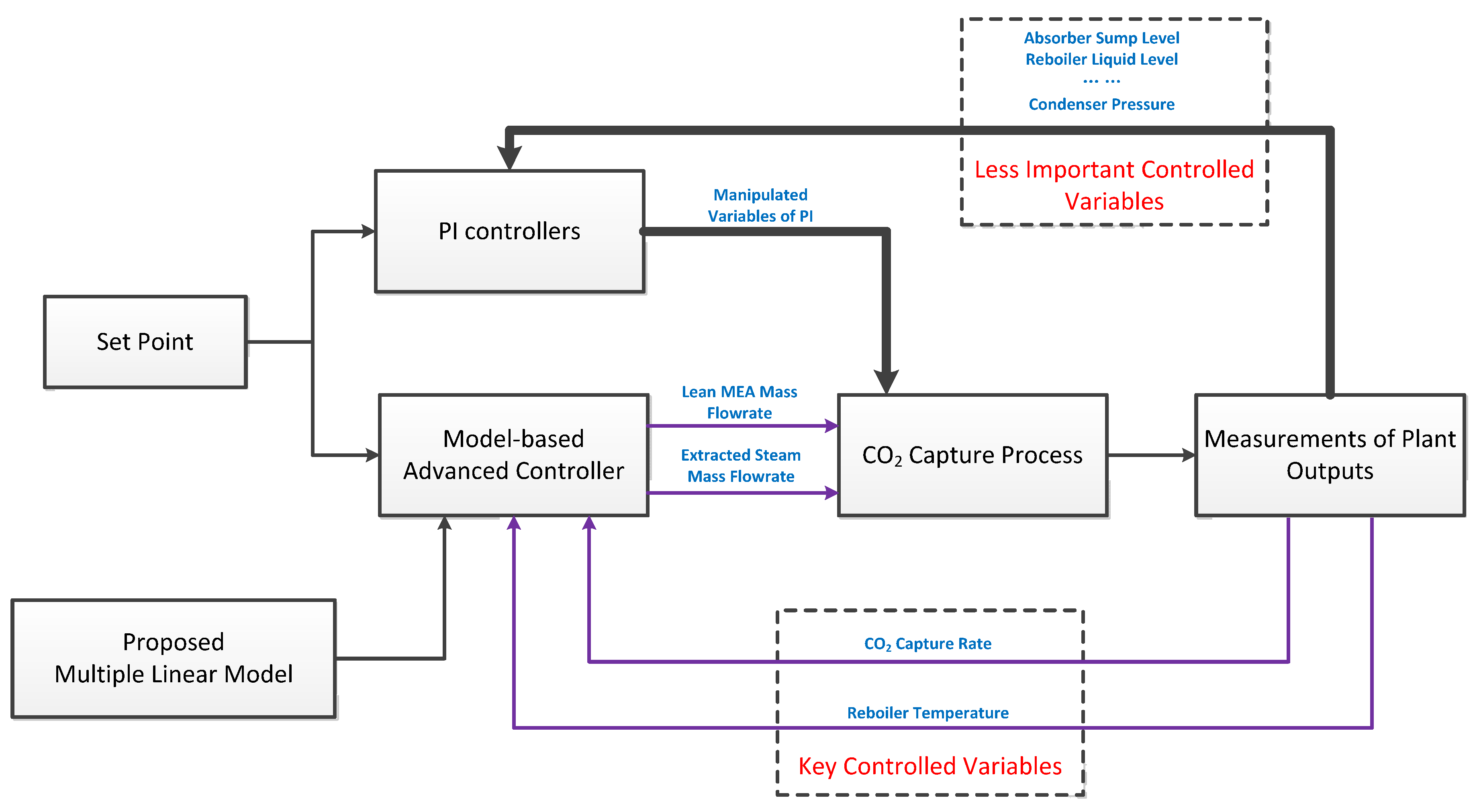
5. Introducing the Multi-Linear Model to Advanced Control System Design
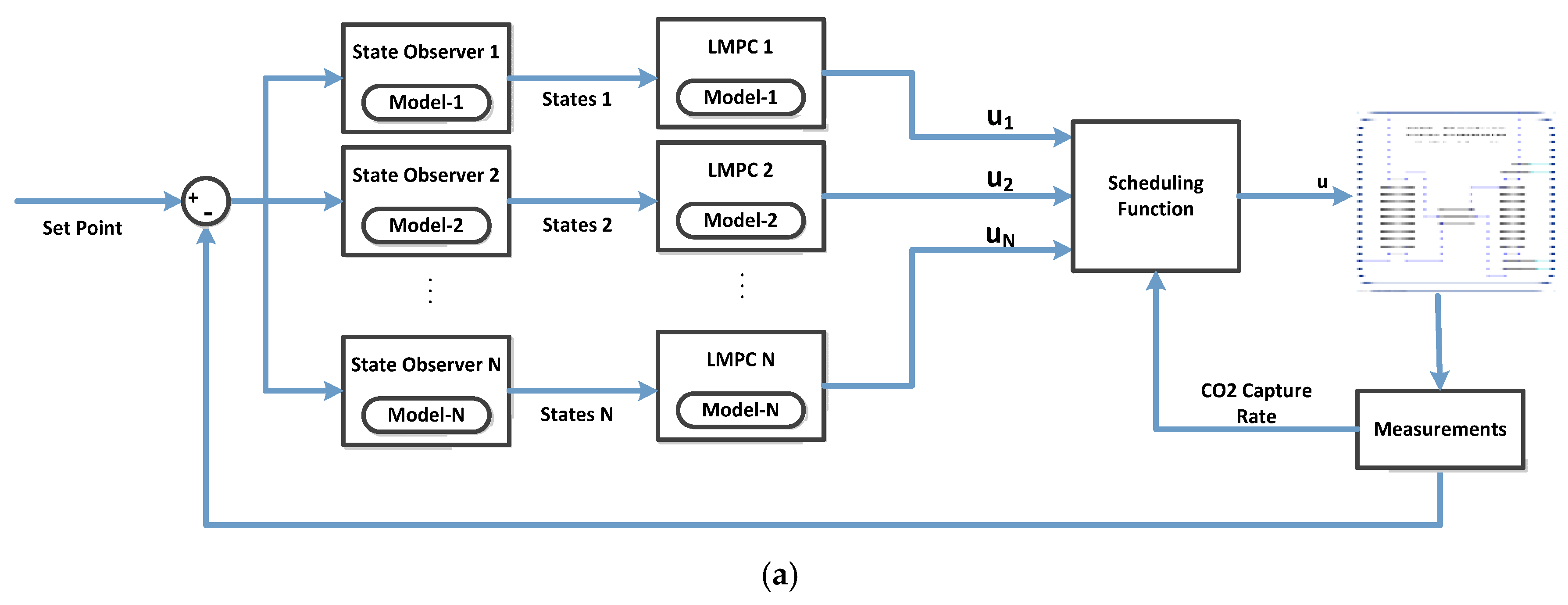
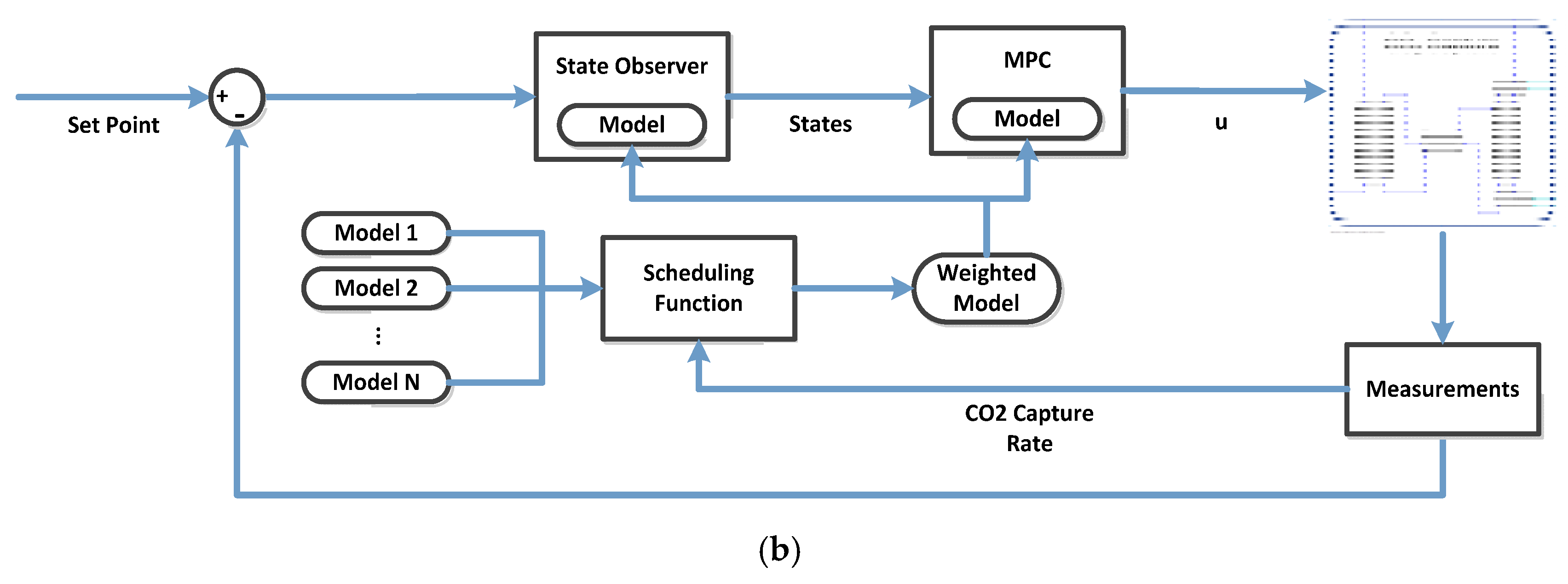
5.1. Multi-Model Predictive Control
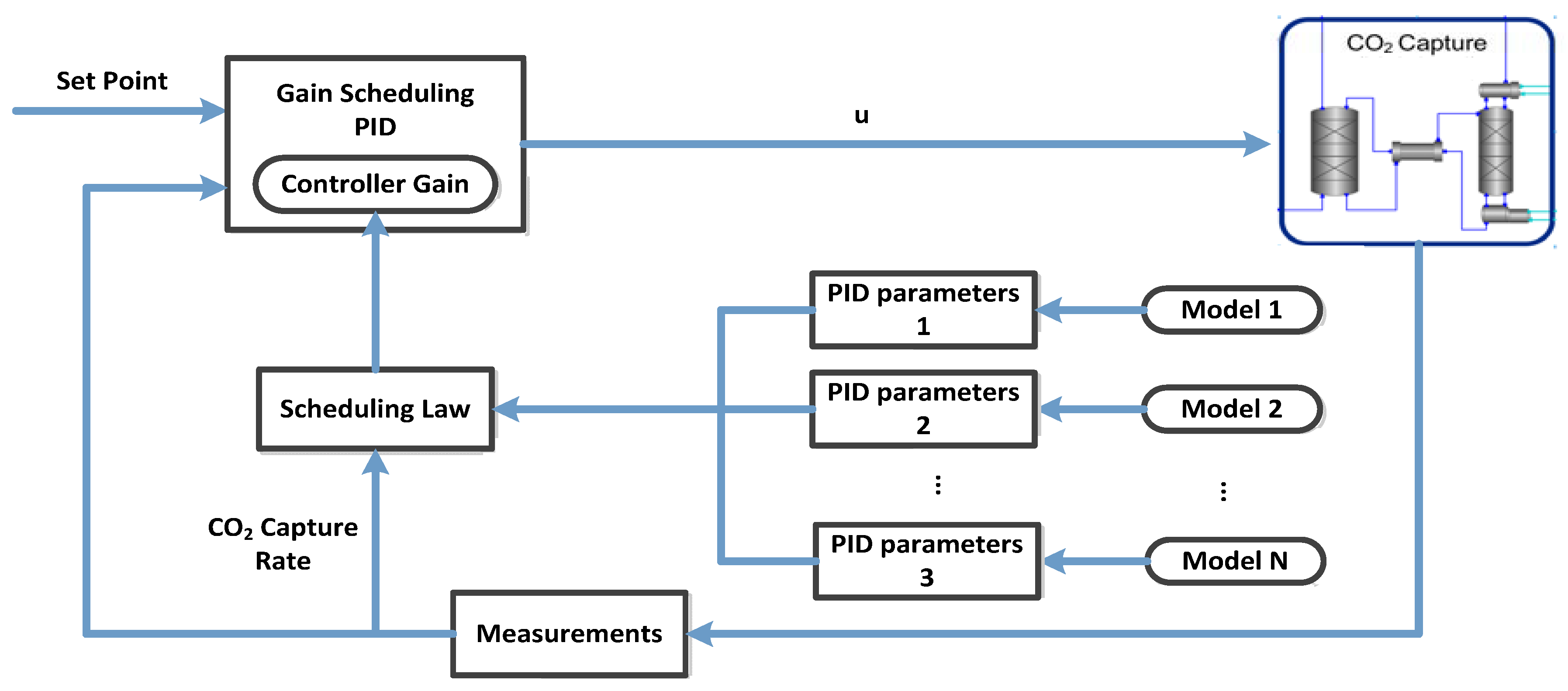
5.2. Gain Scheduling Proportional Integral Derivative Control
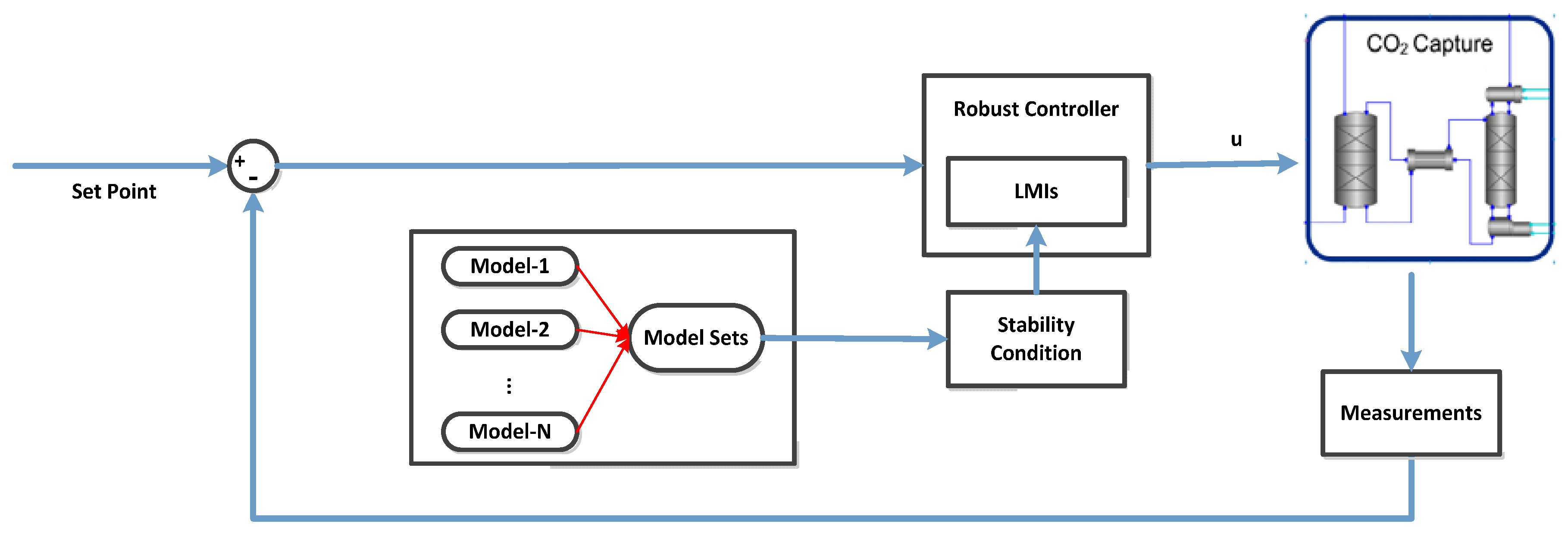
5.3. Robust Control
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bui, M.; Gunawan, I.; Verheyen, V.; Feron, P.; Meuleman, E.; Adeloju, S. Dynamic modelling and optimisation of flexible operation in post-combustion CO2 capture plants—A review. Comput. Chem. Eng. 2014, 61, 245–265. [Google Scholar] [CrossRef]
- Lawal, A.; Wang, M.; Stephenson, P.; Koumpouras, G.; Yeung, H. Dynamic modelling and analysis of post-combustion CO2 chemical absorption process for coal-fired power plants. Fuel 2010, 89, 2791–2801. [Google Scholar] [CrossRef]
- Lawal, A.; Wang, M.; Stephenson, P.; Obi, O. Demonstrating full-scale post-combustion CO2 capture for coal-fired power plants through dynamic modelling and simulation. Fuel 2012, 101, 115–128. [Google Scholar] [CrossRef]
- Nittaya, T.; Douglas, P.L.; Croiset, E.; Ricardez-Sandoval, L.A. Dynamic modelling and control of MEA absorption processes for CO2 capture from power plants. Fuel 2014, 116, 672–691. [Google Scholar] [CrossRef]
- Luu, M.T.; Manaf, N.A.; Abbas, A. Dynamic modelling and control strategies for flexible operation of amine-based post-combustion CO2 capture systems. Int. J. Greenh. Gas Control 2015, 39, 377–389. [Google Scholar] [CrossRef]
- He, Z.; Sahraei, M.H.; Ricardez-Sandoval, L.A. Flexible operation and simultaneous scheduling and control of a CO2 capture plant using model predictive control. Int. J. Greenh. Gas Control 2016, 48, 300–311. [Google Scholar] [CrossRef]
- Wellner, K.; Marx-Schubach, T.; Schmitz, G. Dynamic behavior of coal-fired power plants with postcombustion CO2 capture. Ind. Eng. Chem. Res. 2016, 55, 12038–12045. [Google Scholar] [CrossRef]
- Walters, M.S.; Edgar, T.F.; Rochelle, G.T. Dynamic modeling and control of an intercooled absorber for post-combustion CO2 capture. Chem. Eng. Process. Process Intensif. 2016, 107, 1–10. [Google Scholar] [CrossRef]
- Posch, S.; Haider, M. Dynamic modeling of CO2 absorption from coal-fired power plants into an aqueous monoethanolamine solution. Chem. Eng. Res. Des. 2013, 91, 977–987. [Google Scholar] [CrossRef]
- Ziaii, S.; Rochelle, G.T.; Edgar, T.F. Dynamic modeling to minimize energy use for CO2 capture in power plants by aqueous monoethanolamine. Ind. Eng. Chem. Res. 2009, 48, 6105–6111. [Google Scholar] [CrossRef]
- Mac Dowell, N.; Samsatli, N.J.; Shah, N. Dynamic modelling and analysis of an amine-based post-combustion CO2 capture absorption column. Int. J. Greenh. Gas Control 2013, 12, 247–258. [Google Scholar] [CrossRef]
- Léonard, G.; Mogador, B.C.; Belletante, S.; Heyen, G. Dynamic modelling and control of a pilot plant for post-combustion CO2 capture. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013; Volume 32, pp. 451–456. [Google Scholar]
- Lawal, A.; Wang, M.; Stephenson, P.; Yeung, H. Dynamic modelling of CO2 absorption for post combustion capture in coal-fired power plants. Fuel 2009, 88, 2455–2462. [Google Scholar] [CrossRef]
- Manaf, N.A.; Cousins, A.; Feron, P.; Abbas, A. Dynamic modelling, identification and preliminary control analysis of an amine-based post-combustion CO2 capture pilot plant. J. Clean. Prod. 2016, 113, 635–653. [Google Scholar] [CrossRef]
- Van De Haar, A.; Trapp, C.; Wellner, K.; De Kler, R.; Schmitz, G.; Colonna, P. Dynamics of postcombustion CO2 capture plants: Modeling, validation, and case study. Ind. Eng. Chem. Res. 2017, 56, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Wong, D.S.-H.; Jang, S.-S.; Ou, J.-J. Control strategies for flexible operation of power plant with CO2 capture plant. AIChE J. 2012, 58, 2697–2704. [Google Scholar] [CrossRef]
- Sahraei, M.H.; Ricardez-Sandoval, L.A. Controllability and optimal scheduling of a CO2 capture plant using model predictive control. Int. J. Greenh. Gas Control 2014, 30, 58–71. [Google Scholar] [CrossRef]
- Agbonghae, E.O.; Hughes, K.J.; Ingham, D.B.; Ma, L.; Pourkashanian, M. Optimal process design of commercial-scale amine-based CO2 capture plants. Ind. Eng. Chem. Res. 2014, 53, 14815–14829. [Google Scholar] [CrossRef]
- Arce, A.; Mac Dowell, N.; Shah, N.; Vega, L.F. Flexible operation of solvent regeneration systems for CO2 capture processes using advanced control techniques: Towards operational cost minimisation. Int. J. Greenh. Gas Control 2012, 11, 236–250. [Google Scholar] [CrossRef]
- Garđarsdóttir, S.O.; Montañés, R.M.; Normann, F.; Nord, L.O.; Johnsson, F. Effects of CO2-absorption control strategies on the dynamic performance of a supercritical pulverized-coal-fired power plant. Ind. Eng. Chem. Res. 2017, 56, 4415–4430. [Google Scholar] [CrossRef]
- Afkhamipour, M.; Mofarahi, M. Sensitivity analysis of the rate-based CO2 absorber model using amine solutions (MEA, MDEA and AMP) in packed columns. Int. J. Greenh. Gas Control 2014, 25, 9–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Chen, C.-C.; Plaza, J.M.; Dugas, R.; Rochelle, G.T. Rate-based process modeling study of CO2 capture with aqueous monoethanolamine solution. Ind. Eng. Chem. Res. 2009, 48, 9233–9246. [Google Scholar] [CrossRef]
- Gabrielsen, J.; Svendsen, H.F.; Michelsen, M.L.; Stenby, E.H.; Kontogeorgis, G.M. Experimental validation of a rate-based model for CO2 capture using an AMP solution. Chem. Eng. Sci. 2007, 62, 2397–2413. [Google Scholar] [CrossRef]
- Mores, P.; Scenna, N.; Mussati, S. A rate based model of a packed column for CO2 absorption using aqueous monoethanolamine solution. Int. J. Greenh. Gas Control 2012, 6, 21–36. [Google Scholar] [CrossRef]
- Niu, Z.; Guo, Y.; Zeng, Q.; Lin, W. Experimental studies and rate-based process simulations of CO2 absorption with aqueous ammonia solutions. Ind. Eng. Chem. Res. 2012, 51, 5309–5319. [Google Scholar] [CrossRef]
- Plaza, J.M.; Van Wagener, D.; Rochelle, G.T. Modeling CO2 capture with aqueous monoethanolamine. Int. J. Greenh. Gas Control 2010, 4, 161–166. [Google Scholar] [CrossRef]
- Borhani, T.N.G.; Akbari, V.; Afkhamipour, M.; Hamid, M.K.A.; Manan, Z.A. Comparison of equilibrium and non-equilibrium models of a tray column for post-combustion CO2 capture using DEA-promoted potassium carbonate solution. Chem. Eng. Sci. 2015, 122, 291–298. [Google Scholar] [CrossRef]
- Yokoyama, T. Analysis of reboiler heat duty in MEA process for CO2 capture using equilibrium-staged model. Sep. Purif. Technol. 2012, 94, 97–103. [Google Scholar] [CrossRef]
- Qin, S.J.; Badgwell, T.A. An overview of nonlinear model predictive control applications. In Nonlinear Model Predictive Control; Springer: New York, NY, USA, 2000; pp. 369–392. [Google Scholar]
- Zhang, Q.; Turton, R.; Bhattacharyya, D. Development of model and model-predictive control of an MEA-based postcombustion CO2 capture process. Ind. Eng. Chem. Res. 2016, 55, 1292–1308. [Google Scholar] [CrossRef]
- Sipöcz, N.; Tobiesen, F.A.; Assadi, M. The use of artificial neural network models for CO2 capture plants. Appl. Energy 2011, 88, 2368–2376. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.; Oko, E.; Wang, M. Modelling of a post-combustion CO2 capture process using neural networks. Fuel 2015, 151, 156–163. [Google Scholar] [CrossRef]
- Bai, Z.; Li, F.; Zhang, J.; Oko, E.; Wang, M.; Xiong, Z.; Huang, D. Modelling of a post-combustion CO2 capture process using bootstrap aggregated extreme learning machines. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 2007–2012. [Google Scholar]
- Du, J.; Johansen, T.A. Integrated multilinear model predictive control of nonlinear systems based on gap metric. Ind. Eng. Chem. Res. 2015, 54, 6002–6011. [Google Scholar] [CrossRef]
- Du, J.; Song, C.; Yao, Y.; Li, P. Multilinear model decomposition of MIMO nonlinear systems and its implication for multilinear model-based control. J. Process Control 2013, 23, 271–281. [Google Scholar] [CrossRef]
- Mechleri, E.; Lawal, A.; Ramos, A.; Davison, J.; Mac Dowell, N. Process control strategies for flexible operation of post-combustion CO2 capture plants. Int. J. Greenh. Gas Control 2017, 57, 14–25. [Google Scholar] [CrossRef]
- Putta, K.R.; Knuutila, H.; Svendsen, H.F. Activity based kinetics and mass transfer of CO2 absorption into MEA using penetration theory. Energy Procedia 2014, 63, 1196–1205. [Google Scholar] [CrossRef]
- Chapman, W.G.; Gubbins, K.E.; Jackson, G.; Radosz, M. SAFT: Equation-of-state solution model for associating fluids. Fluid Phase Equilib. 1989, 52, 31–38. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Pan, T.-H.; Wong, D.S.-H.; Jang, S.-S.; Chi, Y.-W.; Yeh, C.-H. Plantwide control of CO2 capture by absorption and stripping using monoethanolamine solution. Ind. Eng. Chem. Res. 2010, 50, 1338–1345. [Google Scholar] [CrossRef]
- Ziegler, J.G. Optimum settings for automatic controllers. Asme Trans 1993, 64, 759–768. [Google Scholar] [CrossRef]
- Katayama, T. Subspace Methods for System Identification; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Wu, X.; Shen, J.; Li, Y.; Lee, K.Y. Data-driven modeling and predictive control for boiler-turbine unit using fuzzy clustering and subspace methods. ISA Trans. 2014, 53, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Zames, G. Unstable systems and feedback: The gap metric. In Proceedings of the Allerton Conference, Monticello, IL, USA, 8–10 October 1980; pp. 380–385. [Google Scholar]
- Qui, L.; Davison, E.J. Feedback stability under simultaneous gap metric uncertainties in plant and controller. Syst. Control Lett. 1992, 18, 9–22. [Google Scholar] [CrossRef]
- Du, J.; Johansen, T.A. A gap metric based weighting method for multimodel predictive control of MIMO nonlinear systems. J. Process Control 2014, 24, 1346–1357. [Google Scholar] [CrossRef]
- Galan, O.; Romagnoli, J.A.; Arkun, Y.; Palazoglu, A. On the use of gap metric for model selection in multilinear model-based control. In Proceedings of the 2000 American Control Conference, Chicago, IL, USA, 28–30 June 2000; Volume 6, pp. 3742–3746. [Google Scholar]
- Tao, X.; Li, D.; Wang, Y.; Li, N.; Li, S. Gap-metric-based multiple-model Predictive control with a polyhedral stability region. Ind. Eng. Chem. Res. 2015, 54, 11319–11329. [Google Scholar] [CrossRef]
- Schach, M.; Schneider, R.; Schramm, H.; Repke, J. Control structure design for CO2-absorption processes with large operating ranges. Energy Technol. 2013, 1, 233–244. [Google Scholar] [CrossRef]
- Wu, X.; Shen, J.; Li, Y.; Lee, K.Y. Fuzzy modeling and stable model predictive tracking control of large-scale power plants. J. Process Control 2014, 24, 1609–1626. [Google Scholar] [CrossRef]
- Camacho, E.F.; Bordons, C. Nonlinear model predictive control. In Model Predictive Control; Springer: New York, NY, USA, 2007; pp. 249–288. [Google Scholar]
- Blanchett, T.P.; Kember, G.C.; Dubay, R. PID gain scheduling using fuzzy logic. ISA Trans. 2000, 39, 317–325. [Google Scholar] [CrossRef]
- El Ghaoui, L.; Niculescu, S. Advances in Linear Matrix Inequality Methods in Control; SIAM: Philadelphia, PA, USA, 2000. [Google Scholar]




| Stream ID | Temperature (K) | Mass Flow (kg/s) | Mass Fraction | |||
|---|---|---|---|---|---|---|
| H2O | CO2 | MEA | N2 | |||
| Lean MEA to Absorber | 313.9 | 0.777 | 0.618 | 0.074 | 0.308 | 0.000 |
| Rich MEA from Absorber | 323.9 | 0.798 | 0.593 | 0.104 | 0.303 | 0.000 |
| Inlet Flue Gas | 314.3 | 0.130 | 0.015 | 0.252 | 0.000 | 0.733 |
| Outlet Flue Gas | 317.5 | 0.108 | 0.060 | 0.060 | 0.000 | 0.879 |
| Controlled Variable | Manipulated Variable | Set Point Value | Kp | KI |
|---|---|---|---|---|
| Absorber sump liquid level | Absorber sump outlet liquid mass flow | 1.25 m | 200 | 0.10 |
| Stripper sump liquid level | Stripper sump outlet liquid mass flow | 1.25 m | 200 | 0.10 |
| Condenser temperature | Cooling water mass flow | 313.15 K | 80 | 0.06 |
| Condenser pressure | Condenser outlet gas mass flow | 1.69 × 105 Pa | 5.5 | 0.15 |
| Condenser liquid level | Condenser outlet liquid mass flow | 0.25 m | 100 | 0.10 |
| Reboiler pressure | Reboiler outlet gas mass flow | 1.79 × 105 Pa | 5.0 | 0.15 |
| Reboiler liquid level | Reboiler outlet liquid mass flow | 0.25 m | 100 | 0.10 |
| Make-up tank liquid level | Tank outlet liquid mass flow | 1 m | 20 | 0.10 |
| Mass fraction of MEA | Make-up MEA mass flow | 0.307 | 10 | 0.05 |
| Groups | CO2 Capture Rate (%) | Flue Gas Mass Flow Rate (kg/s) | Lean MEA Mass Flow Rate (kg/s) | Steam Mass Flow Rate (kg/s) | CO2 Mass Fraction in Outlet Flue Gas (kg/kg) | MEA Mass Fraction in Lean Solvent (kg/kg) |
|---|---|---|---|---|---|---|
| Group I | 50 | 0.13 | 0.4468 | 0.02498 | 0.1350 | 0.3078 |
| Group I | 60 | 0.13 | 0.5572 | 0.03223 | 0.1121 | 0.3071 |
| Group I | 70 | 0.13 | 0.6687 | 0.03987 | 0.0873 | 0.3079 |
| Group I | 80 | 0.13 | 0.7765 | 0.04764 | 0.0603 | 0.3079 |
| Group I | 90 | 0.13 | 0.8841 | 0.05571 | 0.0314 | 0.3078 |
| Group I | 95 | 0.13 | 0.9428 | 0.06012 | 0.0160 | 0.3073 |
| Group II | 80 | 0.07 | 0.4064 | 0.02127 | 0.0602 | 0.3073 |
| Group II | 80 | 0.09 | 0.5257 | 0.03032 | 0.0598 | 0.3076 |
| Group II | 80 | 0.11 | 0.6501 | 0.03876 | 0.0603 | 0.3080 |
| Group II | 80 | 0.13 | 0.7765 | 0.04764 | 0.0601 | 0.3075 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Li, Y.; Wu, X.; Shen, J.; Lee, K.Y. Nonlinearity Analysis and Multi-Model Modeling of an MEA-Based Post-Combustion CO2 Capture Process for Advanced Control Design. Appl. Sci. 2018, 8, 1053. https://doi.org/10.3390/app8071053
Liang X, Li Y, Wu X, Shen J, Lee KY. Nonlinearity Analysis and Multi-Model Modeling of an MEA-Based Post-Combustion CO2 Capture Process for Advanced Control Design. Applied Sciences. 2018; 8(7):1053. https://doi.org/10.3390/app8071053
Chicago/Turabian StyleLiang, Xiufan, Yiguo Li, Xiao Wu, Jiong Shen, and Kwang Y. Lee. 2018. "Nonlinearity Analysis and Multi-Model Modeling of an MEA-Based Post-Combustion CO2 Capture Process for Advanced Control Design" Applied Sciences 8, no. 7: 1053. https://doi.org/10.3390/app8071053
APA StyleLiang, X., Li, Y., Wu, X., Shen, J., & Lee, K. Y. (2018). Nonlinearity Analysis and Multi-Model Modeling of an MEA-Based Post-Combustion CO2 Capture Process for Advanced Control Design. Applied Sciences, 8(7), 1053. https://doi.org/10.3390/app8071053







