Acetic Acid as an Indirect Sink of CO2 for the Synthesis of Polyhydroxyalkanoates (PHA): Comparison with PHA Production Processes Directly Using CO2 as Feedstock
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Organism and Inoculum Preparation
2.2. Culture Media
2.3. Shake Flask Experiments
2.4. Fed-Batch Experiments
2.5. Analytical Methods
2.6. Calculations
3. Results and Discussion
3.1. Homopolymer PHB Production from Acetic Acid
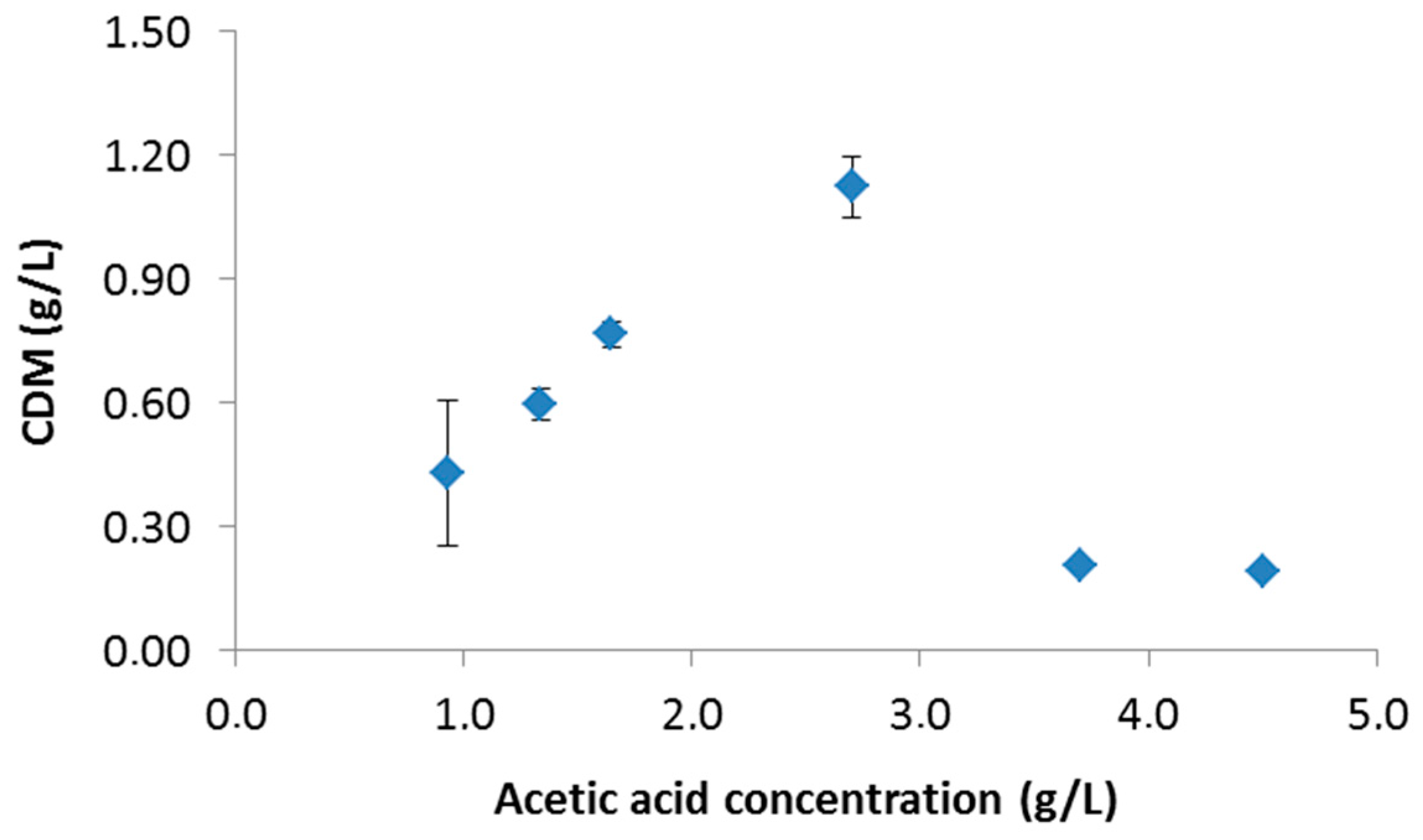
3.1.1. Effect of Initial Acetic Acid Concentration
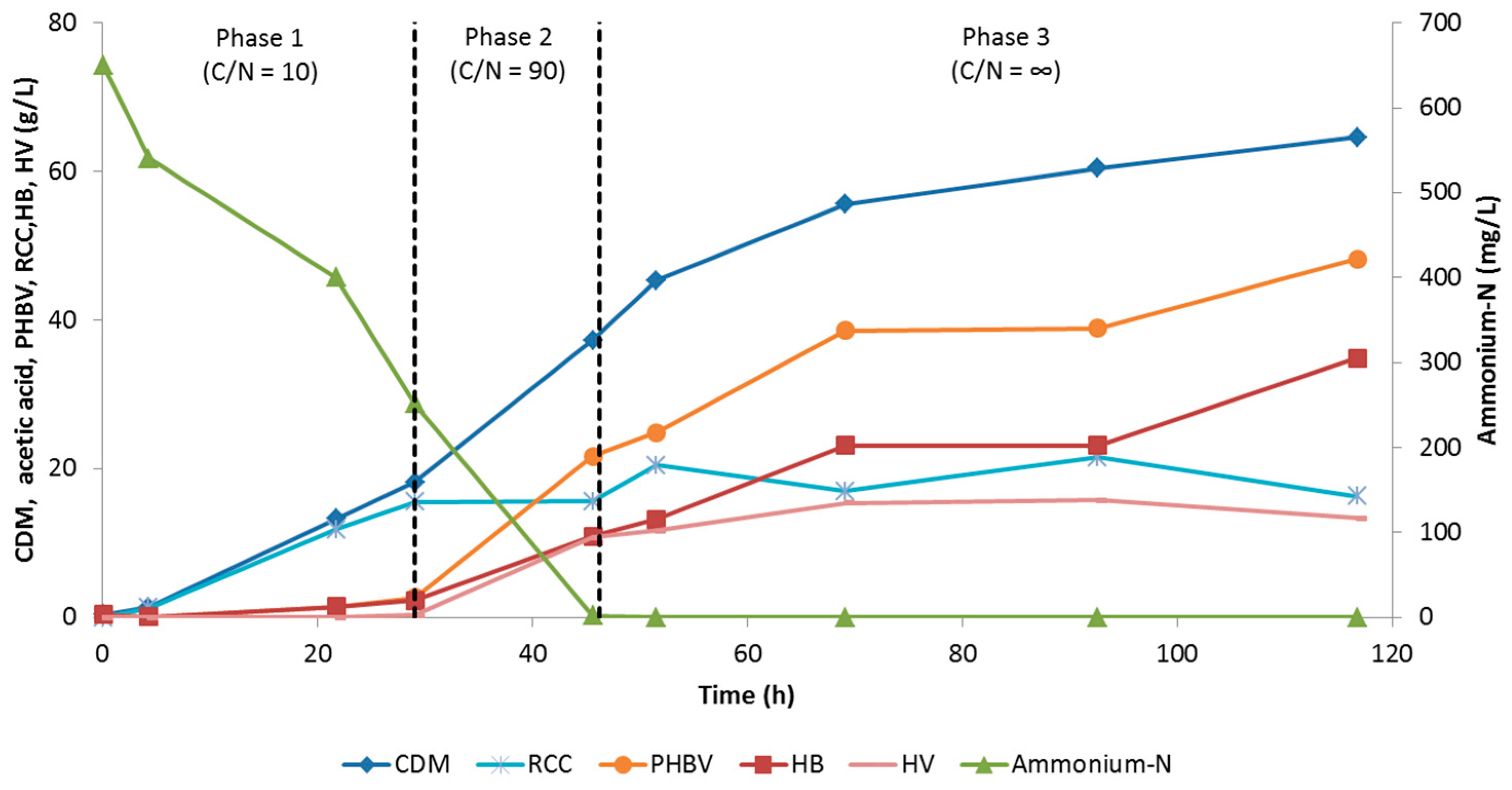
3.1.2. Evaluation of Two-Phase and Three-Phase Fed-Batch Culture
3.2. Copolymer PHBV Production from Acetic Acid and Valeric Acid
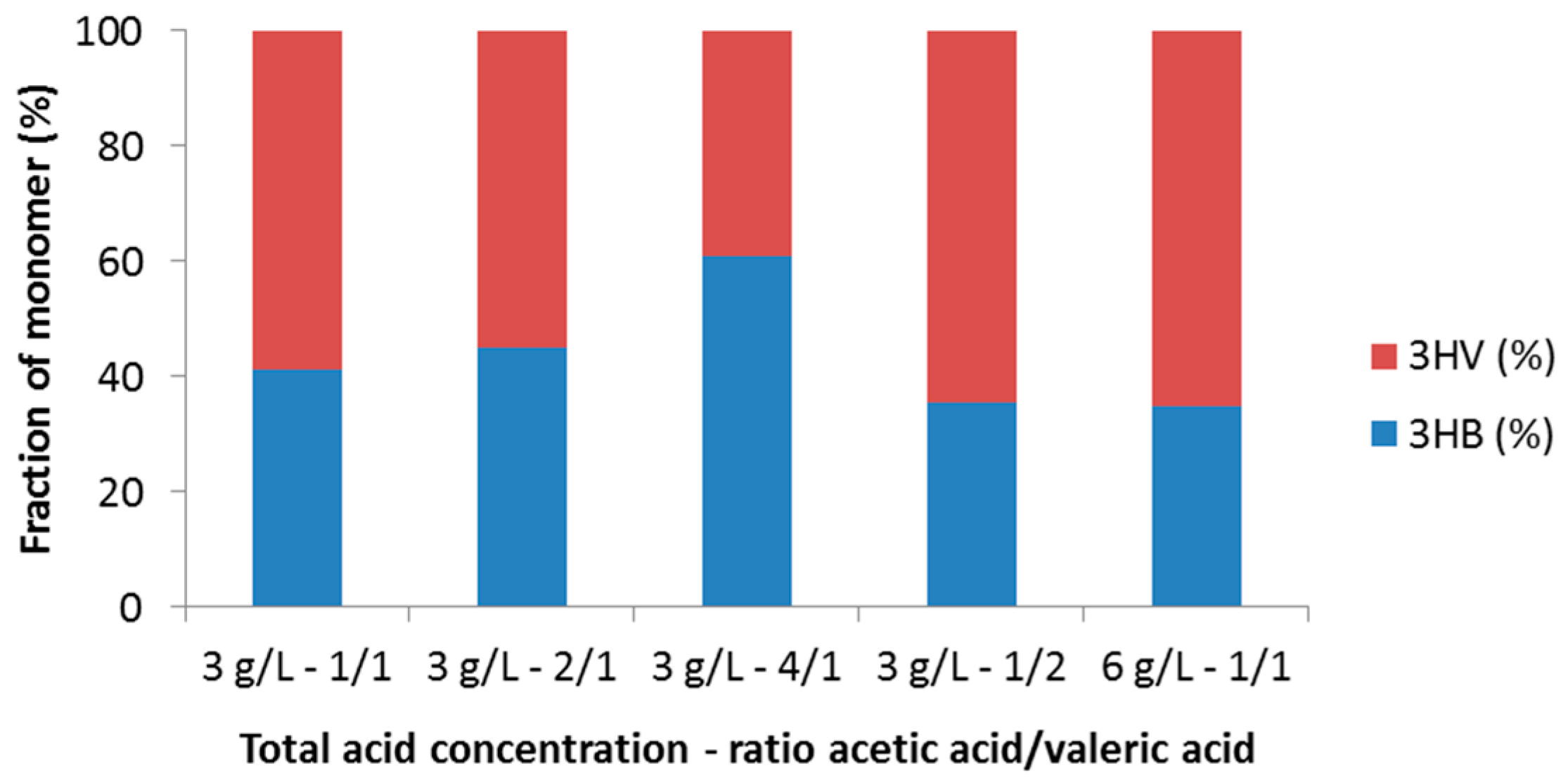
3.2.1. Effect of Initial Acid Concentration and Composition
3.2.2. Evaluation of Three-Phase Fed-Batch Culture
3.3. Evaluation of PHA Production Processes Using CO2 as Feedstock
- the autotrophic–autotrophic process
- the heterotrophic–autotrophic process
- autotrophic–heterotrophic–heterotrophic process
4. Conclusions
- Acetic acid exhibits a toxic effect on cell growth when present above 3 g/L.
- A three-phase fed-batch culture consisting of biomass growth, biomass growth and PHA production, and PHA production outperformed a conventional two-phase cultivation system.
- PHB production by C. necator from acetic acid resulted in the highest PHB concentration reported so far.
- C. necator favoured consumption of valeric acid over acetic acid during growth, while during PHA production, consumption of acetic acid was preferential.
- Production of PHBV by C. necator from acetic acid and valeric acid is promising for attaining a high 3HV fraction in the polymeric chain.
- The indirect conversion of acetic acid from CO2 is an interesting alternative for the direct production of PHA from CO2 in terms of CO2 fixation, H2 consumption, substrate cost, safety and process performance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- 2030 Climate & Energy Framework. Available online: https://ec.europa.eu/clima/policies/strategies/2030_en (accessed on 1 May 2018).
- Carbon Capture Utilisation and Storage. Available online: https://setis.ec.europa.eu/setis-reports/setis-magazine/carbon-capture-utilisation-and-storage/chemical-valorisation-of-co2 (accessed on 1 May 2018).
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Huschner, F.; Grousseau, E.; Brigham, C.J.; Plassmeier, J.; Popovic, M.; Rha, C.; Sinskey, A.J. Development of a feeding strategy for high cell and PHA density fed-batch fermentation of Ralstonia eutropha H16 from organic acids and their salts. Process Biochem. 2015, 50, 165–172. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; De Wever, H. Valorisation of CO2-rich off-gases to biopolymers through biotechnological process. FEMS Microbiol. Lett. 2017, 364, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, A.; Tanaka, K.; Taga, N. Microbial production of poly-D-3-hydroxybutyrate from CO2. Appl. Microbiol. Biotechnol. 2001, 57, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Mozumder, M.S.I.; Garcia-Gonzalez, L.; De Wever, H.; Volcke, E.I.P. Poly(3-hydroxybutyrate) (PHB) production from CO2: Model development and process optimization. Biochem. Eng. J. 2015, 98, 107–116. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Mozumder, M.S.I.; Dubreuil, M.; Volcke, E.I.P.; De Wever, H. Sustainable autotrophic production of polyhydroxybutyrate (PHB) from CO2 using a two-stage cultivation system. Catal. Today 2015, 257, 237–245. [Google Scholar] [CrossRef]
- Ishizaki, A.; Tanaka, K. Production of poly-β-hydroxybutyric acid from carbon dioxide by Alcaligenes eutrophus ATCC 17697T. J. Ferment. Bioeng. 1991, 71, 254–257. [Google Scholar] [CrossRef]
- Ghysels, S.; Mozumder, M.S.I.; De Wever, H.; Volcke, E.I.P.; Garcia-Gonzalez, L. Targeted poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastic production from carbon dioxide. Bioresour. Technol. 2018, 249, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W. Enzymology of the Wood-Ljungdahl pathway of acetogenesis. Ann. N. Y. Acad. Sci. 2008, 1125, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Morgan-Sagastume, F.; Campanari, S.; Villano, M.; Werker, A.; Majone, M. Carbon recovery from wastewater through bioconversion into biodegradable polymers. New Biotechnol. 2017, 37, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Arslan, D.; Steinbusch, K.J.J.; Diels, L.; Hamelers, H.V.M.; Strik, D.P.B.T.B.; Buisman, C.J.N.; De Wever, H. Selective short-chain carboxylates production: A review of control mechanisms to direct mixed culture fermentations. Crit. Rev. Environ. Sci. Technol. 2016, 46, 592–634. [Google Scholar] [CrossRef]
- Wang, J.; Yue, Z.B.; Sheng, G.P.; Yu, H.Q. Kinetic analysis on the production of polyhydroxyalkanoates from volatile fatty acids by Cupriavidus necator with a consideration of substrate inhibition, cell growth, maintenance, and product formation. Biochem. Eng. J. 2010, 49, 422–428. [Google Scholar] [CrossRef]
- Grousseau, E.; Blanchet, E.; Déléris, S.; Albuquerque, M.G.E.; Paul, E.; Uribelarrea, J.L. Phosphorus limitation strategy to increase propionic acid flux towards 3-hydroxyvaleric acid monomers in Cupriavidus necator. Bioresour. Technol. 2014, 153, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Mozumder, M.S.I.; De Wever, H.; Volcke, E.I.P.; Garcia-Gonzalez, L. A robust fed-batch feeding strategy independent of the carbon source for optimal polyhydroxybutyrate production. Process Biochem. 2014, 49, 365–373. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J. Kinetic analysis on inhibited growth and poly(3-hydroxybutyrate) formation of Alcaligenes eutrophus on acetate under nutrient-rich conditions. Process Biochem. 2000, 36, 201–207. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J. Kinetic analysis on formation of poly(3-hydroxybutyrate) from acetic acid by Ralstonia eutropha under chemically defined conditions. J. Ind. Microbiol. Biotechnol. 2001, 26, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Tsuge, T.; Tanaka, K.; Ishizaki, A. Control of acetic acid concentration by pH-stat continuous substrate feeding in heterotrophic culture phase of two-stage cultivation of Alcaligenes eutrophus for production of P(3HB) from CO2, H2, and O2 under non-explosive conditions. Biotechnol. Bioeng. 1999, 62, 625–631. [Google Scholar] [CrossRef]
- Salmond, C.V.; Kroll, R.G.; Booth, I.R. The effect of food preservatives on pH homeostasis in Escherichia coli. J. Gen. Microbiol. 1984, 130, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Lawford, H.G.; Rousseau, J.D. Effects of pH and acetic acid on glucose and xylose metabolism by a genetically engineered ethanologenic Escherichia coli. Appl. Biochem. Biotechnol. 1993, 39, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Roe, A.J.; McLaggan, D.; Davidson, I.; O’Byrne, C.; Booth, I.R. Perturbation of anion balance during inhibition of growth of Escherichia coli by week acids. J. Bacteriol. 1998, 180, 767–772. [Google Scholar] [PubMed]
- Axe, D.D.; Bailey, J.E. Transport of lactate and acetate through the energized cytoplasmic membrane of Escherichia coli. Biotechnol. Bioeng. 1995, 47, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Si, Y.; Wong, W.K.R. Kinetics modeling of inhibition and utilization of mixed volatile fatty acids in the formation of polyhydroxyalkanoates by Ralstonia eutropha. Process Biochem. 2002, 37, 731–738. [Google Scholar] [CrossRef]
- Warnecke, T.; Gill, R.T. Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb. Cell Factories 2005, 4. [Google Scholar] [CrossRef] [PubMed]
- Grousseau, E.; Blanchet, E.; Déléris, S.; Albuquerque, M.G.; Paul, E.; Uribelarrea, J.L. Impact of sustaining a controlled residual growth on polyhydroxybutyrate yield and production kinetics in Cupriavidus necator. Bioresour. Technol. 2013, 148, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shiraishi, M.; Shimizu, K. Metabolic flux analysis for biosynthesis of poly(ß-hydroxybutyric acid) in Alcaligenes eutrophus from various carbon sources. J. Ferment. Bioeng. 1997, 84, 579–587. [Google Scholar] [CrossRef]
- Serafim, L.S.; Lemos, P.C.; Oliveira, R.; Reis, M.A.M. Optimization of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol. Bioeng. 2004, 87, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Marang, L.; Kleerebezem, R.; Muyzer, G.; van Loosdrecht, M.C.M. Effect of temperature and cycle length on microbial competition in PHB-producing sequencing batch reactor. ISME J. 2011, 5, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Jian, Y.; Kleerebezem, R.; Muyzer, G.; van Loosdrecht, M.C.M. Enrichment of a mixed bacterial culture with a high polyhydroxyalkanoate storage capacity. Biomacromolecules 2009, 10, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Kleerebezem, R.; van Loosdrecht, M.C.M. Influence of the C/N ratio on the performance of polyhydroxybutyrate (PHB) producing sequencing batch reactors at short SRTs. Water Res. 2010, 7, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, Z.; Tian, T.; Chen, W. Effects of phosphorus and nitrogen limitation on PHA production in activated sludge. J. Environ. Sci. 2010, 22, 1602–1607. [Google Scholar] [CrossRef]
- Valentino, F.; Karabegovic, L.; Majone, M.; Morgan-Sagastume, F.; Werker, A. Polyhydroxyalkanoate (PHA) storage within a mixed-culture biomass with simultaneous growth as a function of accumulation substrate nitrogen and phosphorus levels. Water Res. 2015, 77, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Salehizadeh, H.; Van Loosdrecht, M.C.M. Production of polyhydroxyalkanoates by mixed culture: Recent trends and biotechnological importance. Biotechnol. Adv. 2004, 22, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.G.E.; Martino, V.; Pollet, E.; Avérous, L.; Reis, M.A.M. Mixed culture polyhydroxyalkanoate (PHA) production from volatile fatty acid (VFA)-rich streams: Effect of substrate composition and feeding regime on PHA productivity, composition and properties. J. Biotechnol. 2011, 151, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.Y.; Kim, M.K.; Kim, G.J.; Chang, H.N.; Park, Y.H.; Park, Y.H. Production of poly(ß-hydroxybutyrate-co-ß-hydroxyvalerate) from glucose and valerate in Alcaligenes eutrophus. Biotechnol. Lett. 1995, 17, 571–574. [Google Scholar] [CrossRef]
- Braunegg, G.; Lefebvre, G.; Genser, K.F. Polyhydroxyalkanoates, biopolyesters from renewable resources: Physiological and engineering aspects. J. Biotechnol. 1998, 65, 127–161. [Google Scholar] [CrossRef]
- Khanna, S.; Srivastava, A.K. Production of poly(3-hydroxybutyric-co-3-hydroxyvaleric acid) having a high hydroxyvalerate content with valeric acid feeding. J. Ind. Microbiol. Biotechnol. 2007, 34, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Tamaki, A.; Kunioka, M.; Soga, K. Production of copolyesters of 3-hydroxybutyrate and 3-hydroxyvalerate by Alcaligenes eutrophus from butyric and pentanoic acids. Appl. Microbiol. Biotechnol. 1988, 28, 330–334. [Google Scholar] [CrossRef]
- Yang, Y.H.; Brigham, C.J.; Budde, C.F.; Boccazzi, P.; Willis, L.B.; Hassan, M.A.; Yusof, Z.A.M.; Rha, C.; Sinskey, A.J. Optimization of growth media components for polyhydroxyalkanoate (PHA) production from organic acids by Ralstonia eutropha. Appl. Microbiol. Biotechnol. 2010, 87, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Gibbons, W.; Muthukumarappan, K. Conversion of volatile fatty acids into polyhydroxyalkanoate by Ralstonia eutropha. J. Appl. Microbiol. 2009, 106, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Kalacheva, G.S. The synthesis of hydroxybutyrate and hydroxyvalerate copolymers by the bacterium Ralstonia eutropha. Microbiology 2005, 74, 54–59. [Google Scholar] [CrossRef]
- Tanaka, K.; Ishizaki, A. Production of poly-d-3-hydroxybutyric acid from carbon dioxide by a two-stage culture method employing Alcaligenes eutrophus ATCC 17697T. J. Ferment. Bioeng. 1994, 77, 425–427. [Google Scholar] [CrossRef]
- Volova, T.G.; Voinov, N.A. Kinetic parameters of a culture of the hydrogen-oxidizing bacterium Ralstonia eutropha grown under conditions favoring polyhydroxybutyrate biosynthesis. Appl. Biochem. Microbiol. 2003, 39, 166–170. [Google Scholar] [CrossRef]
- Volova, T.G.; Kalacheva, G.S.; Gorbunova, O.V.; Zhila, N.O. Dynamics of activity of the key enzymes of polyhydroxyalkanoate metabolism in Ralstonia eutropha B5786. Appl. Biochem. Microbiol. 2004, 40, 170–177. [Google Scholar] [CrossRef]
- Darani, K.K.; Vasheghani-Farahani, E.; Tanaka, K. Hydrogen oxidizing bacteria as poly(hydroxybutyrate) producers. Iran. J. Biotechnol. 2006, 4, 193–196. [Google Scholar]
- Volova, T.G.; Kalacheva, G.S.; Steinbüchel, A. Biosynthesis of multi-component polyhydroxyalkanoates by the bacterium Wautersia eutropha. Macromol. Symp. 2008, 269, 1–7. [Google Scholar] [CrossRef]
- Park, I.; Jho, E.H.; Nam, K. Optimization of carbon dioxide and valeric acid utilization for polyhydroxyalkanoates synthesis by Cupriavidus necator. J. Polym. Environ. 2014, 22, 244–251. [Google Scholar] [CrossRef]
- Volova, T.G.; Kiselev, E.G.; Shishatskaya, E.I.; Zhila, N.O.; Boyandin, A.N.; Syrvacheva, D.A.; Vinogradova, O.N.; Kalacheva, G.S.; Vasiliev, A.D.; Peterson, I.V. Cell growth and accumulation of polyhydroxyalkanoates from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus B-10646. Bioresour. Technol. 2013, 146, 215–222. [Google Scholar] [CrossRef] [PubMed]

| Cmole/Nmole | PHB Production | PHBV Production | |||
|---|---|---|---|---|---|
| Acetic Acid (g/L) | (NH4)2SO4 (g/L) | Acetic Acid (g/L) | Valeric Acid (g/L) | (NH4)2SO4 (g/L) | |
| 10 | 330 | 72.6 | 330 | 0 | 72.6 |
| 90 | 660 | 16 | 700 | 350 | 59.4 |
| ∞ | 660 | 0 | 700 | 350 | 0 |
| Total Acid Concentration (g/L) | C2:C5 Ratio | Acetic Acid (g/L/h) | Valeric Acid (g/L/h) |
|---|---|---|---|
| 3 | 1:1 | 0.047 | 0.108 |
| 3 | 2:1 | 0.156 | 0.129 |
| 3 | 4:1 | 0.243 | 0.074 |
| 3 | 1:2 | 0.048 | 0.158 |
| 6 | 1:1 | 0.095 | 0.151 |
| Cultivation Process | Feedstock | kg CO2/kg PHB | kg H2/kg PHB |
|---|---|---|---|
| Autotrophic-autotrophic | CO2 | 2.84 | 0.96 |
| Heterotrophic-autotrophic | Glucose/CO2 | 1.58 | 0.77 |
| Autotrophic-heterotrophic-heterotrophic | CO2/acetic acid | 2.84 | 0.42 |
| Heterotrophic-heterotrophic | Glucose | 0 (emits 2.81) | 0 |
| Carbon Source Phase 1 Phase 2 Phase 3 | H2:O2:CO2 (vol %) | CDM at Onset of Phase 2 (g/L) | CDM (g/L) | PHB (g/L) | PHB Content (%) | PHB Productivity (g/L/h) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | Acetic acid | Acetic acid | - | 17 | 60 | 43 | 72 | 0.365 | This study |
| Acetic acid | Acetic acid | - | - | 15 | 40 | 30 | 75 | 0.257 | This study |
| Glycerol | CO2 | - | 84.0:2.8:13.2 | 19 | 46 | 28 | 61 | 0.168 | [8] |
| Fructose | CO2 | - | 84.1:6.7:10.3 | 15 | 43 | 24 | 56 | 0.632 | [43] |
| Glucose | CO2 | - | 84.0:2.8:13.2 | 13 | 38 | 24 | 63 | 0.108 | [5] |
| CO2 | CO2 | - | 60:20:10 | 10 | 30 | 22 | 75 | 0.314 | [44] |
| Fructose | CO2 | - | 83.0:5.3:10.6 | 10 | 27 | 15 | 56 | 0.237 | [43] |
| Glycerol | CO2 | - | 84.0:2.8:13.2 | 10 | 18 | 13 | 72 | 0.187 | [8] |
| CO2 | CO2 | - | 60:20:10 | 10 | 12 | 8 | 63 | 0.105 | [45] |
| CO2 | CO2 | - | 70:20:10 | 10 | 16 | 6 | 38 | 0.150 | [46] |
| Carbon Source Phase 1 Phase 2 Phase 3 | H2:O2:CO2 (vol %) | CDM at Onset of Phase 2 (g/L) | CDM (g/L) | PHBV (g/L) | fHV (mol %) | PHBV Content (%) | PHBV Productivity (g/L/h) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | Acetic-valeric acid | Acetic-valeric acid | - | 19 | 65 | 48 | 27 | 74 | 0.413 | This study |
| Glucose | CO2-valeric acid | - | 84:2.8:13.2 | 15 | 32 | 25 | 72 | 78 | 0.480 | [10] |
| CO2 | CO2-valeric acid | - | 60:20:10 | 11 | 18 | 15 | 28 | 80 | 0.188 | [42] |
| CO2 | CO2-valeric acid | - | NS 1 | NS | 7.3 | 6.5 | 42 | 90 | 0.135 | [47] |
| CO2 | CO2-valeric acid | - | 77.78:11.11:11.11 | NS | NS | 1.2 | 32 | NS | 0.005 | [48] |
| CO2 | CO2-valeric acid | - | 70:20:10 | NS | NS | NS | 64 | NS | NS | [49] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Gonzalez, L.; De Wever, H. Acetic Acid as an Indirect Sink of CO2 for the Synthesis of Polyhydroxyalkanoates (PHA): Comparison with PHA Production Processes Directly Using CO2 as Feedstock. Appl. Sci. 2018, 8, 1416. https://doi.org/10.3390/app8091416
Garcia-Gonzalez L, De Wever H. Acetic Acid as an Indirect Sink of CO2 for the Synthesis of Polyhydroxyalkanoates (PHA): Comparison with PHA Production Processes Directly Using CO2 as Feedstock. Applied Sciences. 2018; 8(9):1416. https://doi.org/10.3390/app8091416
Chicago/Turabian StyleGarcia-Gonzalez, Linsey, and Heleen De Wever. 2018. "Acetic Acid as an Indirect Sink of CO2 for the Synthesis of Polyhydroxyalkanoates (PHA): Comparison with PHA Production Processes Directly Using CO2 as Feedstock" Applied Sciences 8, no. 9: 1416. https://doi.org/10.3390/app8091416
APA StyleGarcia-Gonzalez, L., & De Wever, H. (2018). Acetic Acid as an Indirect Sink of CO2 for the Synthesis of Polyhydroxyalkanoates (PHA): Comparison with PHA Production Processes Directly Using CO2 as Feedstock. Applied Sciences, 8(9), 1416. https://doi.org/10.3390/app8091416





