Mesoporous Niobium Oxyhydroxide Catalysts for Cyclohexene Epoxidation Reactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Catalysts
2.2. Catalysts Characterization
2.3. Catalytic Tests
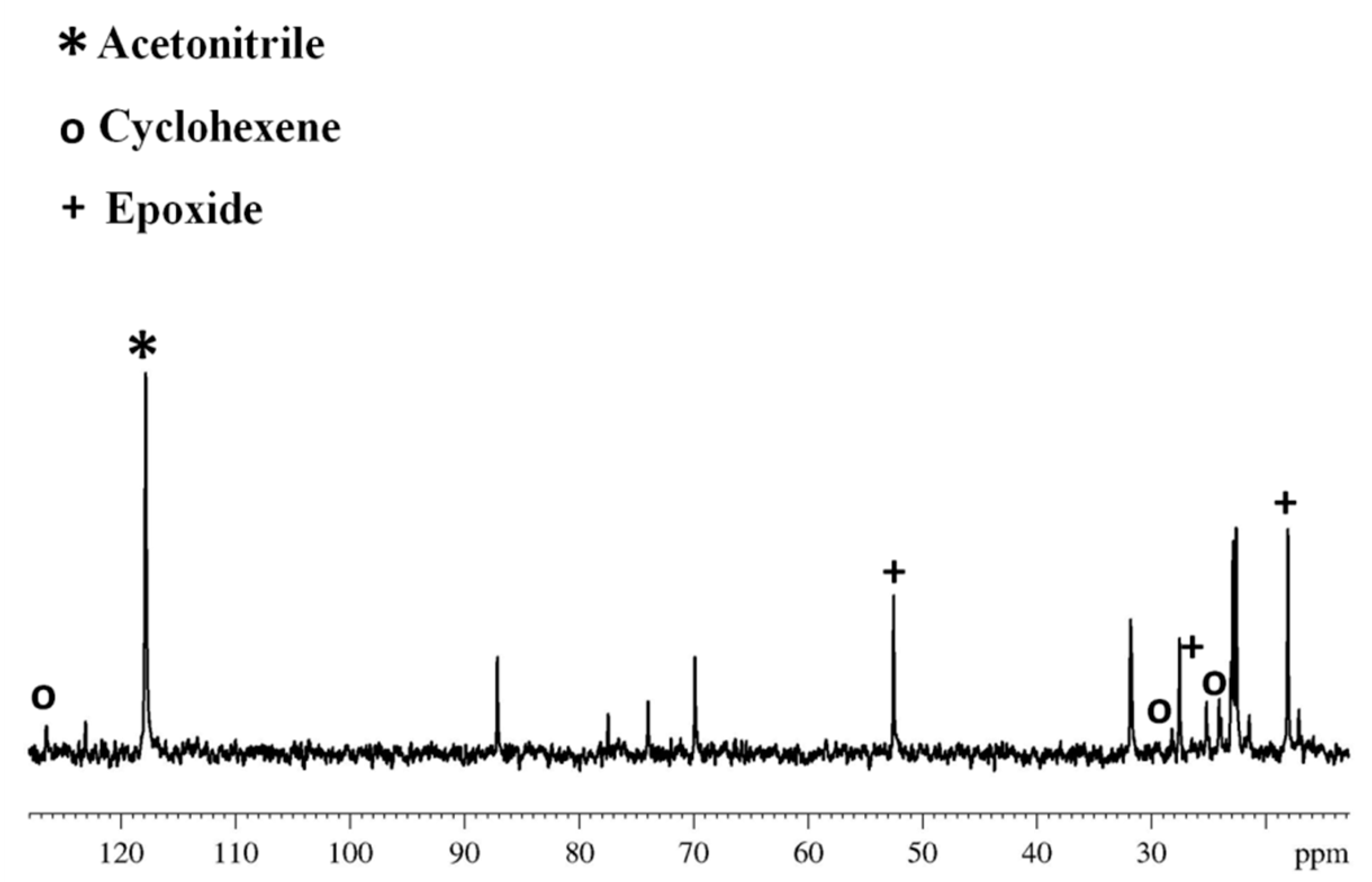
3. Results and Discussion
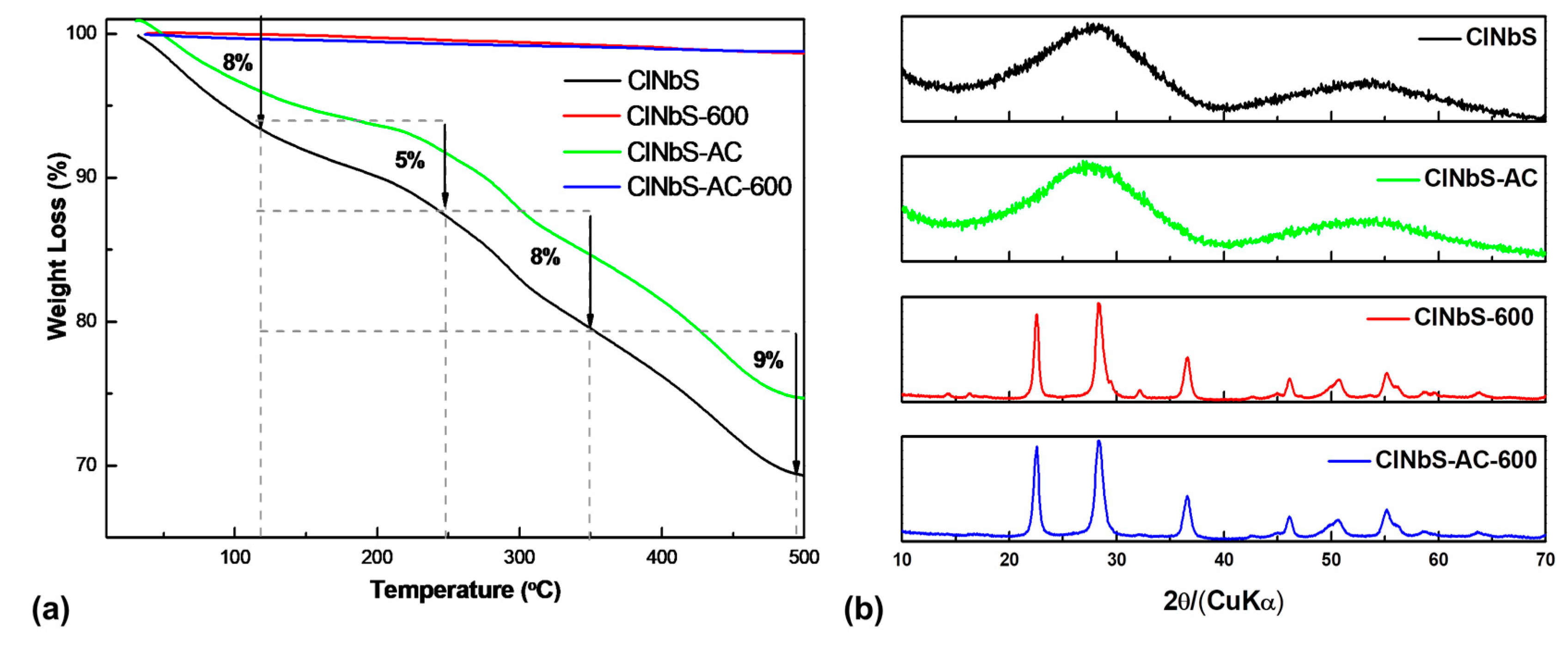
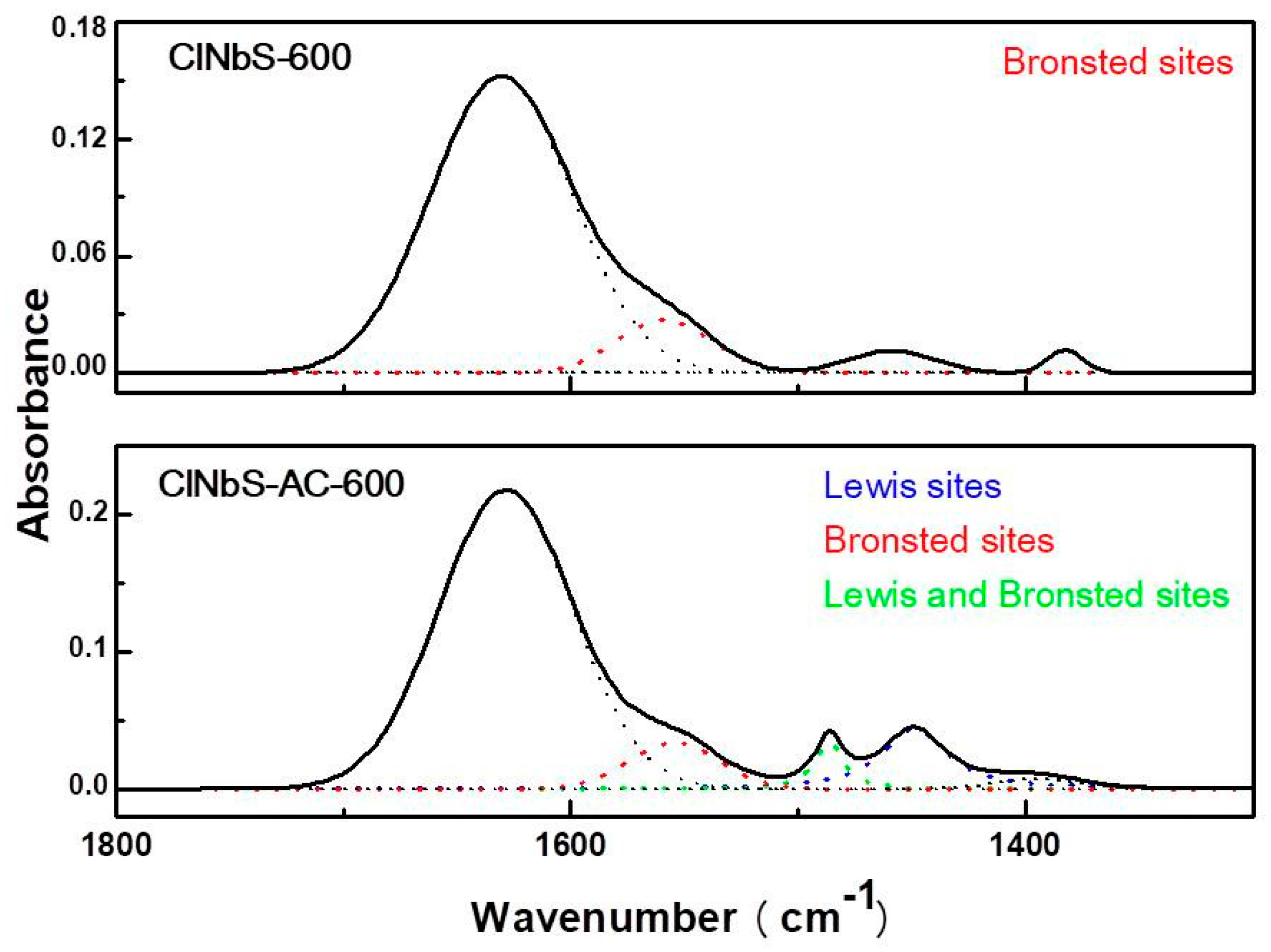
3.1. Characterization of Catalysts
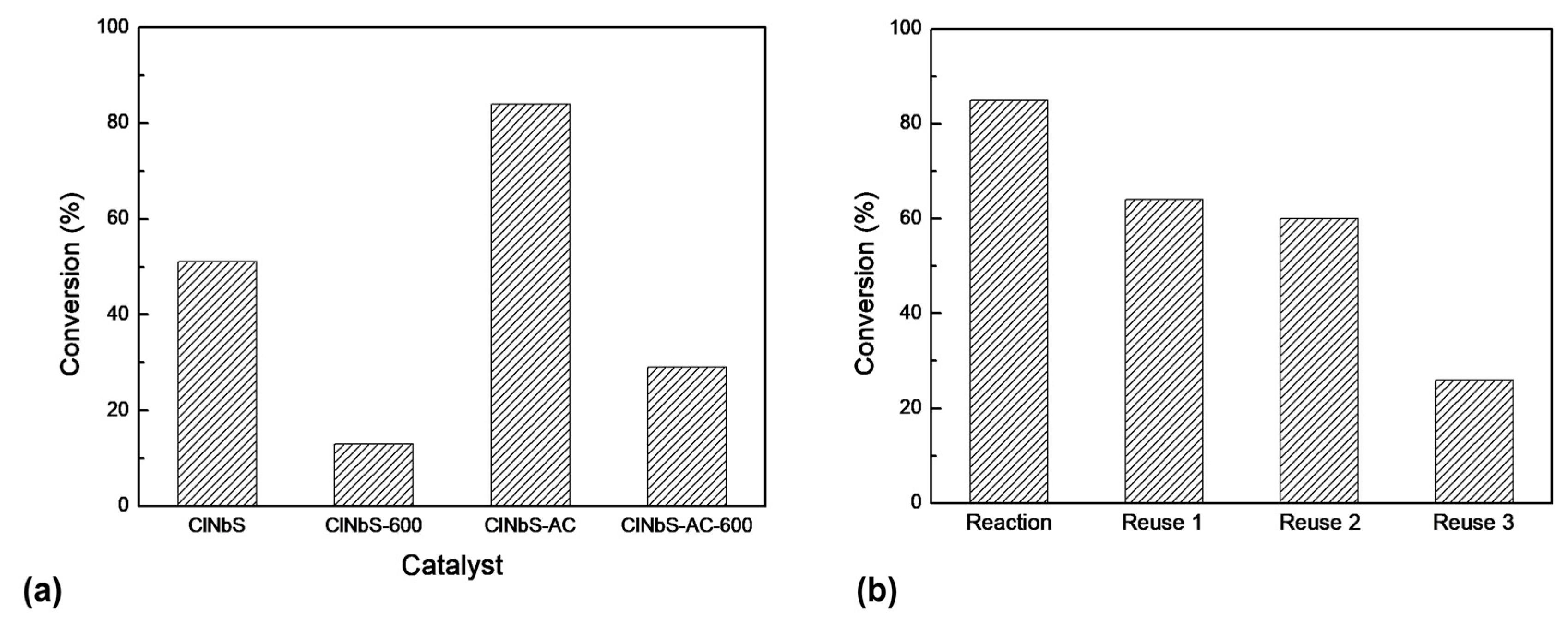
3.2. Epoxidation Reactions
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Marin-Astorga, N.; Martinez, J.J.; Borda, G.; Cubillos, J.; Suarez, D.N.; Rojas, H. Control of the chemoselectivity in the oxidation of geraniol over lanthanum, titanium and niobium catalysts supported on mesoporous silica MCM-41. Top. Catal. 2012, 55, 620–624. [Google Scholar] [CrossRef]
- Yang, G.; Deng, Y.; Wang, J. Non-hydrothermal synthesis and characterization of MCM-41 mesoporous materials from iron ore tailing. Ceram. Int. 2014, 40, 7401–7406. [Google Scholar] [CrossRef]
- De Oliveira, L.C.A.; Costa, N.T.; Pliego, J.R.; Silva, A.C.; de Souza, P.P.; Patrícia, P.S. Amphiphilic niobium oxyhydroxide as a hybrid catalyst for sulfur removal from fuel in a biphasic system. Appl. Catal. B Environ. 2014, 147, 43–48. [Google Scholar] [CrossRef][Green Version]
- Tanabe, K.; Okazaki, S. Various reactions catalyzed by niobium compounds and materials. Appl. Catal. A Gen. 1995, 133, 191–218. [Google Scholar] [CrossRef]
- Mohd Ekhsan, J.; Lee, S.L.; Nur, H. Niobium oxide and phosphoric acid impregnated silica-titania as oxidative-acidic bifunctional catalyst. Appl. Catal. A Gen. 2014, 471, 142–148. [Google Scholar] [CrossRef]
- Chan, X.; Pu, T.; Chen, X.; James, A.; Lee, J.; Parise, J.B.; Kim, D.H.; Kim, T. Effect of niobium oxide phase on the furfuryl alcohol dehydration. Catal. Commun. 2017, 97, 65–69. [Google Scholar] [CrossRef]
- Alexopoulos, K.; Reyniers, M.F.; Marin, G.B. Reaction path analysis of propene selective oxidation over V2O5 and V2O5/TiO2. J. Catal. 2012, 295, 195–206. [Google Scholar] [CrossRef]
- Ramanathan, A.; Zhu, H.; Maheswari, R.; Thapa, P.S.; Subramaniam, B. Comparative study of Nb-incorporated cubic mesoporous silicates as epoxidation catalysts. Ind. Eng. Chem. Res. 2015, 54, 4236–4242. [Google Scholar] [CrossRef]
- Santa A., A.M.; Vergara G., J.C.; Palacio S., L.A.; Echavarría I., A. Limonene epoxidation by molecular sieves zincophosphates and zincochromates. Catal. Today 2008, 133–135, 80–86. [Google Scholar] [CrossRef]
- Moreira, M.A. Produção Enzimática de Peróxi-Ácidos e sua Utilização na Epoxidação de Terpenos; Universidade Federal de Santa Catarina: Florianópolis, Brazil, 2008. [Google Scholar]
- Da Silva, F.P. Oxidação Alílica de Alcenos Catalisada por Nanopartículas de Óxido de Cobalto Suportadas; Universidade de São Paulo: São Paulo, Brazil, 2011. [Google Scholar]
- Di Serio, M.; Turco, R.; Pernice, P.; Aronne, A.; Sannino, F.; Santacesaria, E. Valuation of Nb 2O 5-SiO 2 catalysts in soybean oil epoxidation. Catal. Today 2012, 192, 112–116. [Google Scholar] [CrossRef]
- Nogueira, F.G.E.; Lopes, J.H.; Silva, A.C.; Lago, R.M.; Fabris, J.D.; Oliveira, L.C.A. Catalysts based on clay and iron oxide for oxidation of toluene. Appl. Clay Sci. 2011, 51, 385–389. [Google Scholar] [CrossRef]
- Ingle, R.H.; Vinu, A.; Halligudi, S.B. Alkene epoxidation catalyzed by vanadomolybdophosphoric acids supported on hydrated titania. Catal. Commun. 2008, 9, 931–938. [Google Scholar] [CrossRef]
- Gallo, A.; Tiozzo, C.; Psaro, R.; Carniato, F.; Guidotti, M. Niobium metallocenes deposited onto mesoporous silica via dry impregnation as catalysts for selective epoxidation of alkenes. J. Catal. 2013, 298, 77–83. [Google Scholar] [CrossRef]
- Ziolek, M. Catalytic liquid-phase oxidation in heterogeneous system as green chemistry goal—Advantages and disadvantages of MCM-41 used as catalyst. Catal. Today 2004, 90, 145–150. [Google Scholar] [CrossRef]
- Li, L.; Deng, J.; Yu, R.; Chen, J.; Wang, Z.; Xing, X. Niobium pentoxide hollow nanospheres with enhanced visible light photocatalytic activity. J. Mater. Chem. A 2013, 1, 11894. [Google Scholar] [CrossRef]
- Nakajima, K.; Fukui, T.; Kato, H.; Kitano, M.; Kondo, J.N.; Hayashi, S.; Hara, M. Structure and Acid Catalysis of Mesoporous Nb2O5·nH2O. Chem. Mater. 2010, 22, 3332–3339. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Yang, D.; Li, Y.; Liu, H.; Liu, P.; Wood, B.J.; Zhao, H. Directly Hydrothermal Growth of Single Crystal Nb3O7(OH) Nanorod Film for High Performance Dye-Sensitized Solar Cells. Adv. Mater. 2012, 24, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Ristić, M.; Popović, S.; Musić, S. Sol-gel synthesis and characterization of Nb2O5 powders. Mater. Lett. 2004, 58, 2658–2663. [Google Scholar] [CrossRef]
- Morandin, M.; Gavagnin, R.; Pinna, F.; Strukul, G. Oxidation of Cyclohexene with Hydrogen Peroxide Using Zirconia–Silica Mixed Oxides: Control of the Surface Hydrophilicity and Influence on the Activity of the Catalyst and Hydrogen Peroxide Efficiency. J. Catal. 2002, 212, 193–200. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, C.H.; Wu, L.M.; Lou, J.Y.; Li, N.; Yang, H.M.; Tong, D.S.; Yu, W.H. Catalytic dehydration of glycerol to acrolein over sulfuric acid-activated montmorillonite catalysts. Appl. Clay Sci. 2013, 74, 154–162. [Google Scholar] [CrossRef]
- Chagas, P.; Oliveira, H.S.; Mambrini, R.; Le Hyaric, M.; De Almeida, M.V.; Oliveira, L.C.A. A novel hydrofobic niobium oxyhydroxide as catalyst: Selective cyclohexene oxidation to epoxide. Appl. Catal. A Gen. 2013, 454, 88–92. [Google Scholar] [CrossRef]
- Landmesser, H.; Kosslick, H.; Storek, W.; Fricke, R. Interior surface hydroxyl groups in ordeted mesoporous silicates. Solid State Ion. 1997, 76, 271–277. [Google Scholar] [CrossRef]
- Amgarten, D.R. Determinação do Volume Específico de Poros de Sílicas Cromatográficas por Dessorção de Líquidos em Excesso; Universidade Estadual de Campinas: Campinas, Brazil, 2006. [Google Scholar]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- De Souza, W.F.; Guimarães, I.R.; Guerreiro, M.C.; Oliveira, L.C.A. Catalytic oxidation of sulfur and nitrogen compounds from diesel fuel. Appl. Catal. A Gen. 2009, 360, 205–209. [Google Scholar] [CrossRef]
- Ziolek, M.; Sobczak, I.; Decyk, P.; Sobańska, K.; Pietrzyk, P.; Sojka, Z. Search for reactive intermediates in catalytic oxidation with hydrogen peroxide over amorphous niobium(V) and tantalum(V) oxides. Appl. Catal. B Environ. 2015, 164, 288–296. [Google Scholar] [CrossRef]
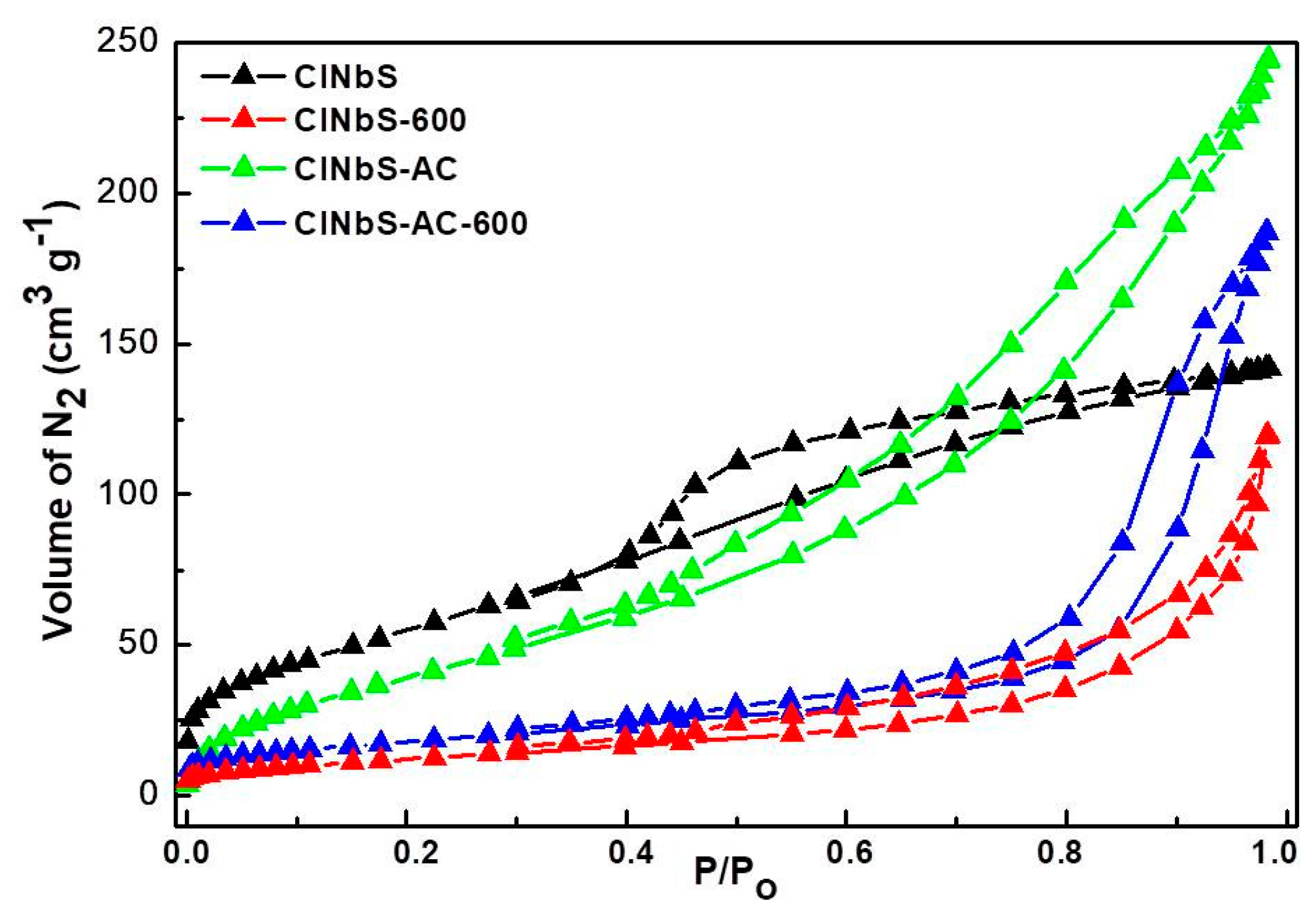
| Catalyst | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) |
|---|---|---|
| ClNbS | 198 | 0.17 |
| ClNbS-600 | 44 | 0.10 |
| ClNbS-AC | 153 | 0.15 |
| ClNbS-AC-600 | 64 | 0.09 |
 | Experimental Chemical Shifts (ppm) | Simulated Chemical Shifts (ppm) |
|---|---|---|
| C1 | 51.22 | 52.07 |
| C2 | 25.36 | 24.50 |
| C3 | 20.53 | 19.47 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padula, I.D.; Chagas, P.; Furst, C.G.; Oliveira, L.C.A. Mesoporous Niobium Oxyhydroxide Catalysts for Cyclohexene Epoxidation Reactions. Appl. Sci. 2018, 8, 881. https://doi.org/10.3390/app8060881
Padula ID, Chagas P, Furst CG, Oliveira LCA. Mesoporous Niobium Oxyhydroxide Catalysts for Cyclohexene Epoxidation Reactions. Applied Sciences. 2018; 8(6):881. https://doi.org/10.3390/app8060881
Chicago/Turabian StylePadula, Izabela D., Poliane Chagas, Carolina G. Furst, and Luiz C. A. Oliveira. 2018. "Mesoporous Niobium Oxyhydroxide Catalysts for Cyclohexene Epoxidation Reactions" Applied Sciences 8, no. 6: 881. https://doi.org/10.3390/app8060881
APA StylePadula, I. D., Chagas, P., Furst, C. G., & Oliveira, L. C. A. (2018). Mesoporous Niobium Oxyhydroxide Catalysts for Cyclohexene Epoxidation Reactions. Applied Sciences, 8(6), 881. https://doi.org/10.3390/app8060881




