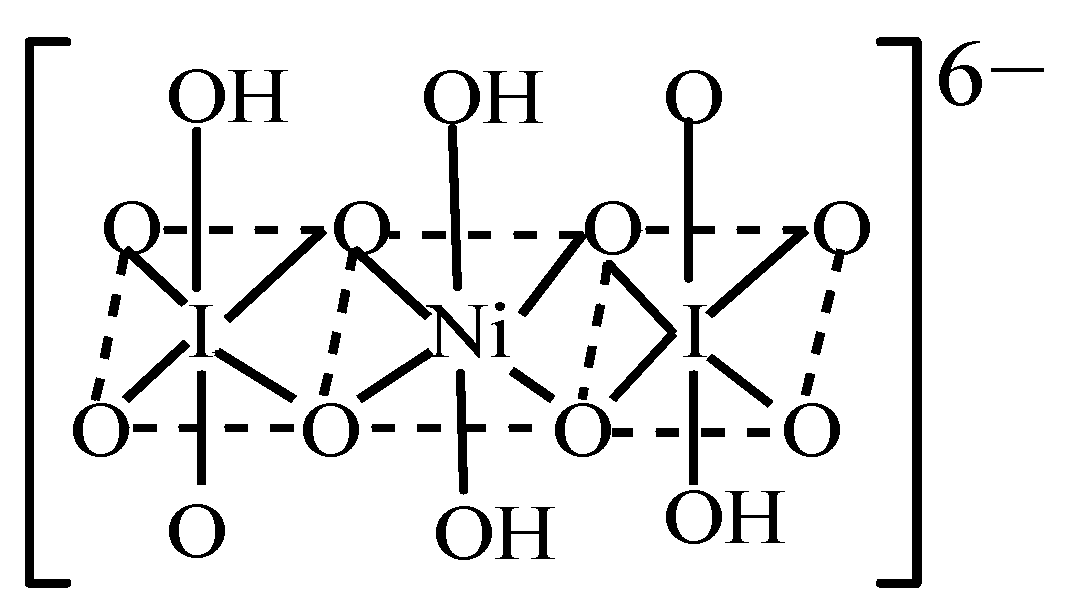
Investigation on a Sensitive Chemiluminescence System Based on Ni(IV) Complex to Determine Two β2-Agonist Drugs in Urine and Swine Feed and Their Mechanism
Abstract
:1. Introduction
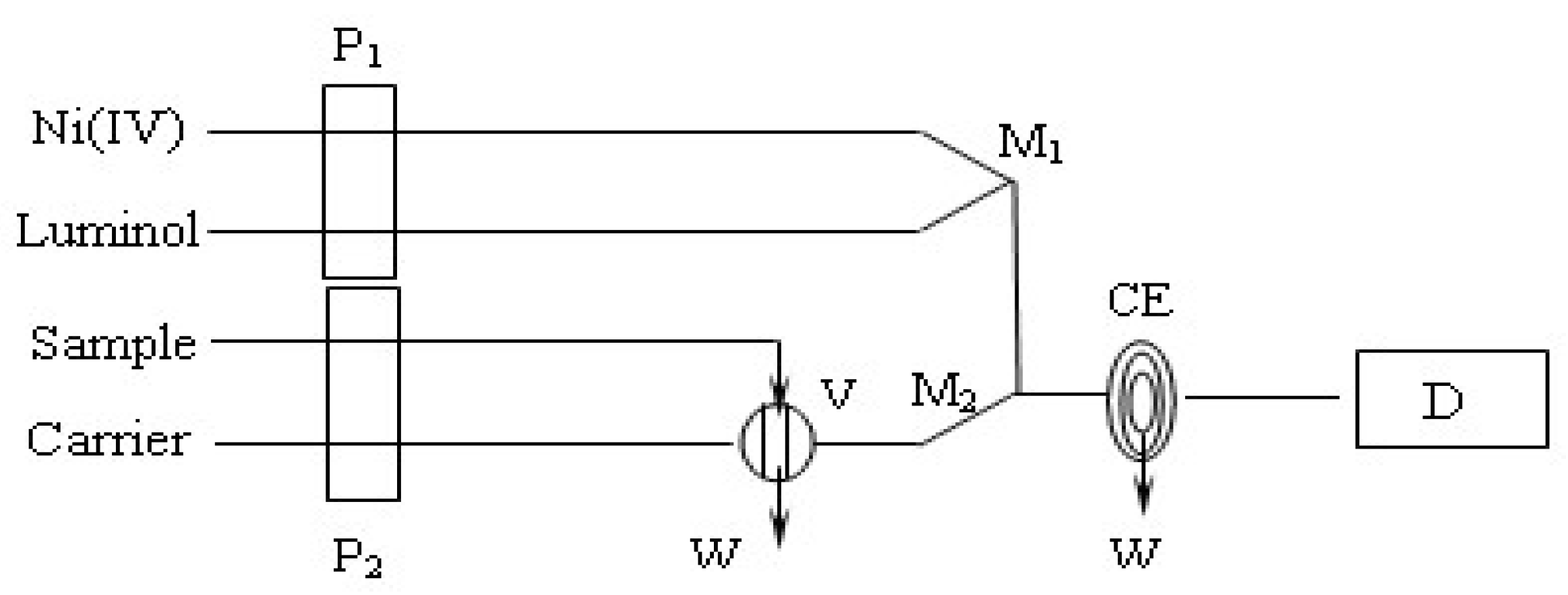
2. Materials and Methods
2.1. Reagents and Solution
2.2. Sample Handling and Extraction Procedure
2.3. Instruments
3. Results
3.1. Optimization of CL Conditions
3.1.1. Effect of Ni(IV) Concentration on CL Intensities
3.1.2. Effect of Luminol Concentration on CL Intensities
3.1.3. Effect of [OH-] in Ni(IV) and Luminol Solutions
3.2. Application of the New CL System
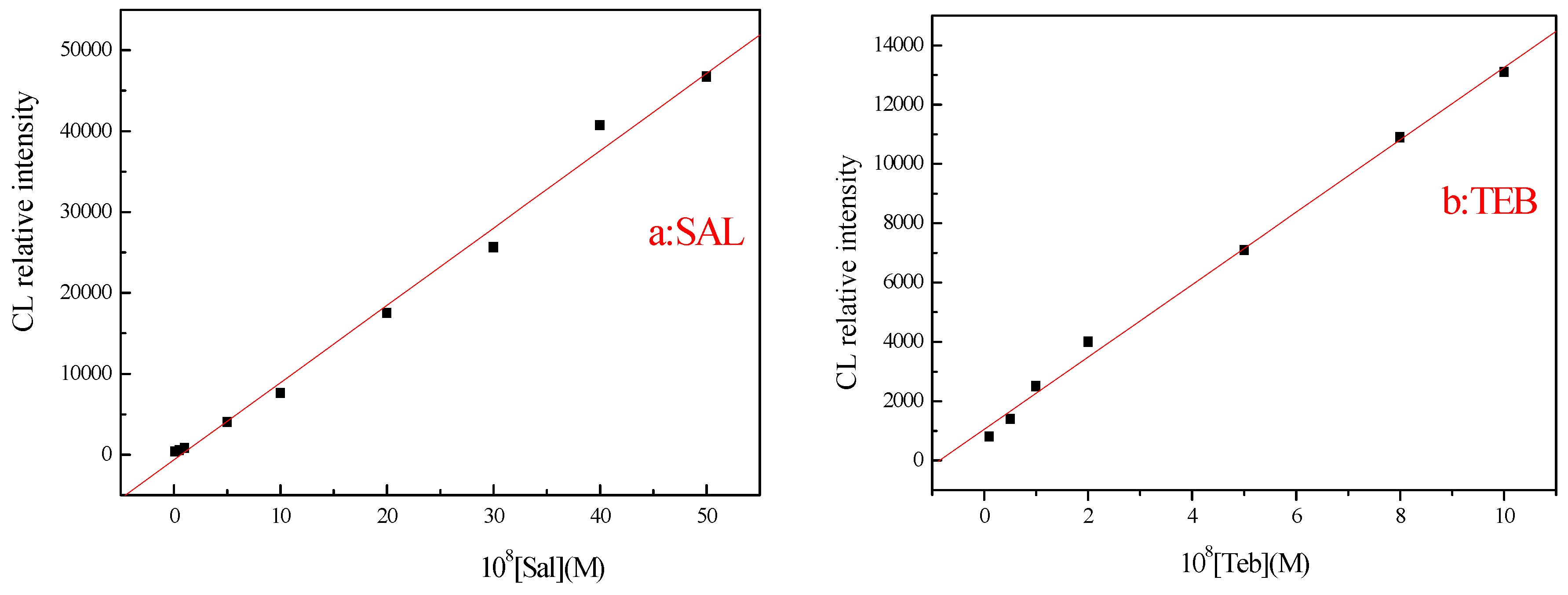
3.2.1. Dynamic Range, Detection Limit, and Relative Standard Deviation
3.2.2. Recovery Experiments and Analysis of Urine and Feed Samples
4. Discussion: Mechanistic Studies of the CL Detection System
4.1. CL Spectra
4.2. Verifying Free Radicals by ESR Spectra
4.3. Reaction Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, M.; Zhang, Y.; Xue, Y.; Hong, X.; Cui, Y.; Liu, Z.; Du, D. Simulataneous determination of â2-agonists clenbuterol and sablutamol in water and swine feed samples by dual-labeled time-resolved fluoroimmunoassay. Food Control. 2017, 73, 1039–1044. [Google Scholar] [CrossRef]
- Zhang, Q.; Ni, Y.; Kokot, S. Competitive interactions of ionic surfactants with salbutamol and bovine serum albumin: A molecular spectroscopy study with implications for salbutamol in food analysis. J. Agric. Food Chem. 2013, 61, 7730–7738. [Google Scholar] [CrossRef] [PubMed]
- Loden, H.; Pettersson, C.; Arvidsson, T.; Amini, A. Quantitative determination of salbutamol in tablets by multiple-injection capillary zone electrophoresis. J. Chromatogr. A 2008, 1207, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fan, L.Y.; Zhang, W.; Cao, C.X. Separation and determination of abused drugs clenbuterol and salbutamol from complex extractants in swine feed by capillary zone electrophoresis with simple pretreatment. Talanta 2008, 76, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, Y.; Yang, T.; Zhang, Y.; Huang-Fu, W. Simultaneous determination of clenbuterol, salbutamol and ractopamine in milk by reversed-phase liquid chromatography tandem mass spectrometry with isotope dilution. J. Chromatogr. A 2010, 1217, 7873–7877. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, Y.; Ding, X.; Huang, C.; Miao, L. Simultaneous determination of ambroxol and salbutamol in human plasma by ultra-performance liquid chromatography–tandem mass spectrometry and its application to a pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Raksawong, P.; Chullasat, K.; Nurerk, P.; Kanatharana, P.; Davis, F.; Bunkoed, O. A hybrid molecularly imprinted polymer coated quantum dot nanocomposite optosensor for highly sensitive and selective determination of salbutamol in animal feeds and meat samples. Anal. Bioanal. Chem. 2017, 409, 4697–4707. [Google Scholar] [CrossRef] [PubMed]
- Antonio, L.C.; Bulhões, L.O.S. Determination of fenoterol and salbutamol in pharmaceutical formulations by electrogenerated chemiluminescence. Talanta 2007, 72, 1746–1751. [Google Scholar]
- Goyal, R.N.; Oyama, M.; Singh, S.P. Fast determination of salbutamol, abused by athletes for doping, in pharmaceuticals and human biological fluids by square wave voltammetry. J. Electroanal. Chem. 2007, 611, 140–148. [Google Scholar] [CrossRef]
- Rao, K.L.N.; Krishnaiah, C.; Babu, K.S.; Reddy, K.P. Development and validation of a stability-indicating LC method for simultaneous determination of related compounds of guaifenesin, terbutaline sulfate and ambroxol HCl in cough syrup formulation. J. Saudi Chem. Soc. 2014, 18, 593–600. [Google Scholar]
- Althanyan, M.S.; Assi, K.H.; Clark, B.J.; Hanaee, J. Microemulsion high performance liquid chromatography (MELC) method for the determination of terbutaline in pharmaceutical preparation. J. Pharm. Biomed. Anal. 2011, 55, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Faiyazuddin, M.; Rauf, A.; Ahmad, N.; Ahmad, S.; Lqbal, Z. A validated HPTLC method for determination of terbutaline sulfate in biological samples: Application to pharmacokinetic study. Saudi Pharm. J. 2011, 19, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, Z.; Zhou, J.; Liu, J.; Song, G. A new voltammetric sensor based on poly(l-arginine)/graphene–Nafion composite film modified electrode for sensitive determination of terbutaline sulfate. J. Electroanal. Chem. 2012, 687, 51–57. [Google Scholar] [CrossRef]
- Kricka, L.J. Clinical applications of chemiluminescence. Anal. Chim. Acta 2003, 500, 279–286. [Google Scholar] [CrossRef]
- Roda, A.; Pasin, I.P.; Mirasoli, M.; Michelini, E.; Guardigli, M. Biotechnological applications of bioluminescence and chemiluminescence. Trends Biotechnol. 2004, 22, 295–303. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhang, L.; He, L.; Liu, G. Chemiluminescence Analysis and its Application in Pharmacology. Prog. Pharm. Sci. 2004, 28, 257–269. [Google Scholar]
- Meseguer-Lloret, S.; Torres-Cartas, S.; Gomez-Benito, M.C. Flow injection photoinduced chemiluminescence determination of imazalil in water samples. Anal. Bioanal. Chem. 2010, 398, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, Y.; Kang, J.; Tang, J.; Zhang, Y. Chemiluminescence determination of terbutaline sulfate in bovine urine and pharmaceutical preparations based on enhancement of the 2-phenyl-4,5-di (2-furyl) imidazole–potassium ferricyanide system. J. Pharm. Biomed. Anal. 2012, 58, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, Y.; Shi, B.; Gao, Z.; Du, Y.; Liu, X.; Lin, B. Determination of beta-agonists in swine hair by μFIA and chemiluminescence. Electrophoresis 2015, 36, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Han, J.; Yang, S.; Wang, Z.; Wang, L.; Fu, Z. Highly sensitive multianalyte immunochromatographic test strip for rapid chemiluminescent detection of ractopamine and salbutamol. Anal. Chim. Acta 2014, 839, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Sun, Y.; Wei, Y. Development of an Analytical Method for the Determination of β2-Agonist Residues in Animal Tissues by High-Performance Liquid Chromatography with On-line Electrogenerated [Cu(HIO6)2]5−-Luminol Chemiluminescence Detection. J. Agric. Food Chem. 2007, 55, 4949–4956. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qian, X.; Zhao, K.; Deng, A.; Li, J. Flow injection chemiluminescent competitive immunoassay for the beta-adrenergic agonist salbutamol using carboxylic resin beads and enzymatic amplification. Sens. Actuators B Chem. 2015, 215, 323–329. [Google Scholar] [CrossRef]
- He, D.; Zhang, Z.; Zhou, H.; Huang, Y. Micro flow sensor on a chip for the determination of terbutaline in human serum based on chemiluminescence and a molecularly imprinted polymer. Talanta 2006, 69, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.A.; Rocha, F.R.P. A Flow-Based Analytical Procedure for Salbutamol Determination Exploiting Chemiluminescence in a Liquid-Core Waveguide. Anal. Lett. 2008, 41, 1579–1591. [Google Scholar] [CrossRef]
- Shi, H.; Shen, S.; Sun, H.; Liu, Z.; Li, L. Oxidaition of L-serine and L-threonine by Bis(hydrogen periodato)argentite(III) Complex anion: a Mechanistic study. J. Inorg. Biochem. 2007, 101, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, J.; Huo, S.; Shen, S.; Kang, W.; Shi, T. Kinetics and Mechanism of Oxidation of the Drug Intermediate 1-(2-Hydroxyethyl)piperidine by Bis(hydrogenperiodato)argentate(III). J. Braz. Chem. Soc. 2013, 24, 1307–1313. [Google Scholar] [CrossRef]
- Shi, H.; Xu, X.; Ding, Y.; Liu, S.; Kang, W. Determination of cortisol in human blood serums by a new Ag(III) complex- luminol chemiluminescent system. Anal. Biochem. 2009, 387, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, H.; Shi, H.; Ma, C.; Cong, B.; Kang, W. Determination of three major catecholamines in human urine by capillary zone electrophoresis with chemiluminescence detection. Anal. Biochem. 2012, 427, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Shi, H.; Lian, K.; Diao, Y.; Chen, Y.; Ma, C.; Kang, W. Highly selective and sensitive determination of several antioxidants in human breast milk using high-performance liquid chromatography based on Ag(III) complex chemiluminescence detection. Food Chem. 2017, 218, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Duan, J.; Li, X.; Xu, X.; Shi, H.; Kang, W. Determination of Sulfonamide Residues in Food by Capillary Zone Electrophoresis with On-Line Chemiluminescence Detection Based on an Ag(III) Complex. Int. J. Mol. Sci. 2017, 18, 1286. [Google Scholar] [CrossRef] [PubMed]
- Murthy, C.P.; Sethurant, B.; Rao, T.N. Oxidation by tetravalent nickel Part 1: Kinetics of electron transfer from some aliphatic alcohols to Ni(IV) in aqueous alkaline media. Z. Phys. Chem. 1986, 267, 1212–1218. [Google Scholar]
- People’s Republic of China Entry and Exit Inspection and Quarantine Industry Standard. Determination of Salbutamol Residues in Animal Feed for Import and Export—Liquid Chromatographic Method; SN/T 1116-2002; China Standard Publishing House: Beijing, China, 2002.
- Safavi, A.; Karimi, M.A. Flow injection determination of cationic surfactants by using N-bromosuccinimide and N-chlorosuccinimide as new oxidizing agents for luminol chemiluminescence. Anal. Chim. Acta 2002, 468, 53–63. [Google Scholar] [CrossRef]
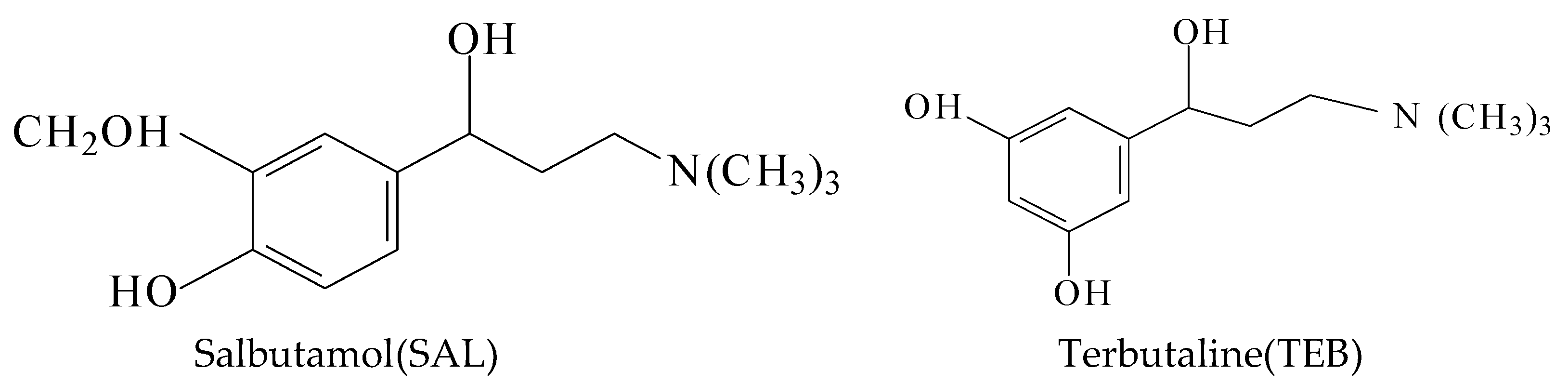
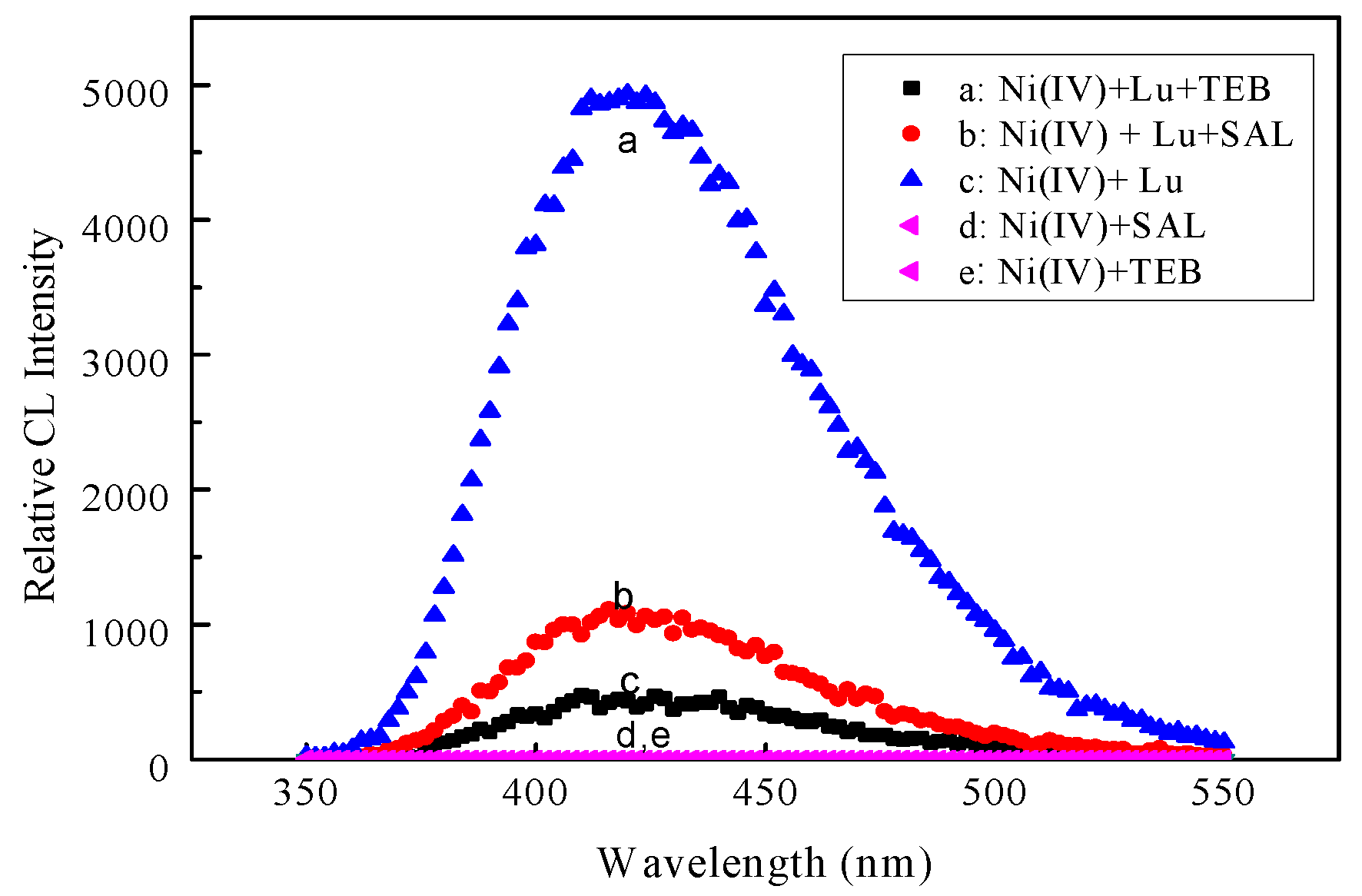
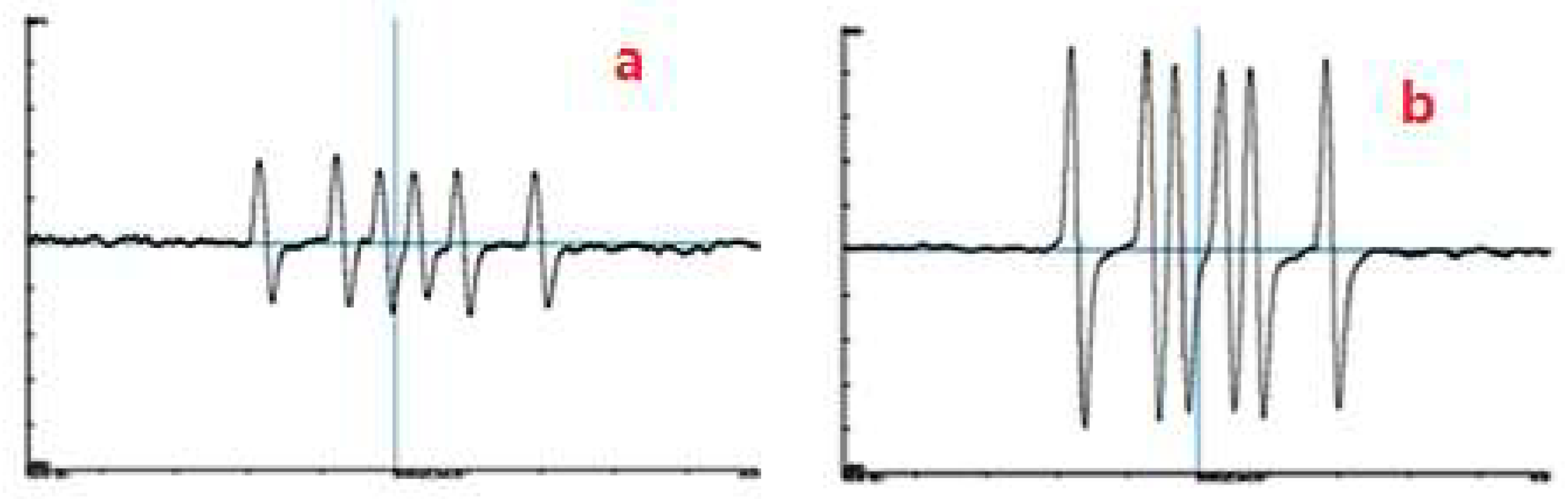
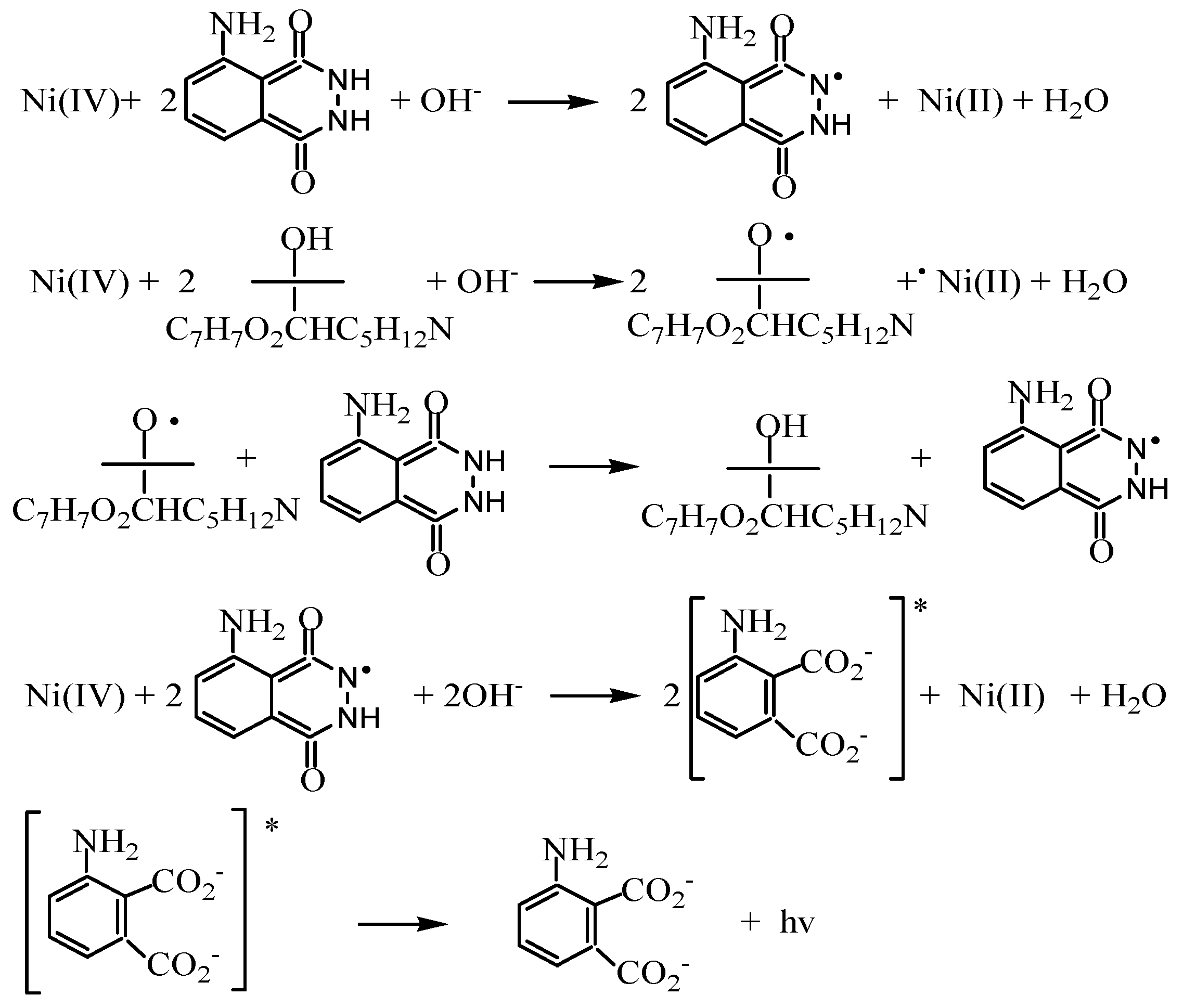
| Variable | Studied Range | Optimum Condition | |
|---|---|---|---|
| TEB | SAL | ||
| [Ni(IV)]/M | 2.0 × 10−8–2.0 × 10−5 | 6.0 ×10−7 | 2.0 × 10−6 |
| [Luminol]/M | 2.0 × 10−8–4.0 × 10−5 | 2.0 × 10−7 | 2.0 × 10−6 |
| [OH− in Ni(IV)]/M | 0.0001–0.05 | 0.003 | 0.001 |
| [OH− in Lu]/M | 0.0001–0.01 | 0.001 | 0.002 |
| Sample | Spiked (×10−8 M) | Found (×10−8 M) | Recovery (%, n = 3) | RSD (%, n = 3) | ||||
|---|---|---|---|---|---|---|---|---|
| SAL | TEB | SAL | TEB | SAL | TEB | SAL | TEB | |
| Urine | 1.0 | 1.0 | 1.05 | 1.08 | 105.0 | 108.0 | 1.8 | 2.0 |
| 10.0 | 5.0 | 10.28 | 4.86 | 102.8 | 97.2 | 1.7 | 2.1 | |
| 40.0 | 8.0 | 38.03 | 8.33 | 95.1 | 104.1 | 1.7 | 1.8 | |
| Feed | 1.0 | 1.0 | 1.03 | 1.05 | 103.0 | 105.0 | 2.0 | 2.1 |
| 10.0 | 5.0 | 9.75 | 4.75 | 97.5 | 95.0 | 1.9 | 2.3 | |
| 40.0 | 8.0 | 39.36 | 7.82 | 98.4 | 97.8 | 1.8 | 2.0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Diao, Y.; Li, X.; Dai, T.; Shi, H.; Li, S. Investigation on a Sensitive Chemiluminescence System Based on Ni(IV) Complex to Determine Two β2-Agonist Drugs in Urine and Swine Feed and Their Mechanism. Appl. Sci. 2018, 8, 663. https://doi.org/10.3390/app8050663
Dong X, Diao Y, Li X, Dai T, Shi H, Li S. Investigation on a Sensitive Chemiluminescence System Based on Ni(IV) Complex to Determine Two β2-Agonist Drugs in Urine and Swine Feed and Their Mechanism. Applied Sciences. 2018; 8(5):663. https://doi.org/10.3390/app8050663
Chicago/Turabian StyleDong, Xiao, Yajie Diao, Xinghua Li, Tingting Dai, Hongmei Shi, and Shan Li. 2018. "Investigation on a Sensitive Chemiluminescence System Based on Ni(IV) Complex to Determine Two β2-Agonist Drugs in Urine and Swine Feed and Their Mechanism" Applied Sciences 8, no. 5: 663. https://doi.org/10.3390/app8050663
APA StyleDong, X., Diao, Y., Li, X., Dai, T., Shi, H., & Li, S. (2018). Investigation on a Sensitive Chemiluminescence System Based on Ni(IV) Complex to Determine Two β2-Agonist Drugs in Urine and Swine Feed and Their Mechanism. Applied Sciences, 8(5), 663. https://doi.org/10.3390/app8050663





