Development of ELISA-Like Fluorescence Assay for Melamine Detection Based on Magnetic Dummy Molecularly Imprinted Polymers
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
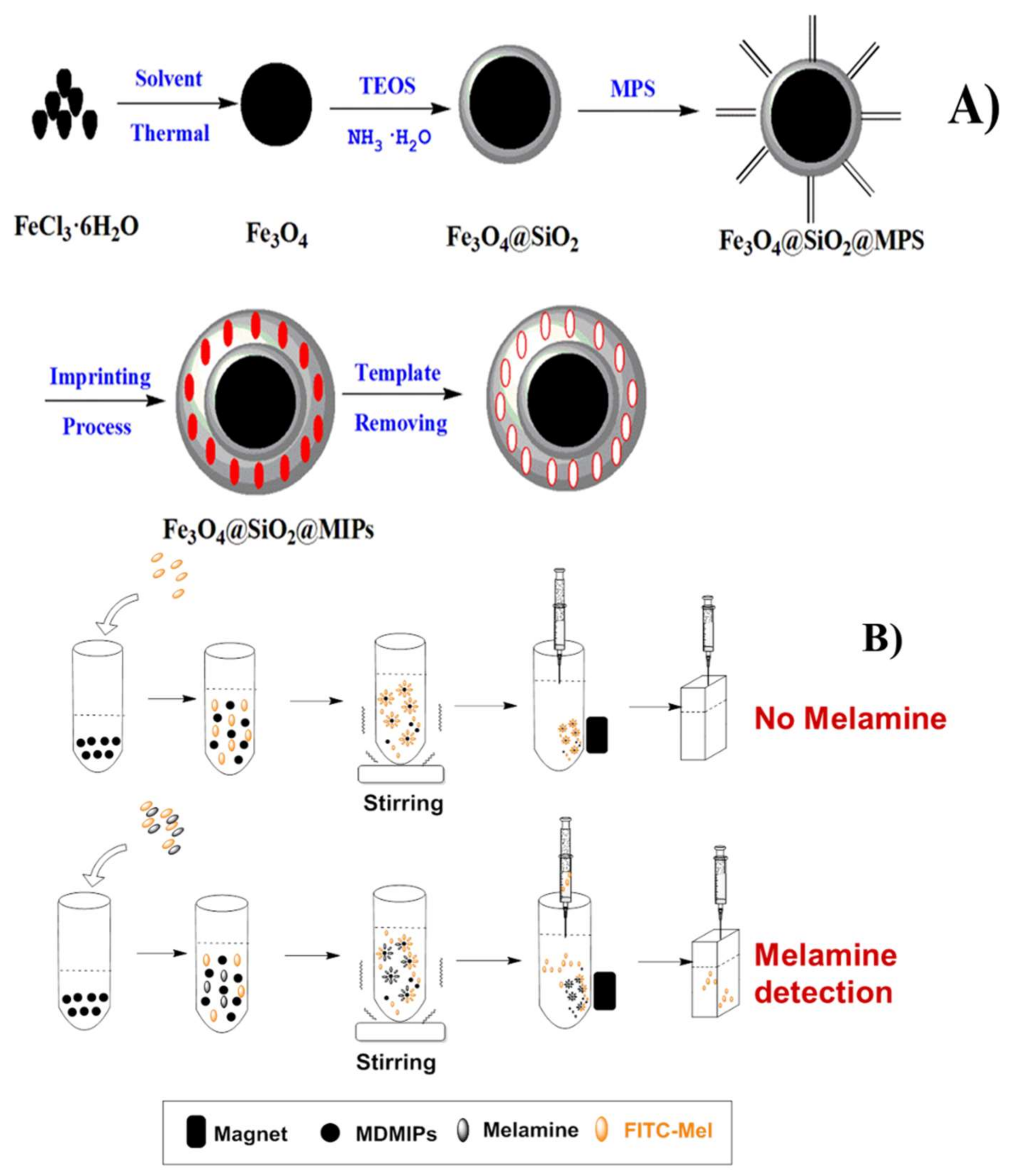
2.2. MDMIP Synthesis
2.3. Synthesis of FITC-Mel Fluorescent Label
2.4. Characterization of MDMIPs
Adsorption Experiments
2.5. Fluorescence Measurements
2.6. Preparation of Milk Samples
3. Results and Discussion
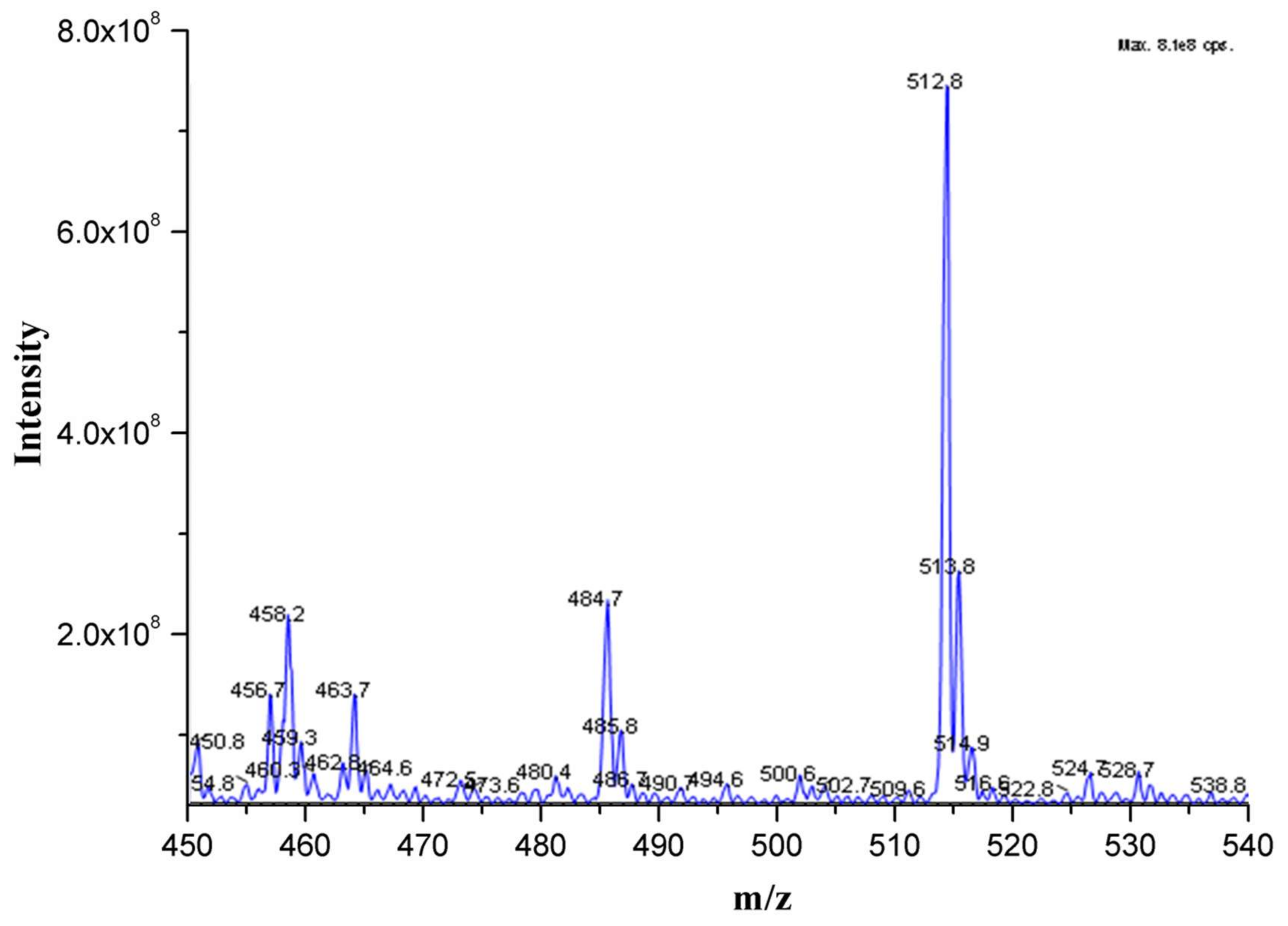
3.1. Mass Spectrum of FITC-Mel
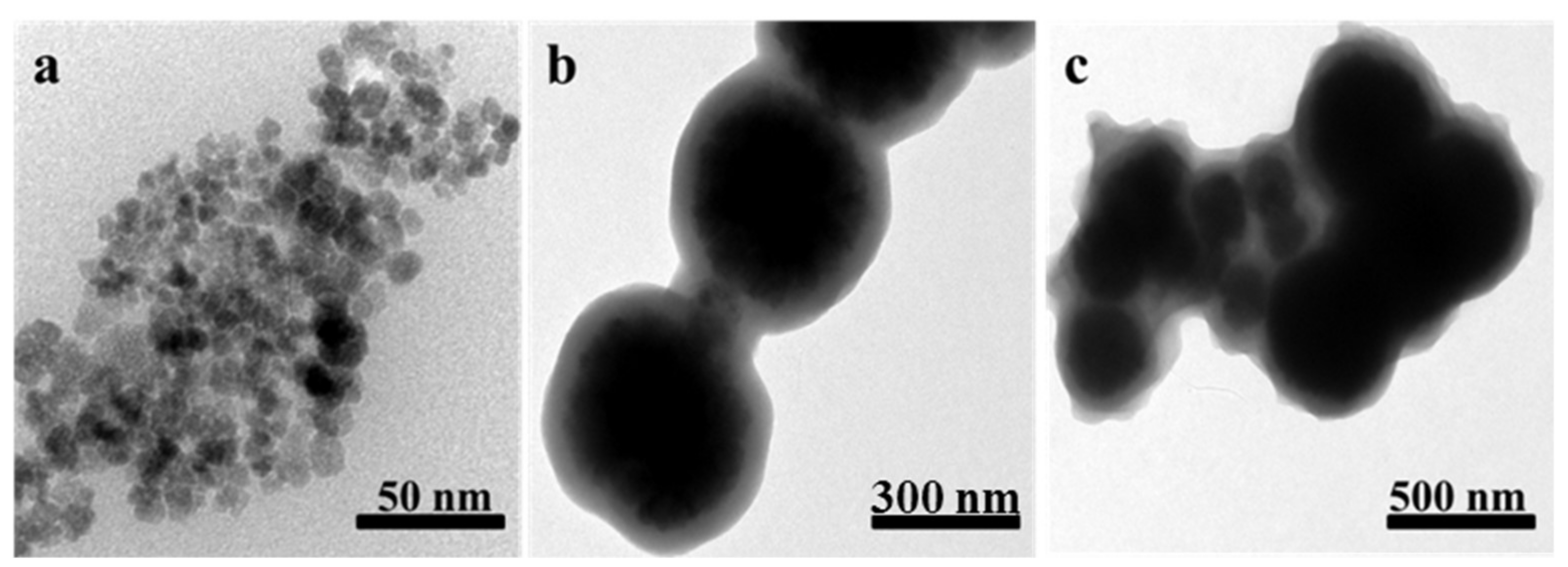
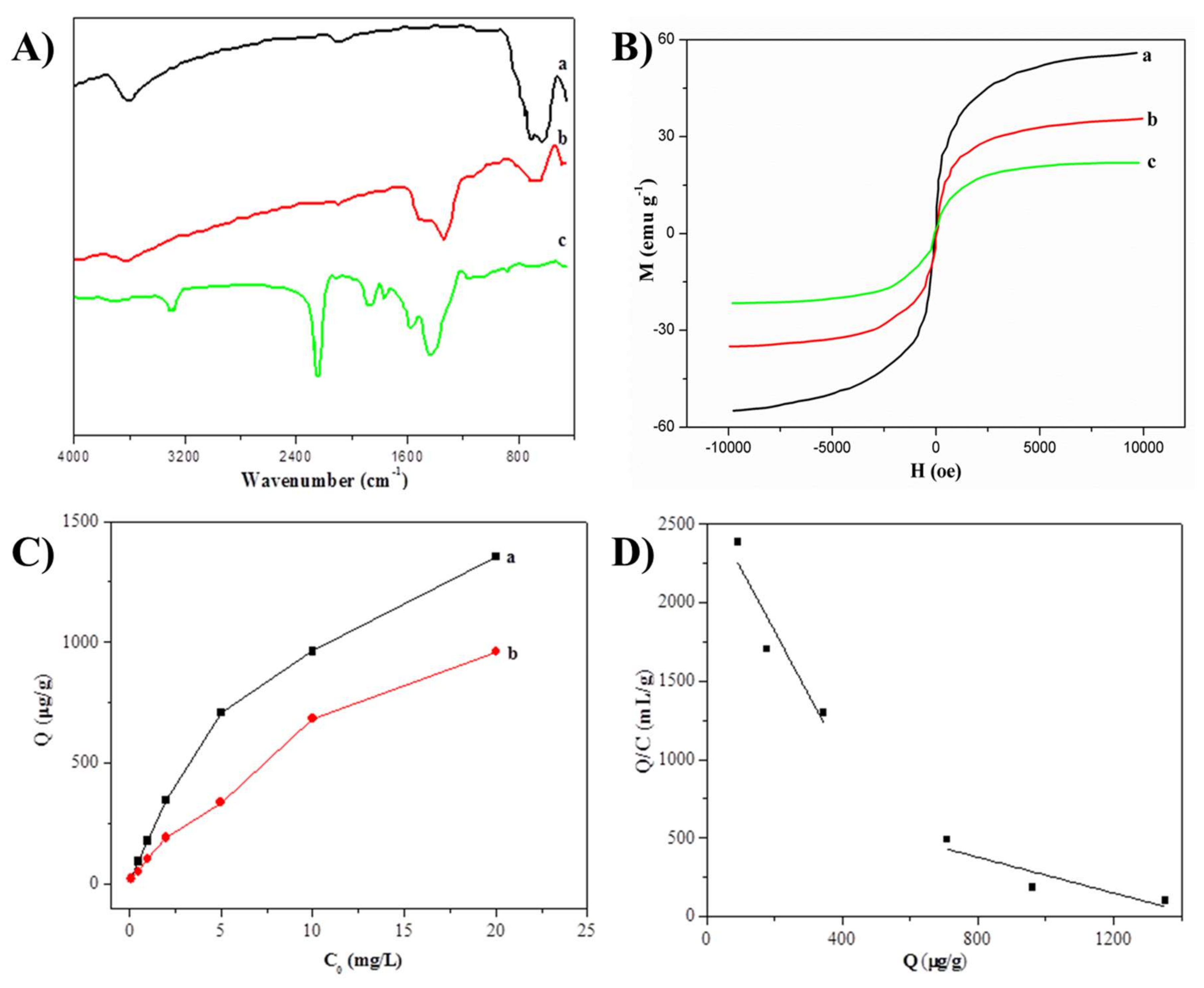
3.2. MDMIP Characterization
3.3. Binding Properties
3.4. Determination of Melamine in Milk Using Fluorescence Assays
3.5. Comparison
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, T.; Yang, X.; She, Y.; Wang, M.; Wang, J.; Zhang, M.; Wang, S.; Jin, F.; Jin, M. Competitive fluorescence assay for specific recognition of atrazine by magnetic molecularly imprinted polymer based on Fe3O4-chitosan. Carbohydr. Polym. 2016, 137, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, T.; Yang, X.; She, Y.; Wang, S.; Wang, J.; Zhang, M.; Jin, F.; Jin, M.; Shao, H. Preparation of a magnetic molecularly imprinted polymer using g-C3N4–Fe3O4 for atrazine adsorption. Mater. Lett. 2015, 160, 472–475. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Sunayama, H. Molecularly Imprinted Polymers. In Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1291–1295. [Google Scholar]
- Ahmadi, M.; Madrakian, T.; Afkhami, A. Solid phase extraction of doxorubicin using molecularly imprinted polymer coated magnetite nanospheres prior to its spectrofluorometric determination. New J. Chem. 2015, 39, 163–171. [Google Scholar] [CrossRef]
- Pavlović, D.M.; Nikšić, K.; Livazović, S.; Brnardić, I.; Anžlovar, A. Preparation and application of sulfaguanidine-imprinted polymer on solid-phase extraction of pharmaceuticals from water. Talanta 2015, 131, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Bai, J.; Peng, Y.; Qie, Z.; Zhao, Y.; Ning, B.; Gao, Z. A core–shell-structured molecularly imprinted polymer on upconverting nanoparticles for selective and sensitive fluorescence sensing of sulfamethazine. Analyst 2015, 140, 5301–5307. [Google Scholar] [CrossRef] [PubMed]
- De Middeleer, G.; Dubruel, P.; De Saeger, S. Characterization of MIP and MIP functionalized surfaces: Current state-of-the-art. TrAC Trends Anal. Chem. 2016, 76, 71–85. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, C.Y.; Wang, X.H.; Li, R.S.; Yang, J.R.; Huang, Y.P.; Liu, Z.S. Macromolecular crowding of molecular imprinting: A facile pathway to produce drug delivery devices for zero-order sustained release. Int. J. Pharm. 2015, 496, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, M.; Shen, X.; Li, S. A Cascade-Reaction Nanoreactor Composed of a Bifunctional Molecularly Imprinted Polymer that Contains Pt Nanoparticles. Chem. Eur. J. 2015, 21, 7532–7539. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Wei, G.; Zhang, Y.; Zeng, Y. Highly sensitive and doubly orientated selective molecularly imprinted electrochemical sensor for Cu2+. Biosens. Bioelectron. 2015, 69, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Deng, Q.; Fang, G.; Wang, J.; Pan, M.; Wang, S.; Pu, Y. Metal-organic frameworks supported surface–imprinted nanoparticles for the sensitive detection of metolcarb. Biosens. Bioelectron. 2016, 79, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Martín-Esteban, A. Recent molecularly imprinted polymer-based sample preparation techniques in environmental analysis. Trends Environ. Anal. Chem. 2016, 9, 8–14. [Google Scholar] [CrossRef]
- Peng, H.; Luo, M.; Xiong, H.; Yu, N.; Ning, F.; Fan, J.; Chen, L. Preparation of photonic-magnetic responsive molecularly imprinted microspheres and their application to fast and selective extraction of 17β-estradiol. J. Chromatogr. A. 2016, 1442, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arabi, M.; Ghaedi, M.; Ostovan, A. Development of dummy molecularly imprinted based on functionalized silica nanoparticles for determination of acrylamide in processed food by matrix solid phase dispersion. Food Chem. 2016, 210, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, Y.; Yan, X.; Liu, S.; Zheng, H. An Effective Method for the Synthesis of Yolk–Shell Magnetic Mesoporous Carbon–Surface Molecularly Imprinted Microspheres. J. Mater. Chem. A 2016, 4, 9807–9815. [Google Scholar] [CrossRef]
- Barahona, F.; Díaz-Álvarez, M.; Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymer-coated hollow fiber membrane for the microextraction of triazines directly from environmental waters. J. Chromatogr. A 2016, 1442, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.M.; Goudini, L.; Piri, F.; Zamani, A.; Saadati, F. Molecular imprinting method for fabricating novel glucose sensor: Polyvinyl acetate electrode reinforced by MnO2/CuO loaded on graphene oxide nanoparticles. Food Chem. 2016, 194, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Gao, R.; He, X.; Chen, L.; Zhang, Y. Synthesis and characterization of the core–shell magnetic molecularly imprinted polymers (Fe3O4@MIPs) adsorbents for effective extraction and determination of sulfonamides in the poultry feed. J. Chromatogr. A 2012, 1245, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Sun, Q.; Li, J.; Tan, Z.; Yan, Y.; Li, C. Magnetic imprinted nanomicrosphere attached to the surface of bacillus using miniemulsion polymerization for selective recognition of 2, 4, 6-trichlorophenol from aqueous solutions. J. Ind. Eng. Chem. 2015, 29, 349–358. [Google Scholar] [CrossRef]
- Tan, L.; He, R.; Chen, K.; Peng, R.; Huang, C.; Yang, R.; Tang, Y. Ultra-high performance liquid chromatography combined with mass spectrometry for determination of aflatoxins using dummy molecularly imprinted polymers deposited on silica-coated magnetic nanoparticles. Microchim. Acta 2016, 183, 1469–1477. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Ghaedi, M.; Purkait, M.K. Novel strategy for synthesis of magnetic dummy molecularly imprinted nanoparticles based on functionalized silica as an efficient sorbent for the determination of acrylamide in potato chips: Optimization by experimental design methodology. Talanta 2016, 154, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Long, Q.; Li, H.; Zhang, Y.; Yao, S. An upconversion fluorescence resonance energy transfer nanosensor for one step detection of melamine in raw milk. Talanta 2015, 136, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Lee, P.T.; Compton, R.G. Electrochemical detection of melamine. Electroanalysis 2014, 26, 1454–1460. [Google Scholar] [CrossRef]
- Jawaid, S.; Talpur, F.N.; Sherazi, S.T.H.; Nizamani, S.M.; Khaskheli, A.A. Rapid detection of melamine adulteration in dairy milk by SB-ATR–Fourier transform infrared spectroscopy. Food Chem. 2013, 141, 3066–3071. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, L.; Santos, L.; Alves, A. Synthesis of a Molecularly Imprinted Polymer for Melamine Analysis in Milk by HPLC with Diode Array Detection. Adv. Polym. Technol. 2015, 34. [Google Scholar] [CrossRef]
- Pan, X.D.; Wu, P.G.; Yang, D.J.; Wang, L.Y.; Shen, X.H.; Zhu, C.Y. Simultaneous determination of melamine and cyanuric acid in dairy products by mixed-mode solid phase extraction and GC–MS. Food Control 2013, 30, 545–548. [Google Scholar] [CrossRef]
- Lin, M.; He, L.; Awika, J.; Yang, L.; Ledoux, D.R.; Li, H.A.; Mustapha, A. Detection of melamine in gluten, chicken feed, and processed foods using surface enhanced Raman spectroscopy and HPLC. J. Food Sci. 2008, 73, T129–T134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Zou, M.Q.; Qi, X.H.; Liu, F.; Zhu, X.H.; Zhao, B.H. Detection of melamine in liquid milk using surface-enhanced Raman scattering spectroscopy. J. Raman Spectrosc. 2010, 41, 1655–1660. [Google Scholar] [CrossRef]
- Miao, P.; Han, K.; Sun, H.; Yin, J.; Zhao, J.; Wang, B.; Tang, Y. Melamine functionalized silver nanoparticles as the probe for electrochemical sensing of clenbuterol. ACS Appl. Mater. Interfaces 2014, 6, 8667–8672. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Yang, H.; Song, J.; Chang, H.; Li, S.; Deng, A. Deng, Sensitivity and specificity enhanced enzyme-linked immunosorbent assay by rational hapten modification and heterogeneous antibody/coating antigen combinations for the detection of melamine in milk, milk powder and feed samples. Talanta 2013, 116, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, H.; Li, Y.; Su, X. Visual and fluorescent detection of acetamiprid based on the inner filter effect of gold nanoparticles on ratiometric fluorescence quantum dots. Anal. Chim. Acta 2014, 852, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, H.; Han, X.; Su, X. A ratiometric fluorescent quantum dots based biosensor for organophosphorus pesticides detection by inner-filter effect. Biosens. Bioelectron. 2015, 74, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, H. One-pot synthesis of mesoporous structured ratiometric fluorescence molecularly imprinted sensor for highly sensitive detection of melamine from milk samples. Biosens. Bioelectron. 2015, 73, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Biyikal, M.; Wagner, R.; Sellergren, B.; Rurack, K. Fluorescent Sensory Microparticles that “Light-up” Consisting of a Silica Core and a Molecularly Imprinted Polymer (MIP) Shell. Angew. Chem. Int. Ed. 2013, 52, 7023–7027. [Google Scholar] [CrossRef] [PubMed]
- Huy, B.T.; Seo, M.H.; Zhang, X.; Lee, Y.I. Selective optosensing of clenbuterol and melamine using molecularly imprinted polymer-capped CdTe quantum dots. Biosens. Bioelectron. 2014, 57, 310–316. [Google Scholar]
- Zhou, Y.; Qu, Z.B.; Zeng, Y.; Zhou, T.; Shi, G. A novel composite of graphene quantum dots and molecularly imprinted polymer for fluorescent detection of paranitrophenol. Biosens. Bioelectron. 2014, 52, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, H.; Shen, H.; Pan, J.; Dai, X.; Yan, Y.; Sellergren, B. Molecularly imprinted fluorescent hollow nanoparticles as sensors for rapid and efficient detection λ-cyhalothrin in environmental water. Biosens. Bioelectron. 2016, 85, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, Z.Z.; Peng, A.H.; Zhong, H.P.; Chen, X.M.; Huang, Z.Y. Biomimetic ELISA detection of malachite green based on magnetic molecularly imprinted polymers. J. Chromatogr. B. 2016, 229, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Urraca, J.L.; Moreno-Bondi, M.C.; Orellana, G.; Sellergren, B.; Hall, A.J. Molecularly imprinted polymers as antibody mimics in automated on-line fluorescent competitive assays. Anal. Chem. 2007, 79, 4915–4923. [Google Scholar] [CrossRef] [PubMed]
- Murase, N.; Taniguchi, S.I.; Takano, E.; Kitayama, Y.; Takeuchi, T. A molecularly imprinted nanocavity-based fluorescence polarization assay platform for cortisol sensing. J. Mater. Chem. B. 2016, 4, 1770–1777. [Google Scholar] [CrossRef]
- Shi, S.; Guo, J.; You, Q.; Chen, X.; Zhang, Y. Selective and simultaneous extraction and determination of hydroxybenzoic acids in aqueous solution by magnetic molecularly imprinted polymers. Chem. Eng. J. 2014, 243, 485–493. [Google Scholar] [CrossRef]
- Ni, Q.; Chen, B.; Dong, S.; Tian, L.; Bai, Q. Preparation of core–shell structure Fe3O4@SiO2 superparamagnetic microspheres immoblized with iminodiacetic acid as immobilized metal ion affinity adsorbents for His-tag protein purification. Biomed. Chromatogr. 2016, 30, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Xing, Y.; Yang, W.; Zhou, Z.; Wang, Y.; Liu, H.; Xu, W. Surface molecular imprinting on hybrid SiO2-coated CdTe nanocrystals for selective optosensing of bisphenol A and its optimal design. Appl. Surf. Sci. 2015, 345, 405–417. [Google Scholar] [CrossRef]
- Hu, Y. Development of Innovative Biosensors for the Determination of Melamine in Milk. Master’s Thesis, The University of British Columbia, Vancouver, BC, Canada, 2015. [Google Scholar]
- Chi, H.; Liu, B.; Guan, G.; Zhang, Z.; Han, M.Y. A simple, reliable and sensitive colorimetric visualization of melamine in milk by unmodified gold nanoparticles. Analyst 2010, 135, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- DU, X.; Zhang, Y.; She, Y.; Liu, G.; Zhao, F.; Wang, J.; Wang, S.; Jin, F.; Shao, H.; Jin, M.; Zheng, L. Fluorescent competitive assay for melamine using dummy molecularly imprinted polymers as antibody mimics. J. Integr. Agric. 2016, 15, 1166–1177. [Google Scholar] [CrossRef]
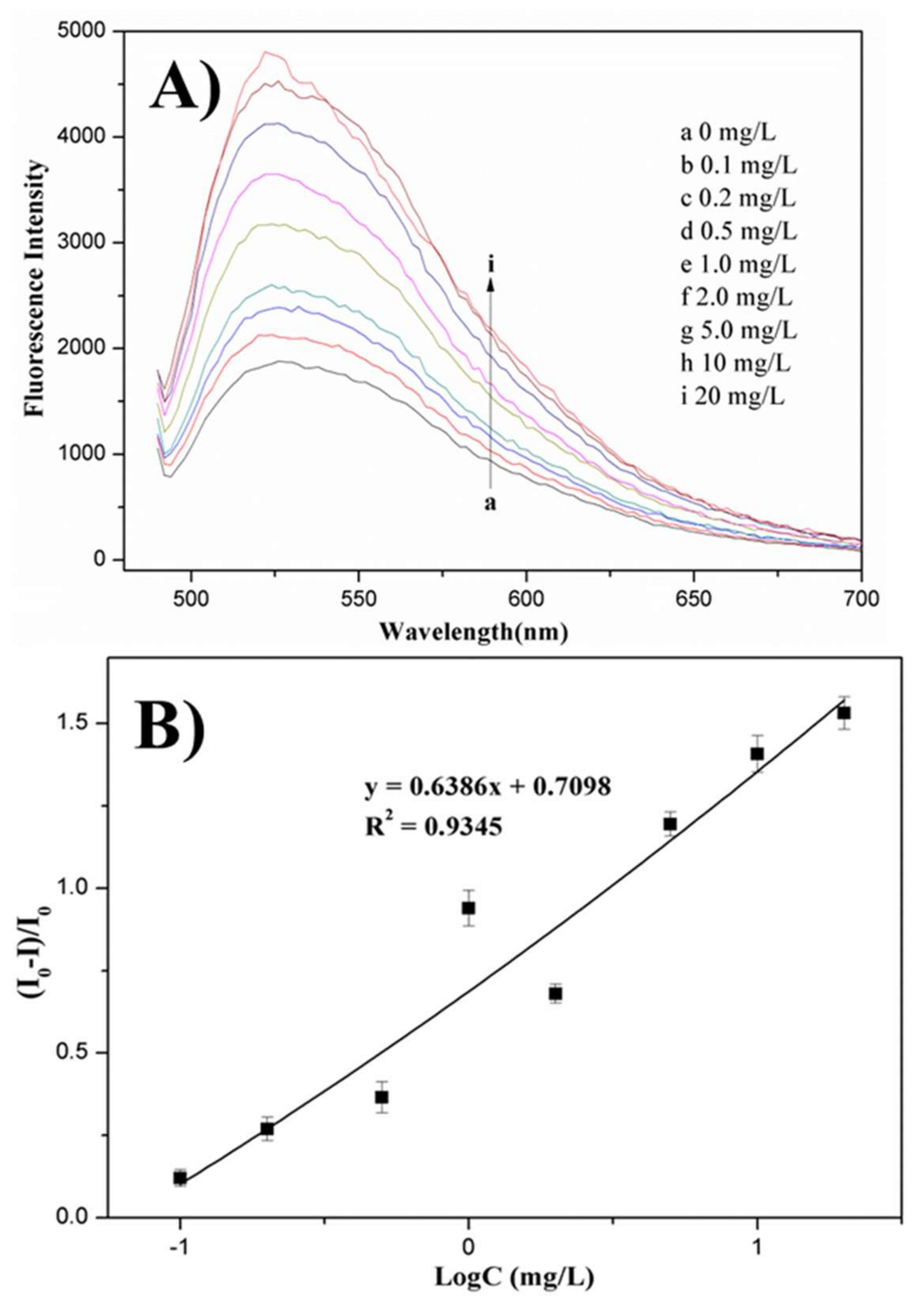
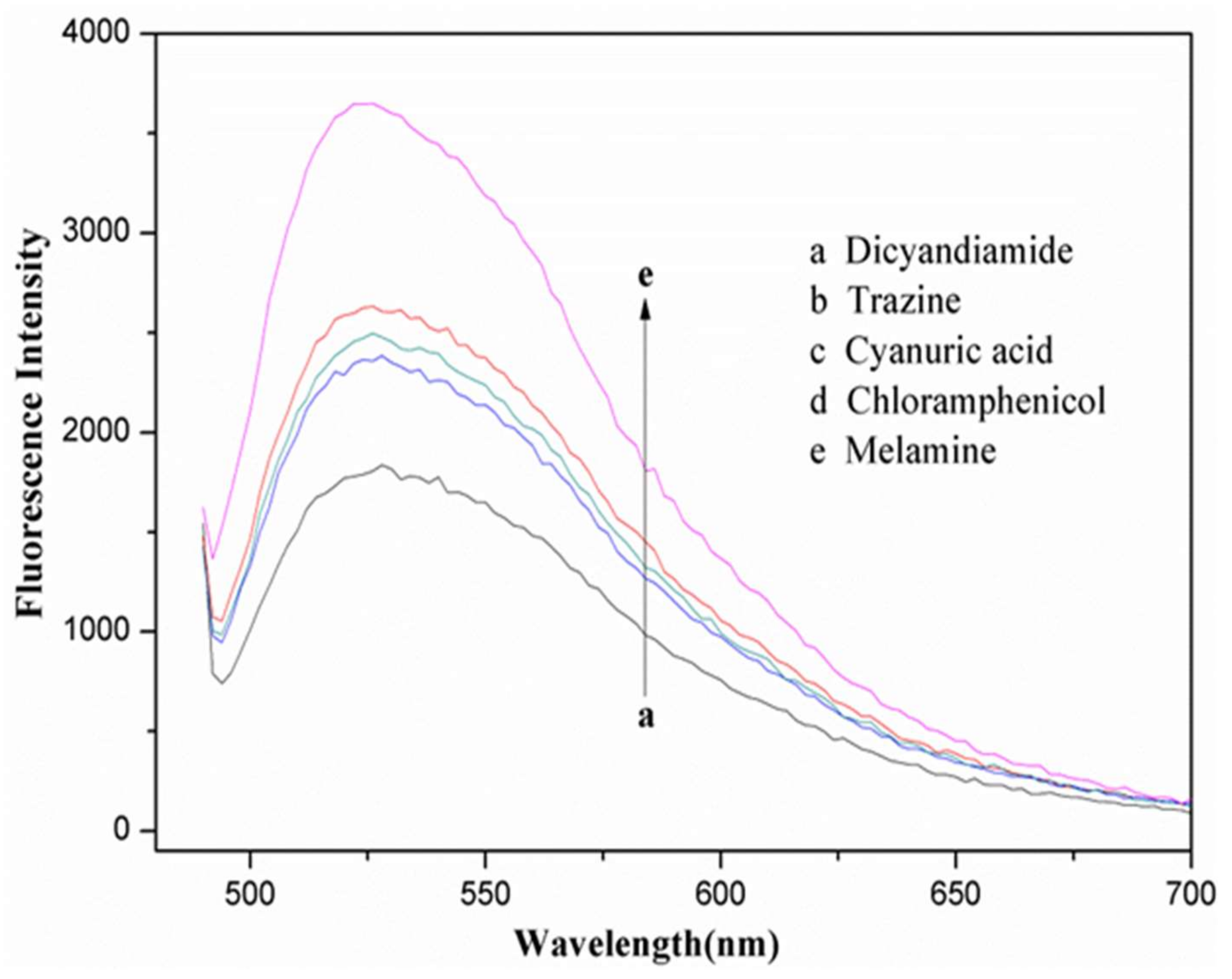
| Added (mg/L) | Fluorescent Found (mg/L) | Recovery, Means ± R.S.D (%, n = 3) | HPLC-MS/MS |
|---|---|---|---|
| 0.25 | 0.175 | 70.2 ± 8.9 | 83.5 ± 4.8 |
| 0.5 | 0.411 | 82.2 ± 12.4 | 82.7 ± 4.1 |
| 1.0 | 0.853 | 85.3 ± 7.6 | 89.6 ± 3.3 |
| 2.5 | 2.32 | 92.7 ± 5.4 | 93.5 ± 2.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; She, Y.; Hong, S.; Wang, J.; Xu, D. Development of ELISA-Like Fluorescence Assay for Melamine Detection Based on Magnetic Dummy Molecularly Imprinted Polymers. Appl. Sci. 2018, 8, 560. https://doi.org/10.3390/app8040560
Liu G, She Y, Hong S, Wang J, Xu D. Development of ELISA-Like Fluorescence Assay for Melamine Detection Based on Magnetic Dummy Molecularly Imprinted Polymers. Applied Sciences. 2018; 8(4):560. https://doi.org/10.3390/app8040560
Chicago/Turabian StyleLiu, Guangyang, Yongxin She, Sihui Hong, Jing Wang, and Donghui Xu. 2018. "Development of ELISA-Like Fluorescence Assay for Melamine Detection Based on Magnetic Dummy Molecularly Imprinted Polymers" Applied Sciences 8, no. 4: 560. https://doi.org/10.3390/app8040560
APA StyleLiu, G., She, Y., Hong, S., Wang, J., & Xu, D. (2018). Development of ELISA-Like Fluorescence Assay for Melamine Detection Based on Magnetic Dummy Molecularly Imprinted Polymers. Applied Sciences, 8(4), 560. https://doi.org/10.3390/app8040560






