Separation of Light Liquid Paraffin C5–C9 with Cuban Volcanic Glass Previously Used in Copper Elimination from Water Solutions
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Volcanic Glass
2.2. Characterization of the Materials
2.3. Cu2+ Adsorption Tests
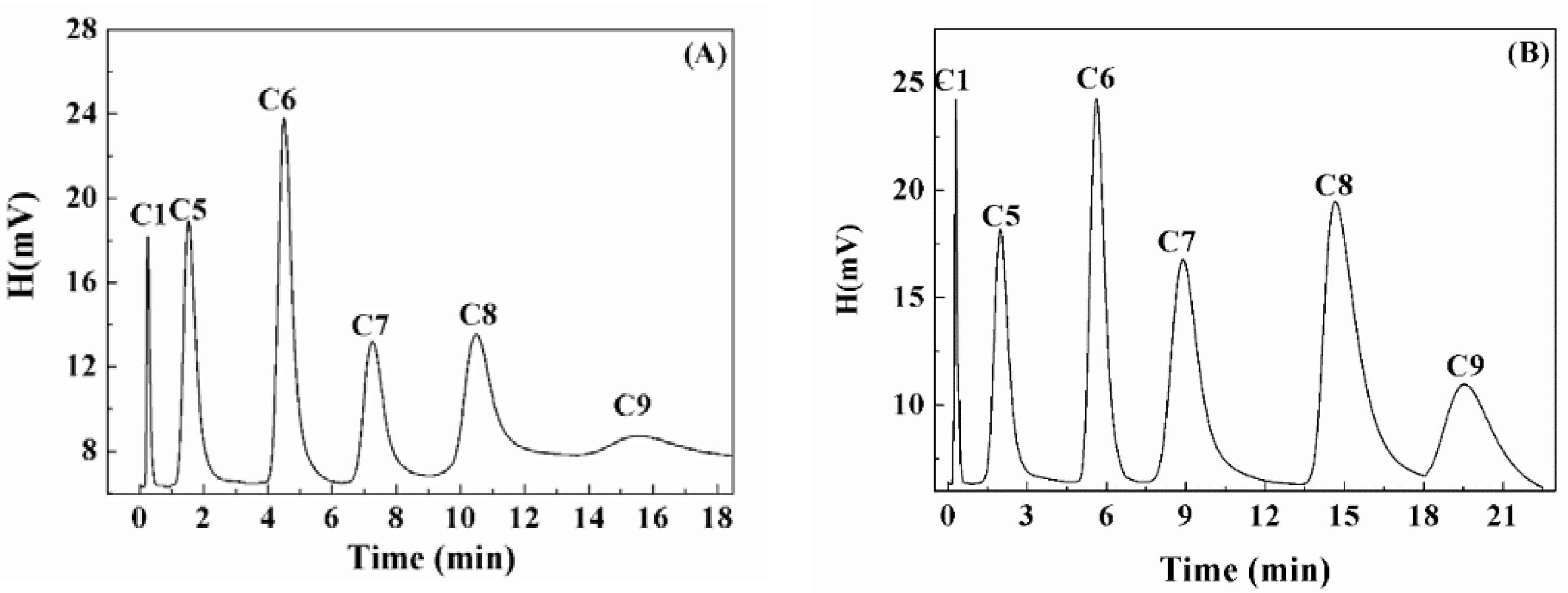
2.4. Separation of N-Paraffin Tests
3. Results and Discussion
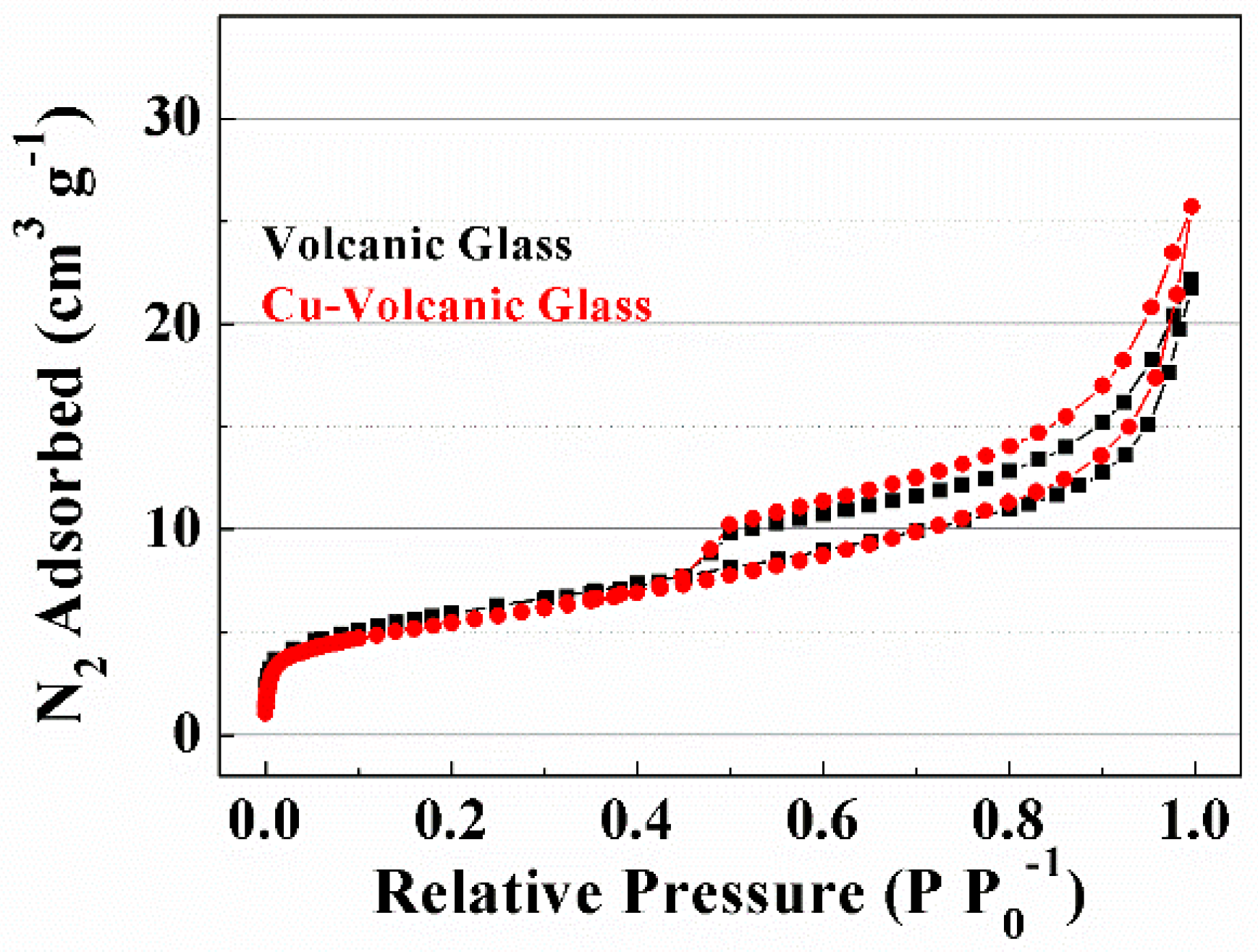
Characterization of Volcanic Glass
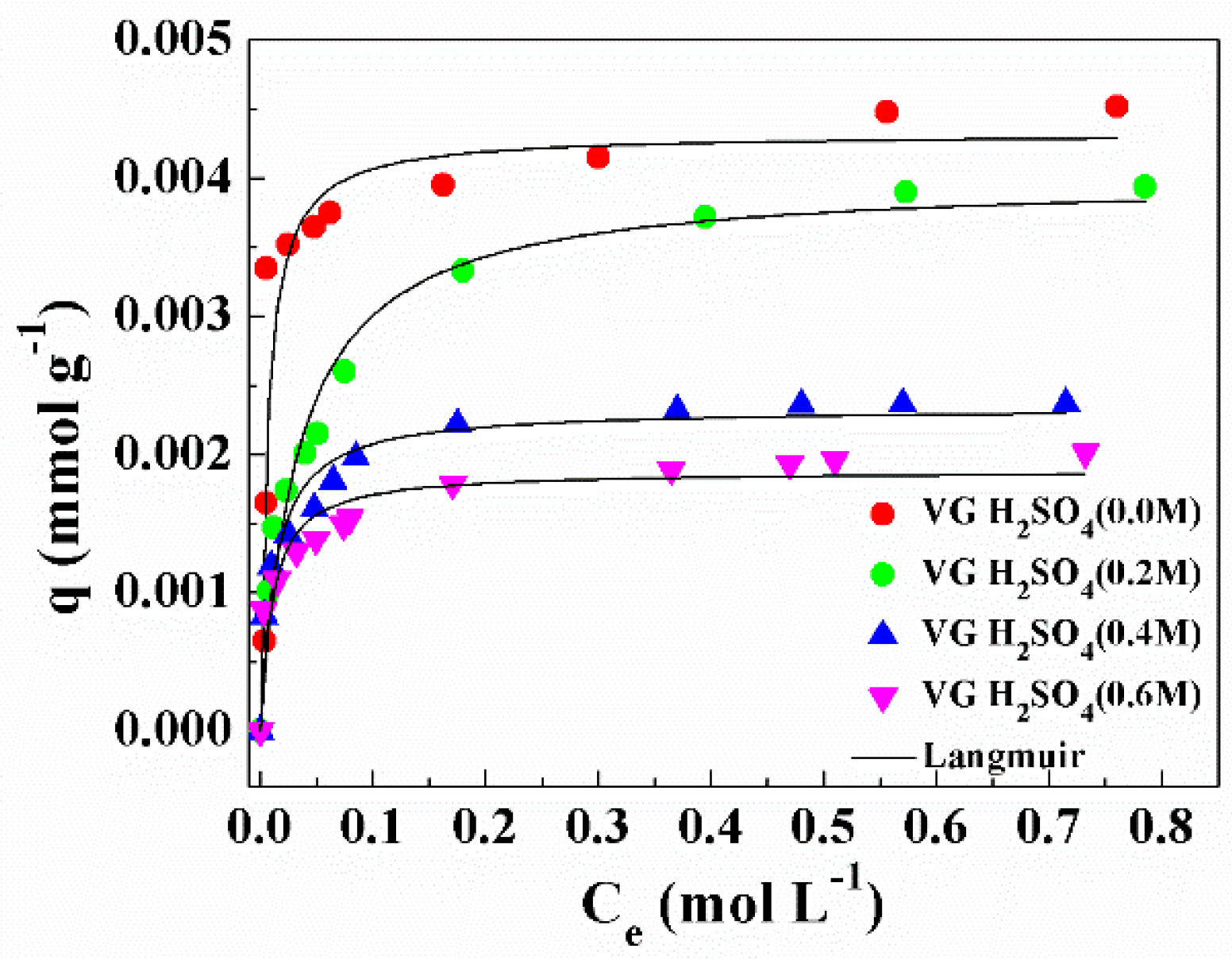
4. Cu2+ Adsorption Capacity of Volcanic Glass
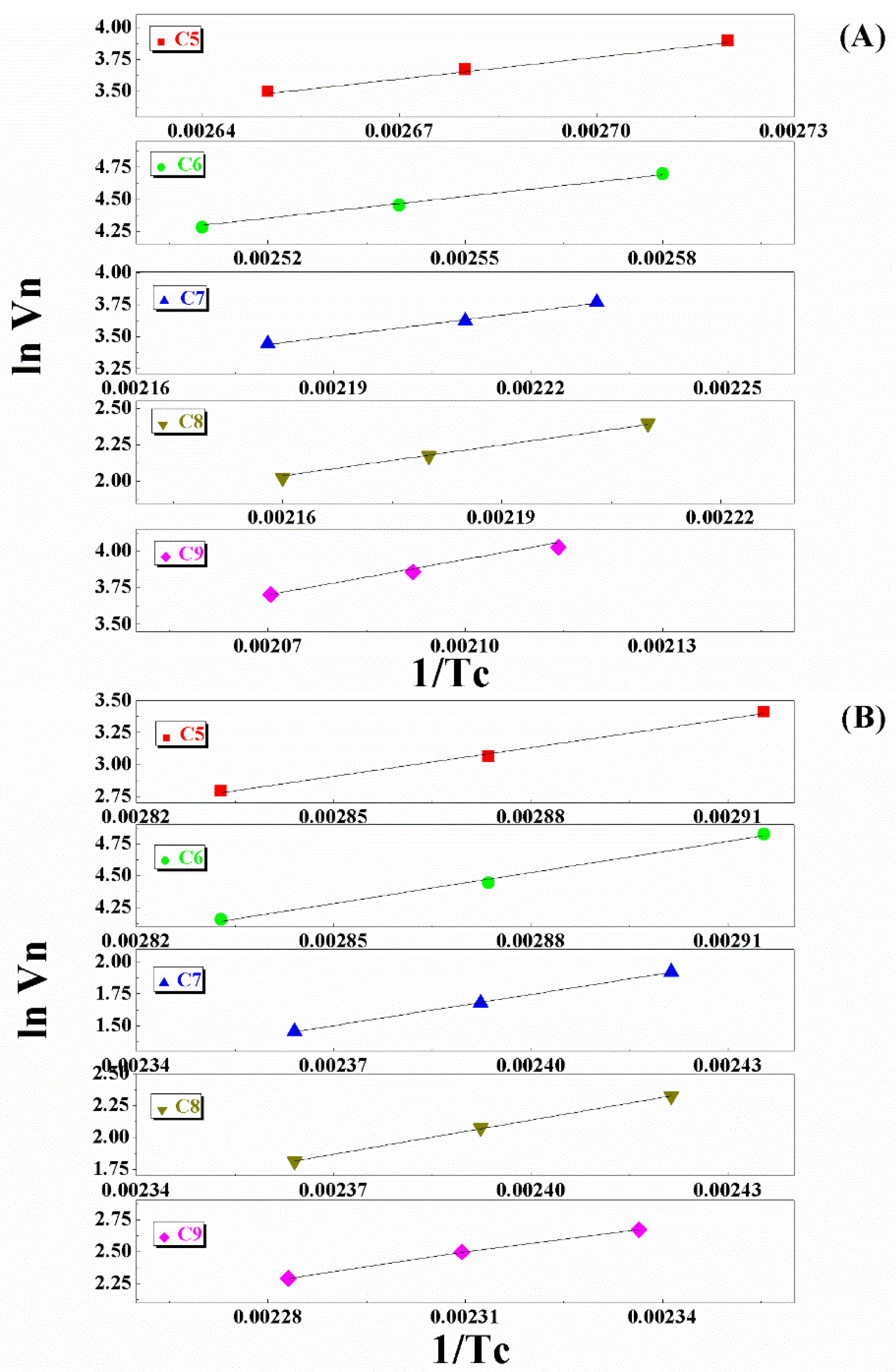
5. Light N-Paraffin Separation in Volcanic Glass
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bryan, P.F. Removal of Propylene from Fuel-Grade Propane. Sep. Purif. Rev. 2004, 33, 157–182. [Google Scholar] [CrossRef]
- Merkel, T.C.; Blanc, R.; Ciobanu, I.; Firat, B.; Suwarlim, A.; Zeid, J. Silver salt facilitated transport membranes for olefin/paraffin separations: Carrier instability and a novel regeneration method. J. Membr. Sci. 2013, 447, 177–189. [Google Scholar] [CrossRef]
- Eldridge, R.B. Olefin/paraffin separation technology: A review. Ind. Eng. Chem. Res. 1993, 32, 2208–2212. [Google Scholar] [CrossRef]
- Azhin, M.; Kaghazchi, T.; Rahmani, M. A review on olefin/paraffin separation using reversible chemical complexation technology. J. Ind. Eng. Chem. 2008, 14, 622–638. [Google Scholar] [CrossRef]
- Faiz, R.; Li, K. Olefin/paraffin separation using membrane based facilitated transport/chemical absorption techniques. Chem. Eng. Sci. 2012, 73, 261–284. [Google Scholar] [CrossRef]
- Ferreira, A.F.P.; Mittelmeijer-Hazeleger, M.C.; Granato, M.A.; Martins, V.F.D.; Rodrigues, A.E.; Rothenberg, G. Sieving di-branched from mono-branched and linear alkanes using ZIF-8: Experimental proof and theoretical explanation. Phys. Chem. Chem. Phys. 2013, 15, 8795–8804. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Farías, T.; de Ménorval, L.C.; Autié-Castro, G.; Yee-Madeira, H.; Contreras, J.L.; Autié-Castro, M. Acid natural clinoptilolite: Structural properties against adsorption/separation of n-paraffins. J. Colloid Interface Sci. 2011, 360, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, Z.; Yuan, H.; Chen, Y.; Kobayashi, N. Evaluation of n-butane gas adsorption performance of composite adsorbents used for carbon canister. Procedia Eng. 2011, 18, 78–85. [Google Scholar] [CrossRef]
- Yahia, S.B.; Ouederni, A. Hydrocarbons gas storage on activated carbons. Int. J. Chem. Eng. Appl. 2012, 3, 220–227. [Google Scholar] [CrossRef]
- Herm, H.; Bloch, E.D.; Long, J.R. Hydrocarbon separation in metal-organic frameworks. Chem. Mater. 2014, 26, 323–338. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Jhung, S.H. Selective adsorption of n-alkanes from n-octane on metal-organic frameworks: Length selectivity. ACS Appl. Mater. Interf. 2016, 8, 6370–6777. [Google Scholar] [CrossRef]
- Rivera, A.; Farías, T.; de Ménorval, L.C.; Ruiz-Salvador, A.R. Preliminary characterization of drug support systems based on natural clinoptilolite. Microporous Mesoporous Mater. 2003, 61, 249–259. [Google Scholar] [CrossRef]
- Elizalde-González, M.P.; Pérez-Cruz, M.A. Interaction between organic vapors and clinoptilolite–mordenite rich tuffs in parent, decationized, and lead exchanged forms. J. Colloid Interface Sci. 2007, 312, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.A.; Corona, L.; González, A.I.; Rojas, F.; Lara, V.H.; Silva, F. Quantitative study of the adsorption of aromatic hydrocarbons (Benzene, Toluene, and p-Xylene) on dealuminated clinoptilolites. Ind. Eng. Chem. Res. 2005, 44, 2908–2916. [Google Scholar] [CrossRef]
- Ruggieri, F.; Marín, V.; Gimeno, D.; Fernández-Turiel, J.L.; García-Vallés, M.; Gutiérrez, L. Application of zeolitic volcanic rocks for arsenic removal from water. Eng. Geol. 2008, 101, 245–250. [Google Scholar] [CrossRef]
- Steinhauser, G.; Bichler, M. Adsorption of ions onto high silica volcanic glass. Appl. Radiat. Isotopes 2008, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dogan, M.; Alkan, M. Removal of methyl violet from aqueous solution by perlite. J. Colloid Interface Sci. 2003, 267, 32–41. [Google Scholar] [CrossRef]
- Alkan, M.; Dogan, M. Adsorption kinetics of Victoria blue onto perlite. Fresenius Environ. Bull. 2003, 12, 418–425. [Google Scholar]
- Dogan, M.; Alkan, M.; Turkylmaz, A.; Ozdemir, Y. Kinetics and mechanism of removal of methylene blue by adsorption onto perlite. J. Hazard. Mater. 2004, 109, 141–148. [Google Scholar] [PubMed]
- Fernández-Hechevarría, H.M.; Labadie-Suárez, J.M.; Santamaría-González, J.; Infantes-Molina, A.; Autié-Castro, G.; Cavalcante, C.L., Jr.; Rodríguez-Castellón, E.; Autié-Castro, M. Adsorption and separation of propane and propylene by Cuban natural volcanic glass. Mater. Chem. Phys. 2015, 168, 132–137. [Google Scholar] [CrossRef]
- Friedman, I.; Long, W. Volcanic glasses, their origins and alteration processes. J. Non-Cryst. Solid 1984, 67, 127–133. [Google Scholar] [CrossRef]
- Buck, B.A. Ancient technology in contemporary surgery. West. J. Med. 1982, 136, 265–269. [Google Scholar] [PubMed]
- Disa, J.J.; Vossoughi, J.; Goldberg, N.H. A comparison of obsidian and surgical steel scalpel wound healing in rats. Plast. Reconstr. Surg. 1993, 92, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Alkan, M.; Dogan, M. Adsorption of copper(II) onto perlite. J. Colloid Interface Sci. 2001, 243, 280–291. [Google Scholar] [CrossRef]
- Tomita, K.; Yamane, H.; Kawano, M. Synthesis of smectite from volcanic glass at low temperature. Clay Clay Miner. 1993, 41, 655–661. [Google Scholar] [CrossRef]
- Yoshida, A.; Inoue, K. Formation of faujasite-type zeolite from ground Shirasu volcanic glass. Zeolites 1986, 6, 467–473. [Google Scholar] [CrossRef]
- Yoshida, A.; Inoue, K. Whiteness in zeolite A prepared from Shirasu volcanic glass. Zeolites 1988, 8, 94–100. [Google Scholar] [CrossRef]
- Rivas, L.; Villegas, S.; Ruf, B.; Peric, I. Removal of metal ions with impact on the environment by wate-insolutble functional copolymer: Synthesis and metal ion uptake properties. J. Chil. Chem. Soc. 2007, 52, 1164–1168. [Google Scholar] [CrossRef]
- Ground Water and Drinking Water. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 16 February 2018).
- Bereket, G.; Aroguz, A.Z.; Ozel, M.Z. Removal of Pb(II), Cd(II), Cu(II), and Zn(II) from aqueous solutions by adsorption on bentonite. J. Colloid Interface Sci. 1997, 187, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Autié-Castro, G.; Autié, M.; Reguera, E.; Santamaría-González, J.; Moreno-Tost, R.; Rodríguez-Castellón, E.; Jiménez-López, A. Adsorption and separation of light alkane hydrocarbons by porous hexacyanocobaltates (III). Surf. Interf. Anal. 2009, 41, 730–734. [Google Scholar] [CrossRef]
- Voelkel, A.; Strzemiecka, B.; Adamska, K.; Milczewaska, K. Inverse gas chromatography as a source of physiochemical data. J. Chromatrogr. A 2009, 1216, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Jan, S.; Waters, K.E. Inverse gas chromatography applications: A review. Adv. Colloid Interface Sci. 2014, 212, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Batko, K.; Voelkel, A. Inverse gas chromatography as a tool for investigation of nanomaterials. J. Colloid Interface Sci. 2007, 315, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Menzel, R.; Lee, A.; Bismarck, A.; Shaffer, M.S.P. Inverse gas chromatography of as-received and modified carbon nanotubes. Langmuir 2009, 25, 8340–8348. [Google Scholar] [CrossRef] [PubMed]
- Heng, J.Y.Y.; Pears, D.F.; Thielmann, F.; Lampke, T.; Bismark, A. Methods to determine surface energies of natural fibres: A review. Compos. Interface 2007, 14, 581–604. [Google Scholar] [CrossRef]
- Murakami, Y.; Enoki, R.; Ogoma, Y.; Kondo, Y. Studies on interaction between silica gel and polymer blend by inverse gas chromatography. Polym. J. 1998, 30, 520–525. [Google Scholar] [CrossRef]
- Abel, M.L.; Chehimi, M.M.; Fricker, F.; Delamar, M.; Brown, A.M.; Watts, J.F. Adsorption of poly(methyl methacrylate) and poly(vinyl chloride) blends onto polypyrrole: Study by X-ray photoelectron spectroscopy, time-of-flight static secondary ion mass spectroscopy, and inverse gas chromatography. J. Chromatogr. A 2002, 969, 273–285. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Farmer, V.C. (Ed.) Infrared Spectra of Minerals; Mineralogical Society: London, UK, 1974; p. 331. [Google Scholar]
- Vilarrasa-García, E.; Cecilia, J.A.; Azevedo, D.C.S.; Cavalcante, C.L., Jr.; Rodríguez-Castellón, E. Evaluation of porous clay heterostructures modified with amine species as adsorbent for the CO2 capture. Microporous Mesoporous Mater. 2017, 249, 25–33. [Google Scholar] [CrossRef]
- Correia, L.M.; Campelo, N.S.; Novaes, D.S.; Cavalcante, C.L., Jr.; Cecilia, J.A.; Rodríguez-Castellón, E.; Vieira, R.S. Characterization and application of dolomite as catalytic precursor for canola and sunflower oils for biodiesel production. Chem. Eng. J. 2015, 269, 35–43. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sool, P.E.; Bomber, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Pekin-Elmer: Eden Praire, NM, USA, 1992. [Google Scholar]
- Kinniburgh, D.G. Adsorption of Inorganic Solid–Liquid Interfaces; Anderson, M.A., Rubin, A.J., Eds.; Ann Arbor Science Publisher: Ann Arbor, MI, USA, 1981; pp. 91–160. [Google Scholar]
- Ghassabzadeh, H.; Torab-Mostaedi, M.; Mohaddespour, A.; Maragheh, M.G.; Ahmadi, S.J.; Zaheri, P. Characterizations of Co(II) and Pb(II) removal process from aqueous solutions using expanded perlite. Desalinitation 2010, 261, 73–79. [Google Scholar] [CrossRef]
- Ghassabzadeh, H.; Mohadespour, A.; Torab-Mostaedic, M.; Zaherib, P.; Maraghehc, M.G.; Taheric, H. Adsorption of Ag, Cu and Hg from aqueous solutions using expanded perlite. J. Hazard. Mater. 2010, 177, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Franco, F.; Pozo, M.; Cecilia, J.A.; Benítez-Guerrero, M.; Pozo, E.; Martín Rubí, J.A. Microwave assisted acid treatment of sepiolite: The role of composition and “crystallinity”. Appl. Clay Sci. 2014, 102, 15–27. [Google Scholar] [CrossRef]
- Franco, F.; Pozo, M.; Cecilia, J.A.; Benítez-Guerrero, M.; Lorente, M. Effectiveness of microwave assisted acid treatment on dioctahedral and trioctahedral smectites. The influence of octahedral composition. Appl. Clay Sci. 2016, 120, 70–80. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Arango-Díaz, A.; Rico-Pérez, V.; Bueno-López, A.; Rodríguez-Castellón, E. The influence of promoters (Zr, La, Tb, Pr) on the catalytic performance of CuO-CeO2 systems for the preferential oxidation of CO in the presence of CO2 and H2O. Catal. Today 2015, 253, 115–125. [Google Scholar] [CrossRef]
- Niua, Y.; Li, K.; Ying, D.; Wang, J.; Jia, J. Novel recyclable adsorbent for the removal of copper(II) and lead(II) from aqueous solution. Bioresour. Technol. 2017, 229, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Ying, D.; Li, K.; Wang, Y.; Jia, J. Fast removal of copper ions from aqueous solution using an eco–friendly fibrous adsorbent. Chemosphere 2016, 161, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Tofighy, M.A.; Mohammadi, T. Copper ions removal from water using functionalized carbon nanotubes–mullite composite as adsorbent. Mater. Res. Bull. 2015, 68, 54–59. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Mo, J.; Glocheux, Y.; Allen, S.; Walker, G.; Mangwandi, C. Preliminary investigation of mixed adsorbents for the removal of copper and methylene blue from aqueous solutions. Chem. Eng. J. 2014, 255, 525–534. [Google Scholar] [CrossRef]
- Abubakar, H.; Ahmad, M.; Hussein, M.Z.; Ibrahim, N.A.; Musa, A.; Saleh, T.A. Nanocomposite of ZnO with montmorillonite for removal of lead and copper ions from aqueous solutions. Process Saf. Environ. Prot. 2017, 109, 97–105. [Google Scholar]
- Grande, C.A.; Araujo, J.D.P.; Cavenati, S.; Firpo, N.; Basaldella, E.; Rodrigues, A.E. New π-complexation adsorbents for propane-propylene separation. Langmuir 2004, 20, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, M.; Palomares, A.E.; Chiesa, M.; Boronat, M.; Giamello, E.; Blasco, T. Evidence of a Cu2+–alkane interaction in Cu-zeolite catalysts crucial for the selective catalytic reduction of NOx with hydrocarbons. ACS Catal. 2017, 7, 3501–3509. [Google Scholar] [CrossRef]


| Sample | SiO2 | Al2O3 | K2O | Fe2O3 | CuO | MgO | CaO | Na2O |
|---|---|---|---|---|---|---|---|---|
| Volcanic Glass | 66.2 | 13.4 | 2.4 | 2.8 | – | 1.3 | 2.5 | 1.7 |
| Cu-Volcanic Glass | 63.3 | 12.2 | 3.6 | 3.4 | 2.2 | 1.9 | 1.8 | 1.2 |
| Sample | O 1s | Si 2p | Al 2p | Mg 2p | Ca 2p | Na 1s | K 1s | Cu 2p |
|---|---|---|---|---|---|---|---|---|
| Volcanic Glass | 61.5 | 19.8 | 3.7 | 2.8 | 1.6 | 0.7 | 0.7 | – |
| Cu-Volcanic Glass | 62.1 | 19.9 | 3.8 | 2.2 | 0.9 | 0.7 | 0.7 | 0.6 |
| Sample | qmax × 103 (mmol g−1) | KL (L g−1) | R |
|---|---|---|---|
| Volcanic Glass (H2SO4 0.0 M) | 4.32 ± 0.27 | 2.5 ± 0.9 | 0.96 |
| Volcanic Glass (H2SO4 0.2 M) | 4.00 ± 0.14 | 1.5 ± 0.5 | 0.97 |
| Volcanic Glass (H2SO4 0.4 M) | 2.34 ± 0.07 | 1.2 ± 0.2 | 0.96 |
| Volcanic Glass (H2SO4 0.6 M) | 1.81 ± 0.08 | 0.5 ± 0.1 | 0.94 |
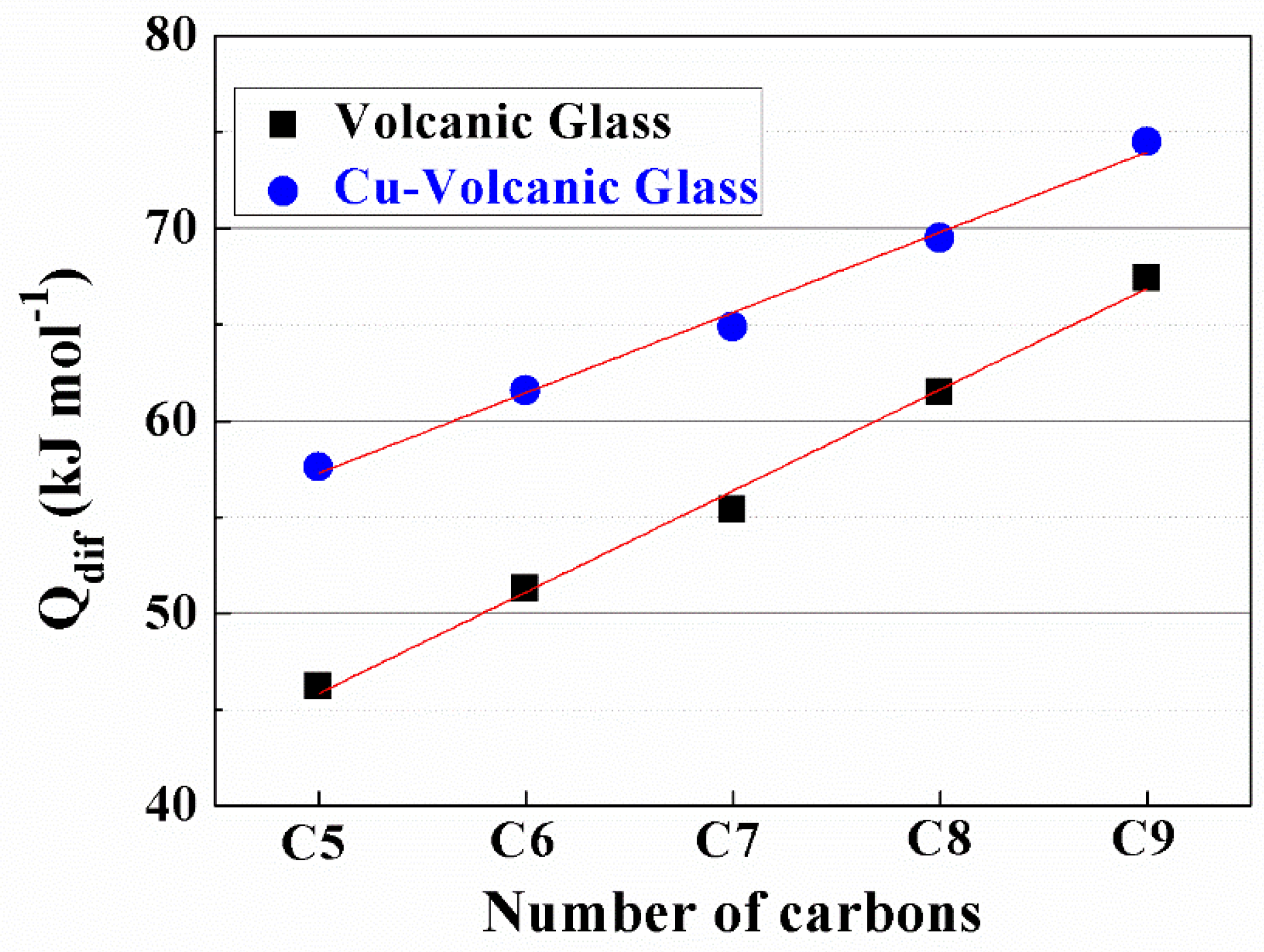
| Sample | N-Paraffin | y = ax + b | r | Qdif | |
|---|---|---|---|---|---|
| a | b | ||||
| Volcanic Glass | C5 | 5560 | −11.23 | 0.995 | 46.2 |
| C6 | 6170 | −11.23 | 0.997 | 51.3 | |
| C7 | 6670 | −11.11 | 0.995 | 55.4 | |
| C8 | 7406 | −13.97 | 0.993 | 61.5 | |
| C9 | 8117 | −13.10 | 0.995 | 67.4 | |
| Cu2+-Volcanic Glass | C5 | 6932 | −16.83 | 0.991 | 57.6 |
| C6 | 7410 | −16.81 | 0.990 | 61.6 | |
| C7 | 7810 | −16.98 | 0.998 | 64.9 | |
| C8 | 8669 | −17.93 | 0.998 | 69.5 | |
| C9 | 8969 | −17.41 | 0.998 | 74.5 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Autie-Pérez, M.; Infantes-Molina, A.; Cecilia, J.A.; Labadie-Suárez, J.M.; Rodríguez-Castellón, E. Separation of Light Liquid Paraffin C5–C9 with Cuban Volcanic Glass Previously Used in Copper Elimination from Water Solutions. Appl. Sci. 2018, 8, 295. https://doi.org/10.3390/app8020295
Autie-Pérez M, Infantes-Molina A, Cecilia JA, Labadie-Suárez JM, Rodríguez-Castellón E. Separation of Light Liquid Paraffin C5–C9 with Cuban Volcanic Glass Previously Used in Copper Elimination from Water Solutions. Applied Sciences. 2018; 8(2):295. https://doi.org/10.3390/app8020295
Chicago/Turabian StyleAutie-Pérez, Miguel, Antonia Infantes-Molina, Juan Antonio Cecilia, Juan M. Labadie-Suárez, and Enrique Rodríguez-Castellón. 2018. "Separation of Light Liquid Paraffin C5–C9 with Cuban Volcanic Glass Previously Used in Copper Elimination from Water Solutions" Applied Sciences 8, no. 2: 295. https://doi.org/10.3390/app8020295
APA StyleAutie-Pérez, M., Infantes-Molina, A., Cecilia, J. A., Labadie-Suárez, J. M., & Rodríguez-Castellón, E. (2018). Separation of Light Liquid Paraffin C5–C9 with Cuban Volcanic Glass Previously Used in Copper Elimination from Water Solutions. Applied Sciences, 8(2), 295. https://doi.org/10.3390/app8020295







