Controlling Chemical Reactions in Confined Environments: Water Dissociation in MOF-74
Abstract
:1. Introduction
2. Methods
2.1. Sample Preparation and In-Situ Infrared Spectroscopy
2.2. Experimental Measurement Conditions
2.3. Computational Details
3. Results and Discussion
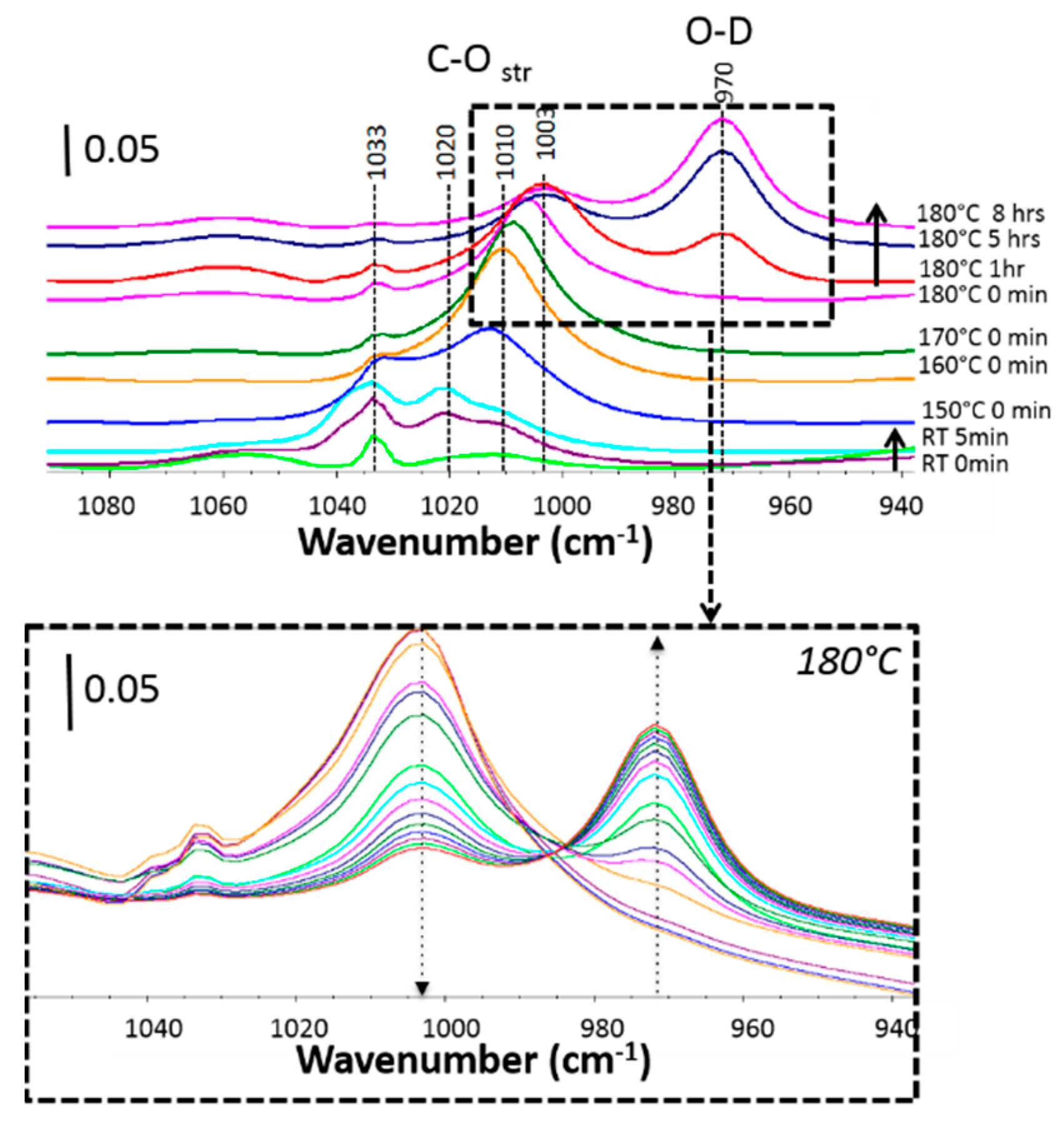
3.1. Experimental Quantification of the Water Dissociation Reaction Rate
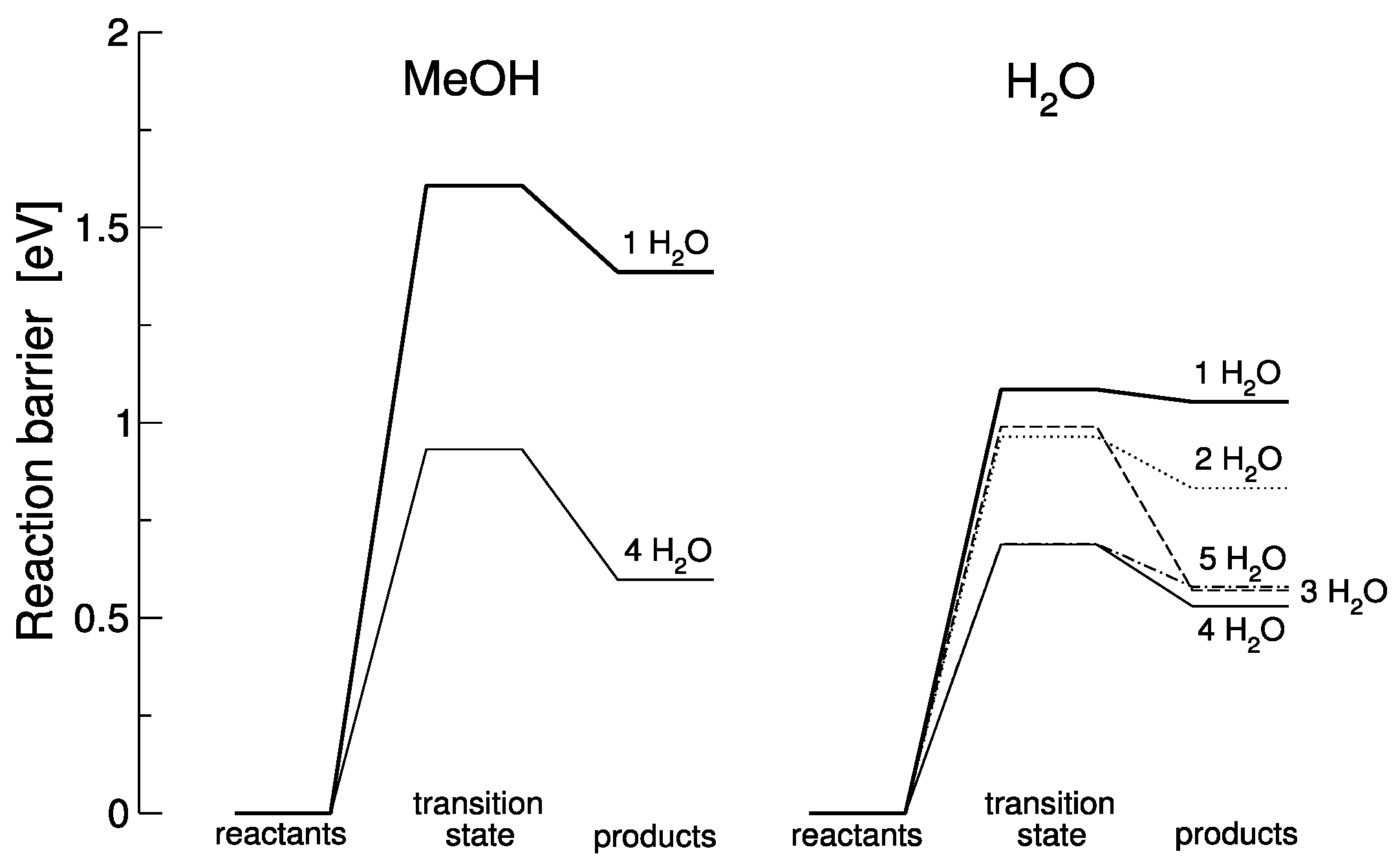
3.2. Theoretical Analysis of the Water Dissociation Reaction Rate
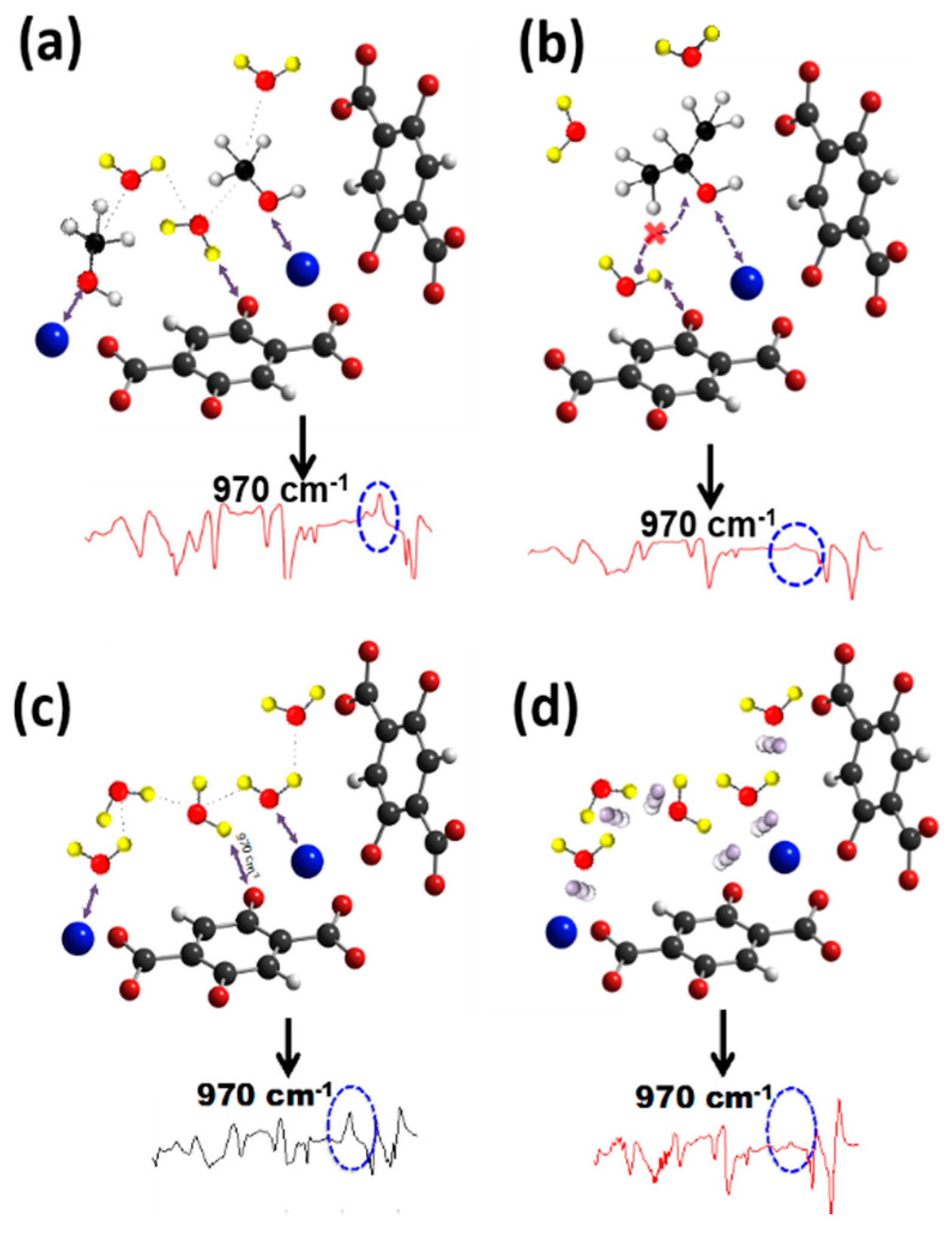
3.3. Stabilization Effect of Additional Water on the Water Dissociation Reaction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Henderson, M.A. The interaction of water with solid surfaces: Fundamental aspects revisited. Surf. Sci. Rep. 2002, 46, 1–308. [Google Scholar] [CrossRef]
- Tan, K.; Nijem, N.; Gao, Y.; Zuluaga, S.; Li, J.; Thonhauser, T.; Chabal, Y.J. Water interactions in metal organic frameworks. CrystEngComm 2015, 17, 247–260. [Google Scholar] [CrossRef]
- Tan, K.; Zuluaga, S.; Gong, Q.; Canepa, P.; Wang, H.; Li, J.; Chabal, Y.J.; Thonhauser, T. Water Reaction Mechanism in Metal Organic Frameworks with Coordinatively Unsaturated Metal Ions: MOF-74. Chem. Mater. 2014, 26, 6886–6895. [Google Scholar] [CrossRef]
- Ward, M.D. Confined systems: The bright side of MOFs. Nat. Chem. 2010, 2, 610–611. [Google Scholar] [CrossRef] [PubMed]
- Hass, K.C.; Schneider, W.F.; Curioni, A.; Andreoni, W. The Chemistry of Water on Alumina Surfaces: Reaction Dynamics from First Principles. Science 1998, 282, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.E., Jr. How Minerals React with Water. Science 2001, 294, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Bikondoa, O.; Pang, C.L.; Ithnin, R.; Muryn, C.A.; Onishi, H.; Thornton, G. Imaging water dissociation on TiO2(110). Nat. Mater. 2006, 5, 189–192. [Google Scholar] [CrossRef]
- Song, Z.; Fan, J.; Xu, H. Strain-induced water dissociation on supported ultrathin oxide films. Sci. Rep. 2016, 6, 22853–22858. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Goniakowski, J.; Suzanne, J. Partial Dissociation of Water Molecules in the (3 × 2) Water Monolayer Deposited on the MgO (100) Surface. Phys. Rev. Lett. 1998, 81, 1271–1273. [Google Scholar] [CrossRef]
- Odelius, M. Mixed Molecular and Dissociative Water Adsorption on MgO[100]. Phys. Rev. Lett. 1999, 82, 3919–3922. [Google Scholar] [CrossRef]
- Carrasco, J.; Hodgson, A.; Michaelides, A. A molecular perspective of water at metal interfaces. Nat. Mater. 2012, 11, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Goodman, D.W. High-pressure catalytic reactions over single-crystal metal surfaces. Surf. Sci. Rep. 1991, 14, 1–107. [Google Scholar] [CrossRef]
- Tan, K.; Canepa, P.; Gong, Q.; Liu, J.; Johnson, D.H.; Dyevoich, A.; Thallapally, P.K.; Thonhauser, T.; Li, J.; Chabal, Y.J. Mechanism of Preferential Adsorption of SO2 into Two Microporous Paddle Wheel Frameworks M(bdc)(ted)0.5. Chem. Mater. 2013, 25, 4653–4662. [Google Scholar] [CrossRef]
- Zuluaga, S.; Fuentes-Fernandez, E.M.; Tan, K.; Li, J.; Chabal, Y.J.; Thonhauser, T. Cluster assisted water dissociation mechanism in MOF-74 and controlling it using helium. J. Mater. Chem. A 2016, 4, 11524–11530. [Google Scholar] [CrossRef]
- Canepa, P.; Nijem, N.; Chabal, Y.J.; Thonhauser, T. Diffusion of Small Molecules in Metal Organic Framework Materials. Phys. Rev. Lett. 2013, 110, 026102. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen Storage in Metal–Organic Frameworks. Chem. Rev. 2011, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2011, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Gong, Q.H.; Olson, D.H.; Li, J. Commensurate Adsorption of Hydrocarbons and Alcohols in Microporous Metal Organic Frameworks. Chem. Rev. 2012, 112, 836–868. [Google Scholar] [CrossRef] [PubMed]
- Kizzie, A.C.; Wong-Foy, A.G.; Matzger, A.J. Effect of Humidity on the Performance of Microporous Coordination Polymers as Adsorbents for CO2 Capture. Langmuir 2011, 27, 6368–6373. [Google Scholar] [CrossRef] [PubMed]
- Dietzel, P.D.C.; Johnsen, R.E.; Fjellvag, H.; Bordiga, S.; Groppo, E.; Chavan, S.; Blom, R. Adsorption properties and structure of CO2 adsorbed on open coordination sites of metal-organic framework Ni2(dhtp) from gas adsorption, IR spectroscopy and X-ray diffraction. Chem. Commun. 2008, 0, 5125–5127. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, W.; Yildirim, T. High-Capacity Methane Storage in Metal Organic Frameworks M2(dhtp): The Important Role of Open Metal Sites. J. Am. Chem. Soc. 2009, 131, 4995–5000. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kabbour, H.; Brown, C.M.; Neumann, D.A.; Ahn, C.C. Increasing the Density of Adsorbed Hydrogen with Coordinatively Unsaturated Metal Centers in Metal Organic Frameworks. Langmuir 2008, 24, 4772–4777. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.D.; Hudson, M.R.; Mason, J.A.; Chavan, S.; Crocellà, V.; Howe, J.D.; Lee, K.; Dzubak, A.L.; Queen, W.L.; Zadrozny, J.M. Reversible CO Binding Enables Tunable CO/H2 and CO/N2 Separations in Metal–Organic Frameworks with Exposed Divalent Metal Cations. J. Am. Chem. Soc. 2014, 136, 10752–10761. [Google Scholar] [CrossRef] [PubMed]
- Bonino, F.; Chavan, S.; Vitillo, J.G.; Groppo, E.; Agostini, G.; Lamberti, C.; Dietzel, P.D.; Prestipino, C.; Bordiga, S. Local Structure of CPO-27-Ni Metallorganic Framework upon Dehydration and Coordination of NO. Chem. Mater. 2008, 20, 4957–4968. [Google Scholar] [CrossRef]
- Canepa, P.; Arter, C.A.; Conwill, E.M.; Johnson, D.H.; Shoemaker, B.A.; Soliman, K.Z.; Thonhauser, T. High-throughput screening of small-molecule adsorption in MOF. J. Mater. Chem. A 2013, 1, 13597–13604. [Google Scholar] [CrossRef]
- Tan, K.; Zuluaga, S.; Fuentes, E.; Mattson, E.C.; Veyan, J.-F.; Wang, H.; Li, J.; Thonhauser, T.; Chabal, Y.J. Trapping gases in metal organic frameworks with a selective surface molecular barrier layer. Nat. Commun. 2016, 7, 13871–13879. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Zuluaga, S.; Gong, Q.; Gao, Y.; Nijem, N.; Li, J.; Thonhauser, T.; Chabal, Y.J. Competitive Coadsorption of CO2 with H2O, NH3, SO2, NO, NO2, N2, O2, and CH4 in M-MOF-74 (M = Mg, Co, Ni): The Role of Hydrogen Bonding. Chem. Mater. 2015, 27, 2203–2217. [Google Scholar] [CrossRef]
- Zuluaga, S.; Fuentes-Fernandez, E.M.A.; Tan, K.; Arter, C.A.; Li, J.; Chabal, Y.J.; Thonhauser, T. Chemistry in confined spaces: Reactivity of the Zn-MOF-74 channels. J. Mater. Chem. A 2016, 4, 13176–13182. [Google Scholar] [CrossRef]
- Zuluaga, S.; Fuentes-Fernandez, E.M.A.; Tan, K.; Xu, F.; Li, J.; Chabal, Y.J.; Thonhauser, T. Understanding and controlling water stability of MOF-74. J. Mater. Chem. A 2016, 4, 5176–5183. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 1169–1186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Berland, K.; Cooper, V.R.; Lee, K.; Schröder, E.; Thonhauser, T.; Hyldgaard, P.; Lundqvist, B.I. van der Waals forces in density functional theory: A review of the vdW-DF method. Rep. Prog. Phys. 2015, 78, 066501. [Google Scholar] [CrossRef] [PubMed]
- Langreth, D.; Lundqvist, B.I.; Chakarova-Käck, S.D.; Cooper, V.; Dion, M.; Hyldgaard, P.; Kelkkanen, A.; Kleis, J.; Kong, L.; Li, S. A density functional for sparse matter. J. Phys. Condens. Matter 2009, 21, 084203. [Google Scholar] [CrossRef] [PubMed]
- Thonhauser, T.; Cooper, V.R.; Li, S.; Puzder, A.; Hyldgaard, P.; Langreth, D.C. Van der Waals density functional: Self-consistent potential and the nature of the van der Waals bond. Phys. Rev. B 2007, 76, 125112. [Google Scholar] [CrossRef]
- Thonhauser, T.; Zuluaga, S.; Arter, C.; Berland, K.; Schröder, E.; Hyldgaard, P. Spin Signature of Nonlocal Correlation Binding in Metal-Organic Frameworks. Phys. Rev. B 2015, 115, 136402. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Uberuaga, B.P.; Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Falk, M.; Whalley, E. Infrared Spectra of Methanol and Deuterated Methanols in Gas, Liquid, and Solid Phases. J. Chem. Phys. 1961, 34, 1554–1568. [Google Scholar] [CrossRef]
- Canepa, P.; Hanson, R.M.; Ugliengo, P.; Alfredsson, M. J-ICE: A new Jmol interface for handling and visualizing crystallographic and electronic properties. J. Appl. Crystallogr. 2011, 44, 225–229. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Xu, D.; Chung, J.D.; Kaviany, M.; Huang, B. H2O Adsorption/Desorption in MOF-74: Ab Initio Molecular Dynamics and Experiments. J. Phys. Chem. C 2015, 119, 13021–13031. [Google Scholar] [CrossRef]
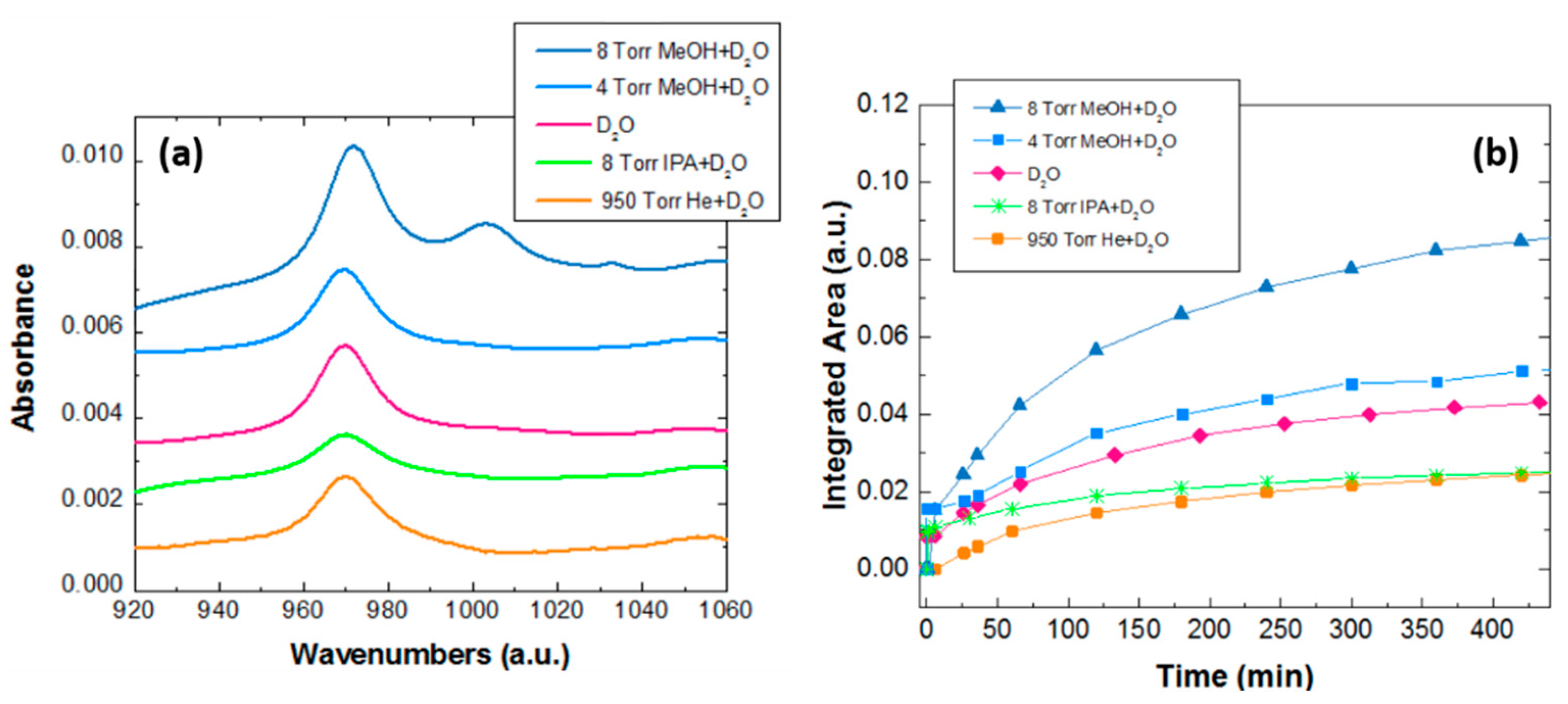
| D2O + Guest Molecules | Percentage of D2O Dissociated after 8 h (%) |
|---|---|
| 8 Torr D2O | 100 |
| 4 Torr MeOH + 8 Torr D2O | 122 |
| 8Torr MeOH + 8 Torr D2O | 195 |
| 8 Torr IPA + 8 Torr D2O | 61 |
| 950 Torr He + 8 Torr D2O | 61 |
| Binding Energies of Reactant and Products Inside Zn-MOF-74 | ||
|---|---|---|
| Functional | Zn Metal Center Eb (eV) | Organic Linker Eb (eV) |
| MeOH | −0.780 | DNB |
| H2O | −0.612 | −0.610 |
| OH | −2.442 | DNB |
| H | −2.002 | −1.932 |
| Water Systems | No. H2O | Initial State (eV) | Transition State (eV) | Final State (eV) | Forward Barrier (eV) | Backward Barrier (eV) |
|---|---|---|---|---|---|---|
| MeOH + H2O | 1 | 0 | 1.607 | 1.386 | 1.607 | 0.220 |
| 4 | 0 | 0.932 | 0.598 | 0.932 | 0.333 | |
| H2O | 1 | 0 | 1.085 | 1.054 | 1.085 | 0.031 |
| 2 | 0 | 0.964 | 0.832 | 0.964 | 0.132 | |
| 3 | 0 | 0.990 | 0.571 | 0.990 | 0.418 | |
| 4 | 0 | 0.688 | 0.530 | 0.688 | 0.158 | |
| 5 | 0 | 0.690 | 0.580 | 0.690 | 0.110 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes-Fernandez, E.M.A.; Jensen, S.; Tan, K.; Zuluaga, S.; Wang, H.; Li, J.; Thonhauser, T.; Chabal, Y.J. Controlling Chemical Reactions in Confined Environments: Water Dissociation in MOF-74. Appl. Sci. 2018, 8, 270. https://doi.org/10.3390/app8020270
Fuentes-Fernandez EMA, Jensen S, Tan K, Zuluaga S, Wang H, Li J, Thonhauser T, Chabal YJ. Controlling Chemical Reactions in Confined Environments: Water Dissociation in MOF-74. Applied Sciences. 2018; 8(2):270. https://doi.org/10.3390/app8020270
Chicago/Turabian StyleFuentes-Fernandez, Erika M. A., Stephanie Jensen, Kui Tan, Sebastian Zuluaga, Hao Wang, Jing Li, Timo Thonhauser, and Yves J. Chabal. 2018. "Controlling Chemical Reactions in Confined Environments: Water Dissociation in MOF-74" Applied Sciences 8, no. 2: 270. https://doi.org/10.3390/app8020270
APA StyleFuentes-Fernandez, E. M. A., Jensen, S., Tan, K., Zuluaga, S., Wang, H., Li, J., Thonhauser, T., & Chabal, Y. J. (2018). Controlling Chemical Reactions in Confined Environments: Water Dissociation in MOF-74. Applied Sciences, 8(2), 270. https://doi.org/10.3390/app8020270




