Hydrogen Production from Methanol Steam Reforming over TiO2 and CeO2 Pillared Clay Supported Au Catalysts
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Catalytic Activity Measurements
3. Results and Discussion
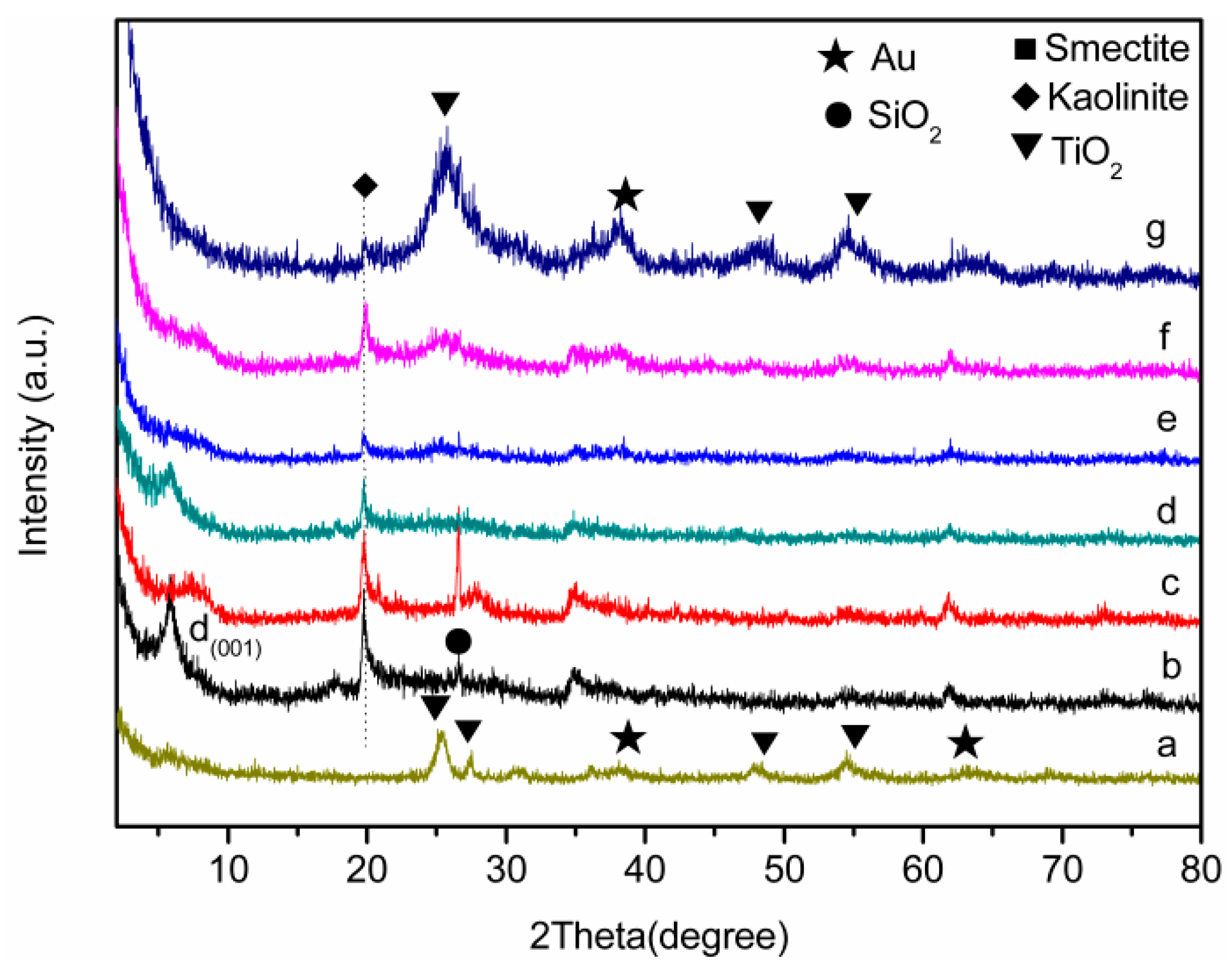
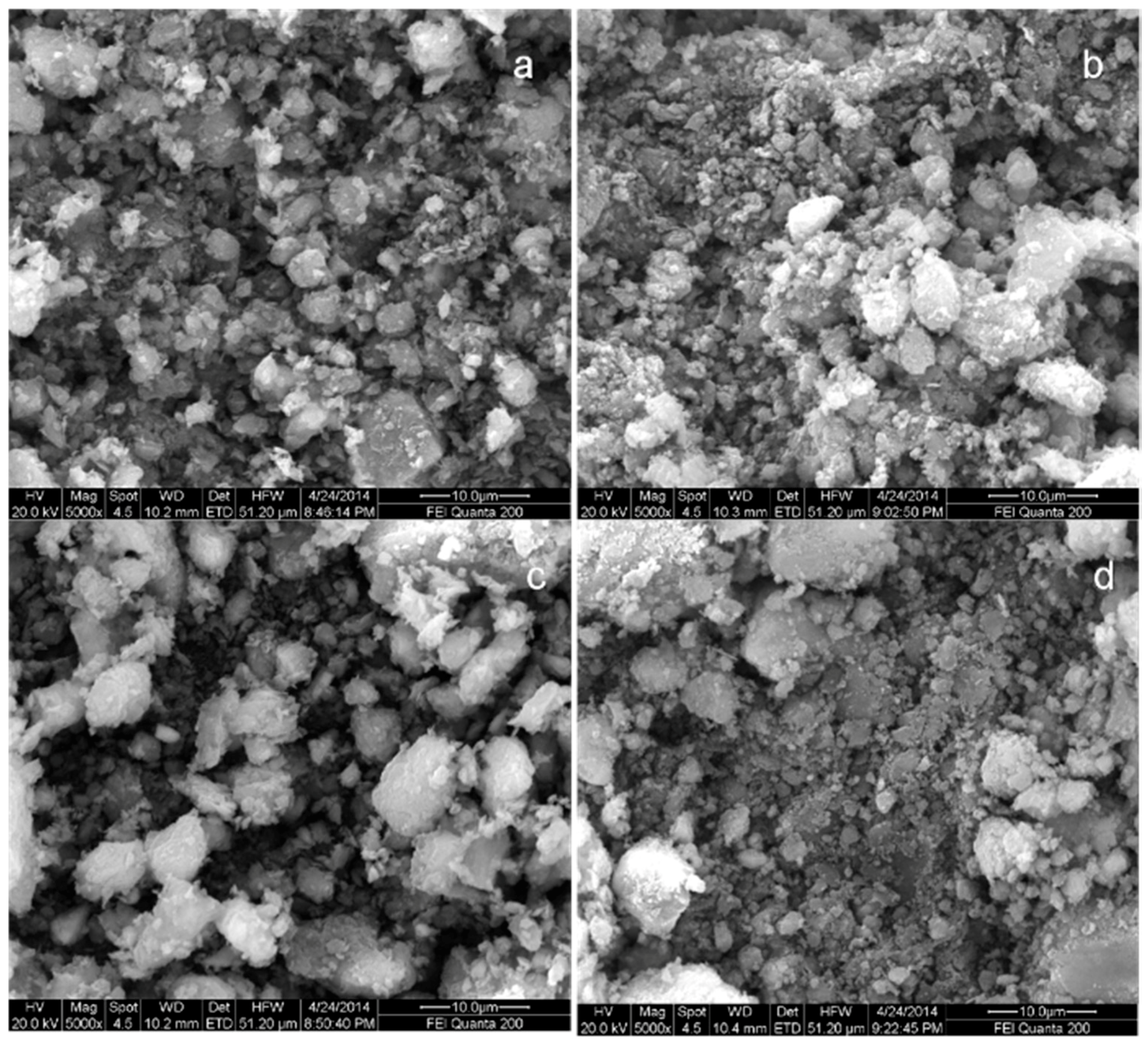
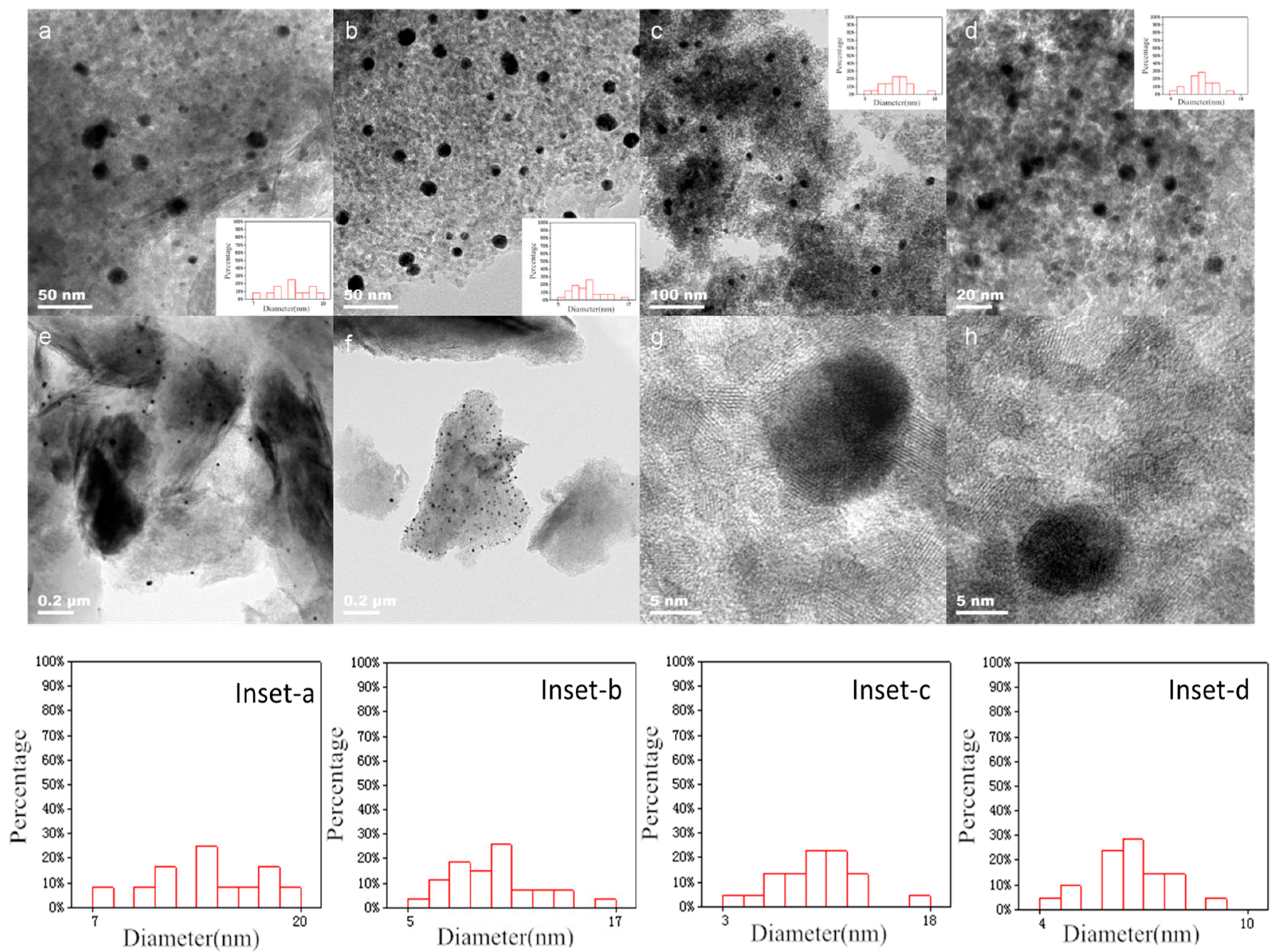
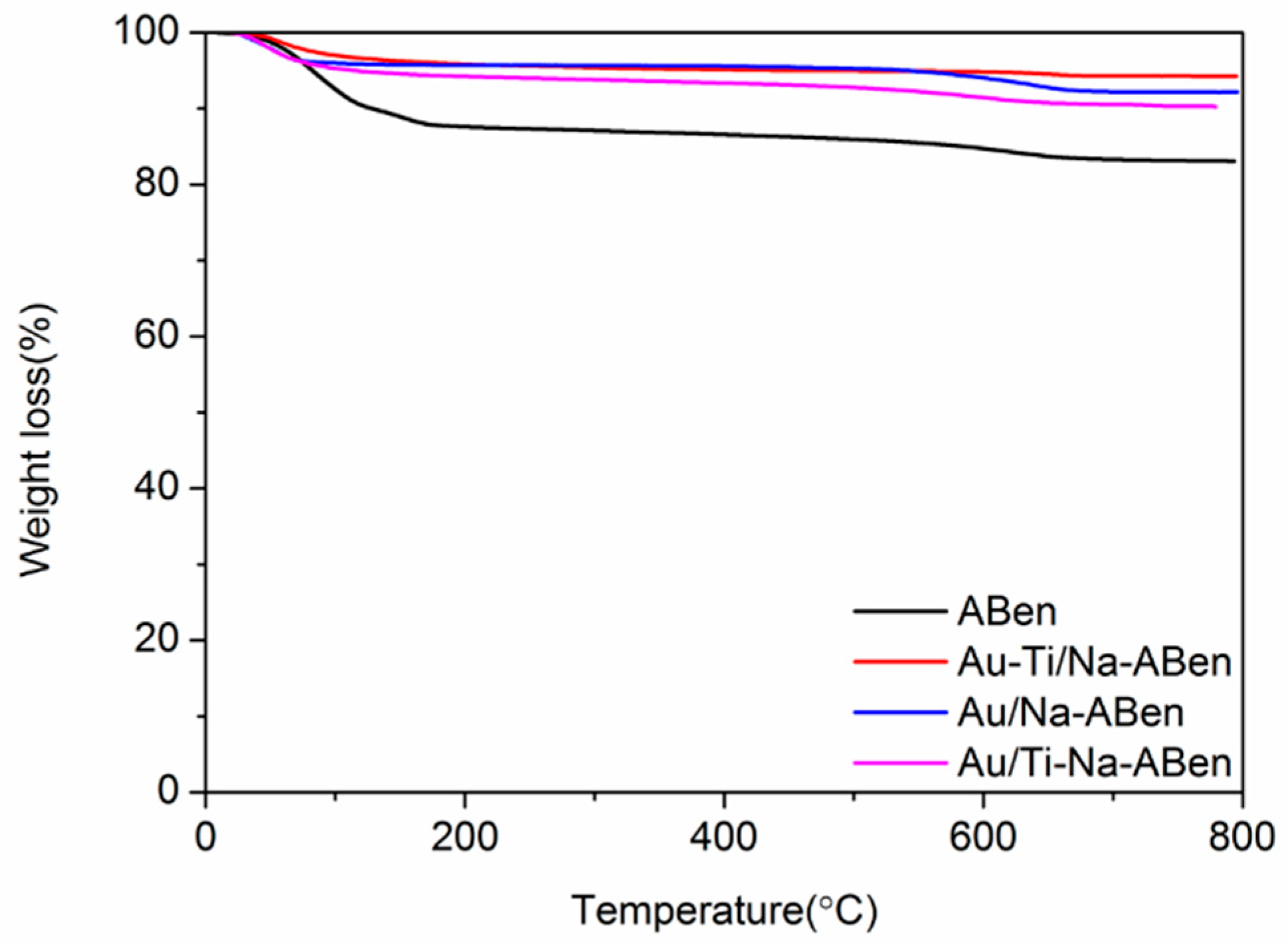
3.1. Characterization
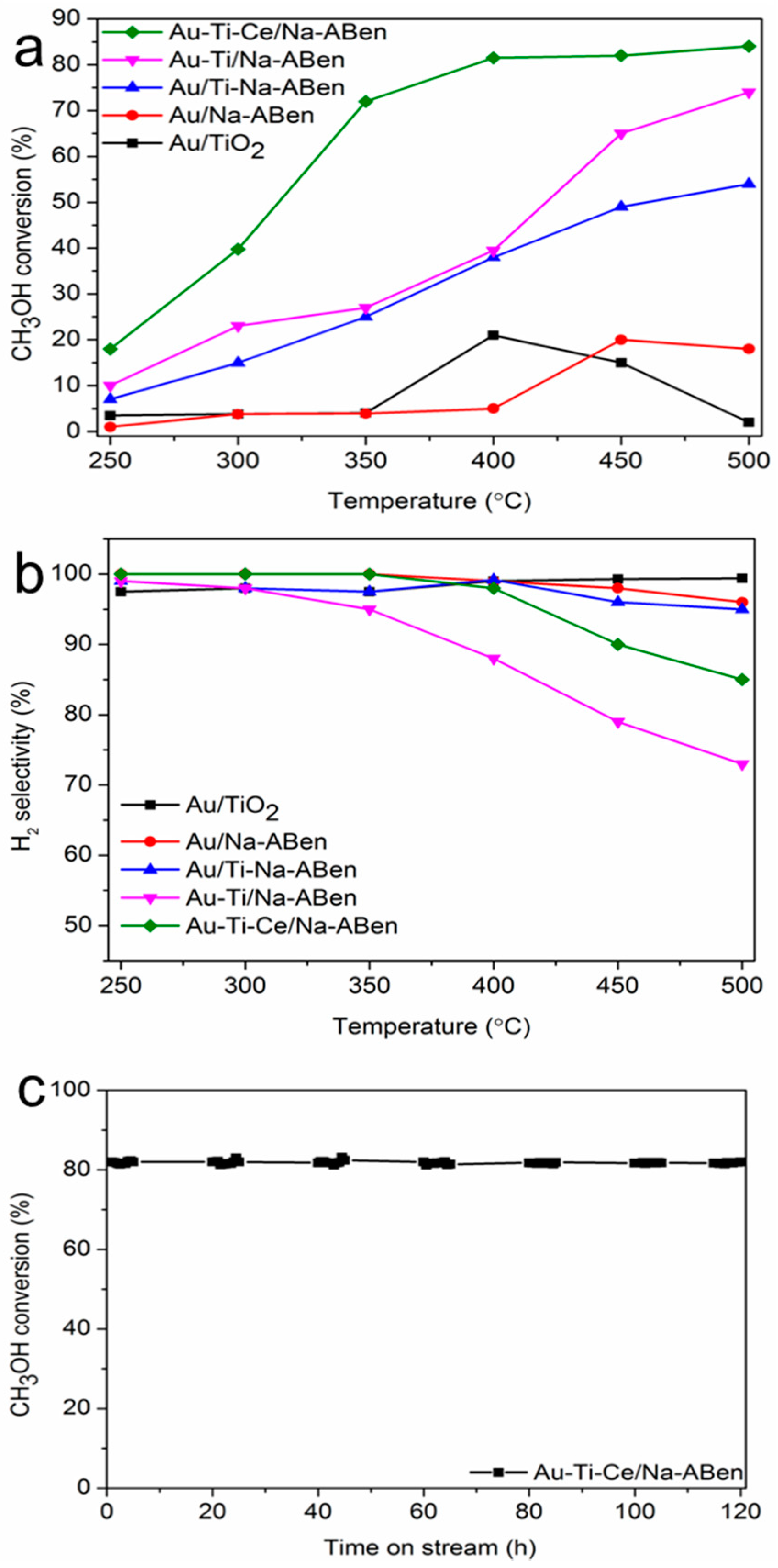
3.2. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Momirlan, M.; Veziroglu, T.N. Current status of hydrogen energy. Renew. Sustain. Energy Rev. 2002, 6, 141–179. [Google Scholar] [CrossRef]
- El-Shahat, A. Hydrogen Fuel Cells Progressing as Power Source. Nat. Gas Electr. 2016, 33, 7–11. [Google Scholar] [CrossRef]
- Bockris, J.O. Hydrogen. Materials 2011, 4, 2073–2091. [Google Scholar] [CrossRef] [PubMed]
- Momirlan, M.; Veziroglu, T.N. The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet. Int. J. Hydrog. Energy 2005, 30, 795–802. [Google Scholar] [CrossRef]
- Schlapbach, L.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F.; Brandon, N.P. Hydrogen and fuel cells: Towards a sustainable energy future. Energy Policy 2008, 36, 4356–4362. [Google Scholar] [CrossRef]
- Liu, N.; Yuan, Z.; Wang, S.; Zhang, C.; Wang, S.; Li, D. Characterization and performance of a ZnO–ZnCr2O4/CeO2–ZrO2 monolithic catalyst for methanol auto-thermal reforming process. Int. J. Hydrog. Energy 2008, 33, 1643–1651. [Google Scholar] [CrossRef]
- Hamelinck, C.N.; Faaij, A.P.C. Future prospects for production of methanol and hydrogen from biomass. J. Power Sources 2002, 111, 1–22. [Google Scholar] [CrossRef]
- Agrell, J.; Birgersson, H.; Boutonnet, M.; Melián-Cabrera, I.; Navarro, R.M.; Fierro, J.L.G. Production of hydrogen from methanol over Cu/ZnO catalysts promoted by ZrO2 and Al2O3. J. Catal. 2003, 219, 389–403. [Google Scholar] [CrossRef]
- Hong, X.; Ren, S. Selective hydrogen production from methanol oxidative steam reforming over Zn–Cr catalysts with or without Cu loading. Int. J. Hydrog. Energy 2008, 33, 700–708. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. B 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Lwin, Y.; Daud, W.R.W.; Mohamad, A.B.; Yaakob, Z. Hydrogen production from steam–methanol reforming: Thermodynamic analysis. Int. J. Hydrog. Energy 2000, 25, 47–53. [Google Scholar] [CrossRef]
- Patel, S.; Pant, K.K. Activity and stability enhancement of copper–alumina catalysts using cerium and zinc promoters for the selective production of hydrogen via steam reforming of methanol. J. Power Sources 2006, 159, 139–143. [Google Scholar] [CrossRef]
- Onsuratoom, S.; Puangpetch, T.; Chavadej, S. Comparative investigation of hydrogen production over Ag-, Ni-, and Cu-loaded mesoporous-assembled TiO2–ZrO2 mixed oxide nanocrystal photocatalysts. Chem. Eng. J. 2011, 173, 667–675. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, C.; Chen, W. Hydrogen production from steam reforming of ethanol over Ni/MgO-CeO2 catalyst at low temperature. J. Rare Earths 2009, 27, 948–954. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Li, M.; Liu, Y.; Bai, X. Co/CeO2 for ethanol steam reforming: Effect of ceria morphology. J. Rare Earths 2013, 31, 565–571. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, H.; Wei, Y.; Li, K.; Cheng, X. Hydrogen and syngas production from two-step steam reforming of methane over CeO2-Fe2O3 oxygen carrier. J. Rare Earths 2010, 28, 907–913. [Google Scholar] [CrossRef]
- Xu, X.; Shuai, K.; Xu, B. Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen. Catalysts 2017, 7, 183. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Production of hydrogen via combined steam reforming of methanol over CuO–CeO2 catalysts. Catal. Commun. 2004, 5, 231–235. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kang, M. Hydrogen production from methanol/water decomposition in a liquid photosystem using the anatase structure of Cu loaded. Int. J. Hydrog. Energy 2007, 32, 3841–3848. [Google Scholar] [CrossRef]
- Yao, C.-Z.; Wang, L.-C.; Liu, Y.-M.; Wu, G.-S.; Cao, Y.; Dai, W.-L.; He, H.-Y.; Fan, K.-N. Effect of preparation method on the hydrogen production from methanol steam reforming over binary Cu/ZrO2 catalysts. Appl. Catal. A 2006, 297, 151–158. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, K.I.; Kim, T.H.; Ko, C.H.; Park, H.C.; Song, I.K. Hydrogen production by steam reforming of methanol in a micro-channel reactor coated with Cu/ZnO/ZrO2/Al2O3 catalyst. J. Power Sources 2006, 159, 1296–1299. [Google Scholar] [CrossRef]
- Fornari, A.C.; Menechini Neto, R.; Lenzi, G.G.; dos Santos, O.A.A.; de Matos Jorge, L.M. Utilization of sol-gel CuO-ZnO-Al2O3 catalysts in the methanol steam reforming for hydrogen production. Can. J. Chem. Eng. 2017, 95, 2258–2271. [Google Scholar] [CrossRef]
- Patel, N.; Kale, A.; Miotello, A. Improved dehydrogenation of ammonia borane over Co-P-B coating on Ni: A single catalyst for both hydrolysis and thermolysis. Appl. Catal. B 2012, 111–112, 178–184. [Google Scholar] [CrossRef]
- Suwa, Y.; Ito, S.-I.; Kameoka, S.; Tomishige, K.; Kunimori, K. Comparative study between Zn–Pd/C and Pd/ZnO catalysts for steam reforming of methanol. Appl. Catal. A 2004, 267, 9–16. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Bunnak, N.; Manuspiya, H. A comprehensive review on modified clay based composite for energy based materials. Renew. Sustain. Energy Rev. 2016, 61, 466–472. [Google Scholar] [CrossRef]
- Figueras, F. Pillared Clays as Catalysts. Catal. Rev. 1988, 30, 457–499. [Google Scholar] [CrossRef]
- Karakaya, M.Ç.; Karakaya, N.; Bakır, S. Some properties and potential applications of the Na- and Ca-bentonites of ordu (N.E. Turkey). Appl. Clay Sci. 2011, 54, 159–165. [Google Scholar] [CrossRef]
- Ding, Z.; Kloprogge, J.T.; Frost, R.L.; Lu, G.Q.; Zhu, H.Y. Porous Clays and Pillared Clays-Based Catalysts. Part 2: A Review of the Catalytic and Molecular Sieve Applications. J. Porous Mater. 2001, 8, 273–293. [Google Scholar] [CrossRef]
- Cool, P.; Vansant, E.F. Pillared Clays: Preparation, Characterization and Applications. In Synthesis; Springer: Berlin/Heidelberg, Germany, 1998; pp. 265–288. [Google Scholar]
- Kloprogge, J.T. Synthesis of Smectites and Porous Pillared Clay Catalysts: A Review. J. Porous Mater. 1998, 5, 5–41. [Google Scholar] [CrossRef]
- Chmielarz, L.; Piwowarska, Z.; Kuśtrowski, P.; Gil, B.; Adamski, A.; Dudek, B.; Michalik, M. Porous clay heterostructures (PCHs) intercalated with silica-titania pillars and modified with transition metals as catalysts for the DeNOx process. Appl. Catal. B 2009, 91, 449–459. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Cui, H.; Liu, B.; Gao, X.; Wang, D.; Liang, P. La(III)-loaded bentonite/chitosan beads for defluoridation from aqueous solution. J. Rare Earths 2014, 32, 458–466. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Wang, L.; Zhang, L.; Xu, Y. Effects of chitosan-RE3+-bentonite on growth of Chlorella vulgaris. J. Rare Earths 2010, 28, 149–153. [Google Scholar] [CrossRef]
- Chmielarz, L.; Gil, B.; Kuśtrowski, P.; Piwowarska, Z.; Dudek, B.; Michalik, M. Montmorillonite-based porous clay heterostructures (PCHs) intercalated with silica–titania pillars–synthesis and characterization. J. Solid State Chem. 2009, 182, 1094–1104. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, X.; Yang, C.; Niu, C.; Wang, J.; Su, X. Nano γ-Fe2O3/bentonite magnetic composites: Synthesis, characterization and application as adsorbents. J. Alloys Compd. 2016, 688, 1019–1027. [Google Scholar] [CrossRef]
- Vicente, M.A.; Belver, C.; Trujillano, R.; Rives, V.; Álvarez, A.C.; Lambert, J.F.; Korili, S.A.; Gandı́a, L.M.; Gil, A. Preparation and characterisation of Mn- and Co-supported catalysts derived from Al-pillared clays and Mn- and Co-complexes. Appl. Catal. A 2004, 267, 47–58. [Google Scholar] [CrossRef]
- Castillo, H.L.D.; Grange, P. Preparation and catalytic activity of titanium pillared montmorillonite. Appl. Catal. A 1993, 103, 23–34. [Google Scholar] [CrossRef]
- Nafees, M.; Waseem, A. Organoclays as Sorbent Material for Phenolic Compounds: A Review. Clean Soil Air Water 2014, 42, 1500–1508. [Google Scholar] [CrossRef]
- Yang, H.-C.; Chang, F.-W.; Roselin, L.S. Hydrogen production by partial oxidation of methanol over Au/CuO/ZnO catalysts. J. Mol. Catal. A Chem. 2007, 276, 184–190. [Google Scholar] [CrossRef]
- Barrer, R.M.; MacLeod, D.M. Activation of montmorillonite by ion exchange and sorption complexes of tetra-alkyl ammonium montmorillonites. Trans. Faraday Soc. 1955, 51, 1290–1300. [Google Scholar] [CrossRef]
- Tadros, T.F.; Martini, G.; Bahranowski, K.; Serwicka, E.M. Magnetic Resonance in Colloid and Interface ScienceESR study of vanadium-doped alumina- and titania-pillared montmorillonites. Colloids Surf. A Physicochem. Eng. Asp. 1993, 72, 153–160. [Google Scholar]
- Phukan, A.; Ganguli, J.N.; Dutta, D.K. ZnCl2-Zn2+-Montmorillonite composite: Efficient solid acid catalyst for benzylation of benzene. J. Mol. Catal. A Chem. 2003, 202, 279–287. [Google Scholar] [CrossRef]
- Feng, J.; Wong, R.S.K.; Hu, X.; Yue, P.L. Discoloration and mineralization of Orange II by using Fe3+-doped TiO2 and bentonite clay-based Fe nanocatalysts. Catal. Today 2004, 98, 441–446. [Google Scholar] [CrossRef]
- Zuo, S.; Zhou, R. Al-pillared clays supported rare earths and palladium catalysts for deep oxidation of low concentration of benzene. Appl. Surf. Sci. 2006, 253, 2508–2514. [Google Scholar] [CrossRef]
- Mo, Z.; Zhang, P.; Zuo, D.; Sun, Y.; Chen, H. Synthesis and characterization of polyaniline nanorods/Ce(OH)3–Pr2O3/montmorillonite composites through reverse micelle template. Mater. Res. Bull. 2008, 43, 1664–1669. [Google Scholar] [CrossRef]
- Chen, C.-C.; Kuo, P.-L. Gold nanoparticles prepared using polyethylenimine adsorbed onto montmorillonite. J. Colloid Interface Sci. 2006, 293, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Razakamanantsoa, A.R.; Barast, G.; Djeran-maigre, I. Hydraulic performance of activated calcium bentonite treated by polyionic charged polymer. Appl. Clay Sci. 2012, 59–60, 103–114. [Google Scholar] [CrossRef]
- Khenifi, A.; Zohra, B.; Kahina, B.; Houari, H.; Zoubir, D. Removal of 2,4-DCP from wastewater by CTAB/bentonite using one-step and two-step methods: A comparative study. Chem. Eng. J. 2009, 146, 345–354. [Google Scholar] [CrossRef]
- Tanaka, A.; Hashimoto, K.; Kominami, H. Preparation of Au/CeO2 Exhibiting Strong Surface Plasmon Resonance Effective for Selective or Chemoselective Oxidation of Alcohols to Aldehydes or Ketones in Aqueous Suspensions under Irradiation by Green Light. J. Am. Chem. Soc. 2012, 134, 14526–14533. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, R.; Bellardita, M.; Palmisano, L.; Scirè, S. A comparison between photocatalytic and catalytic oxidation of 2-Propanol over Au/TiO2–CeO2 catalysts. J. Mol. Catal. A Chem. 2016, 415, 56–64. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, K.; Zong, L.; Tong, S.; Wang, X.; Feng, G. Gold supported on ceria nanotubes for CO oxidation. Appl. Surf. Sci. 2017, 416, 183–190. [Google Scholar] [CrossRef]
- Freakley, S.J.; He, Q.; Kiely, C.J.; Hutchings, G.J. Gold Catalysis: A Reflection on Where We are Now. Catal. Lett. 2014, 145, 71–79. [Google Scholar] [CrossRef]
- Fiorenza, R.; Bellardita, M.; D’Urso, L.; Compagnini, G.; Palmisano, L.; Scirè, S. Au/TiO2-CeO2 Catalysts for Photocatalytic Water Splitting and VOCs Oxidation Reactions. Catalysts 2016, 6, 121. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, K.; Zong, L.; Tong, S.; Wang, X.; Zhou, J.; Lu, Z.-H.; Feng, G. Control synthesis of CeO2 nanomaterials supported gold for catalytic oxidation of carbon monoxide. Mol. Catal. 2017, 442, 173–180. [Google Scholar] [CrossRef]
- Scirè, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Drits, V.A.; Eberl, D.D.; Srodon, J. XRD measurement of mean thickness, thickness distribution and strain for illite and illite-smectite crystallites by the Bertaut-Warren-Averbach technique. Clays Clay Miner. 1998, 46, 38–50. [Google Scholar] [CrossRef]
- Go´mez-Sainero, L.M.; Baker, R.T.; Vizcaíno, A.J.; Francis, S.F.; Calles, J.A.; Metcalfe, I.S.; Rodriguez, J.J. Steam Reforming of Methanol with Sm2O3-CeO2-Supported Palladium Catalysts Influence of the Thermal Treatments of Catalyst and Support. Ind. Eng. Chem. Res. 2009, 48, 8364–8372. [Google Scholar] [CrossRef]
- Ou, T.-C.; Chang, F.-W.; Roselin, L.S. Production of hydrogen via partial oxidation of methanol over bimetallic Au–Cu/TiO2 catalysts. J. Mol. Catal. A Chem. 2008, 293, 8–16. [Google Scholar] [CrossRef]
- Booij, E.; Kloprogge, J.T.; Rob van Veen, J.A. Large pore REE/Al pillared bentonites: Preparation, structural aspects and catalytic properties. Appl. Clay Sci. 1996, 11, 155–162. [Google Scholar] [CrossRef]
- Auprêtre, F.; Descorme, C.; Duprez, D. Bio-ethanol catalytic steam reforming over supported metal catalysts. Catal. Commun. 2002, 3, 263–267. [Google Scholar] [CrossRef]
- Xi, H.; Hou, X.; Liu, Y.; Qing, S.; Gao, Z. Cu-Al spinel oxide as an efficient catalyst for methanol steam reforming. Angew. Chem. 2014, 53, 11886–11889. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qing, S.; Hou, X.; Qin, F.; Wang, X.; Gao, Z.; Xiang, H. Temperature dependence of Cu-Al spinel formation and its catalytic performance in methanol steam reforming. Catal. Sci. Technol. 2017, 7, 5069–5078. [Google Scholar] [CrossRef]
| Sample | Au (%) | DAu a (%) | TOFCH3OH b (s−1) | |
|---|---|---|---|---|
| ICP | XPS | |||
| Au/Na-ABen | 0.51 | 0.054 | 8.3 | 0.05 |
| Au/Ti-Na-ABen | 0.54 | 0.116 | 10.0 | 0.32 |
| Au-Ti/Na-ABen | 1.30 | 0.094 | 12.5 | 0.33 |
| Au-Ti-Ce/Na-ABen | 1.34 | 0.086 | 16.7 | 0.41 |
| Sample | 2θ | d (Å) |
|---|---|---|
| Ben | 6.930 | 12.73 |
| Na-ABen | 5.924 | 14.91 |
| Au/Na-ABen | 7.313 | 12.08 |
| Ti-Na-ABen | 5.840 | 15.12 |
| Au/Ti-Na-ABen | 5.783 | 15.27 |
| Au-Ti/Na-ABen | 5.581 | 15.82 |
| Au-Ti-Ce/Na-ABen | 5.410 | 16.30 |
| Sample | Average Pore Diameter (nm) | Pore Volume (cm3/g) | SBET (m2/g) |
|---|---|---|---|
| Au/TiO2 | 7.95 | 0.123 | 62.0 |
| Ben | 6.68 | 0.059 | 35.5 |
| Na-ABen | 4.64 | 0.156 | 134.3 |
| Au/Na-ABen | 5.60 | 0.158 | 112.9 |
| Ti-Na-ABen | 5.48 | 0.289 | 210.6 |
| Au/Ti-Na-ABen | 5.90 | 0.283 | 191.8 |
| Au-Ti/Na-ABen | 9.91 | 0.621 | 250.7 |
| Au-Ti-Ce/Na-ABen | 9.64 | 0.677 | 281.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Huang, C.; Zong, L.; Lu, K.; Wang, X.; Cai, J. Hydrogen Production from Methanol Steam Reforming over TiO2 and CeO2 Pillared Clay Supported Au Catalysts. Appl. Sci. 2018, 8, 176. https://doi.org/10.3390/app8020176
Zhang R, Huang C, Zong L, Lu K, Wang X, Cai J. Hydrogen Production from Methanol Steam Reforming over TiO2 and CeO2 Pillared Clay Supported Au Catalysts. Applied Sciences. 2018; 8(2):176. https://doi.org/10.3390/app8020176
Chicago/Turabian StyleZhang, Rongbin, Chuanqing Huang, Lijuan Zong, Kun Lu, Xuewen Wang, and Jianxin Cai. 2018. "Hydrogen Production from Methanol Steam Reforming over TiO2 and CeO2 Pillared Clay Supported Au Catalysts" Applied Sciences 8, no. 2: 176. https://doi.org/10.3390/app8020176
APA StyleZhang, R., Huang, C., Zong, L., Lu, K., Wang, X., & Cai, J. (2018). Hydrogen Production from Methanol Steam Reforming over TiO2 and CeO2 Pillared Clay Supported Au Catalysts. Applied Sciences, 8(2), 176. https://doi.org/10.3390/app8020176




