Combustion of Hydrogen Enriched Methane and Biogases Containing Hydrogen in a Controlled Auto-Ignition Engine
Abstract
1. Introduction
2. Methods
2.1. CAI Engine
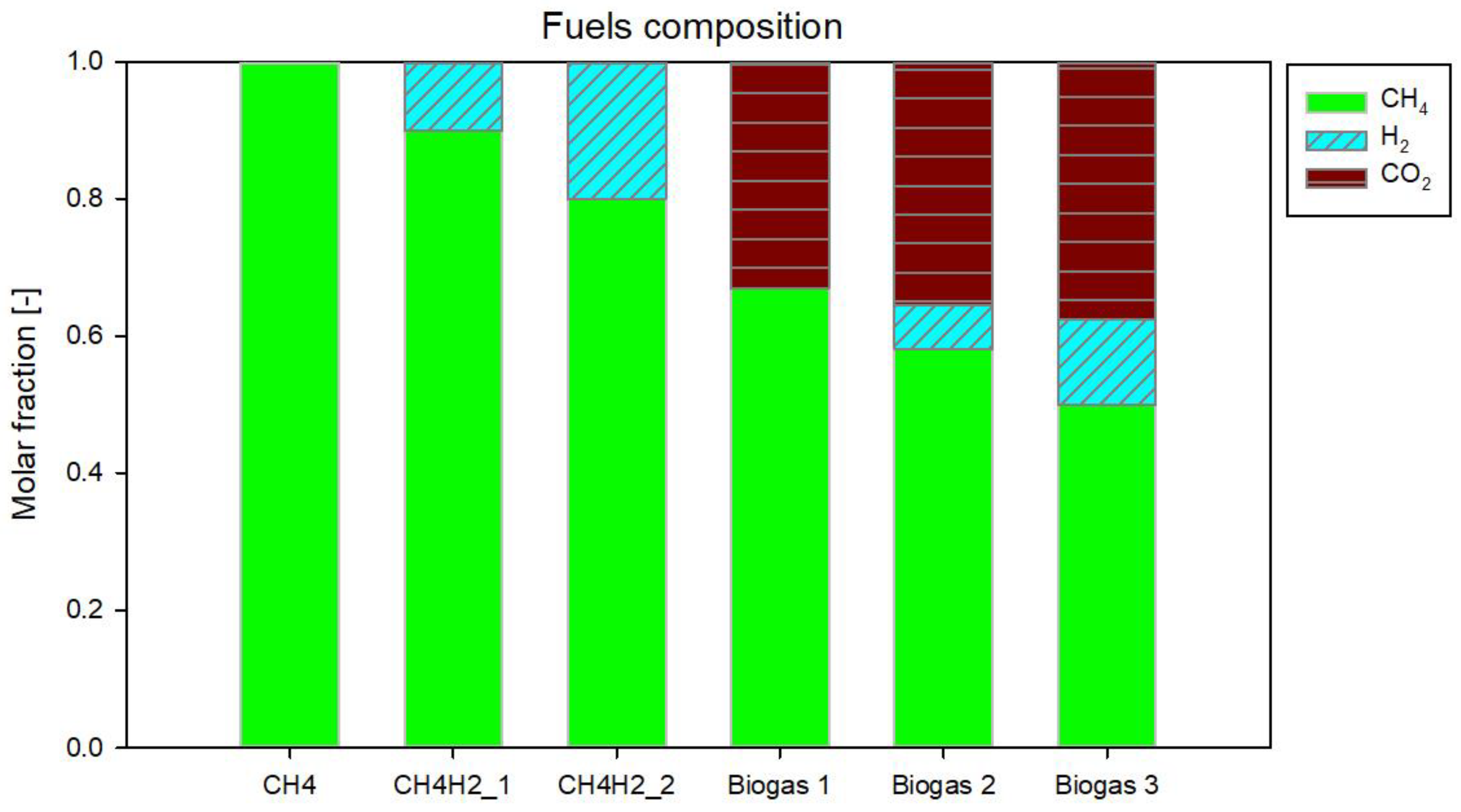
2.2. Fuels
2.3. Engine Operating Parameters
3. Results and Discussion
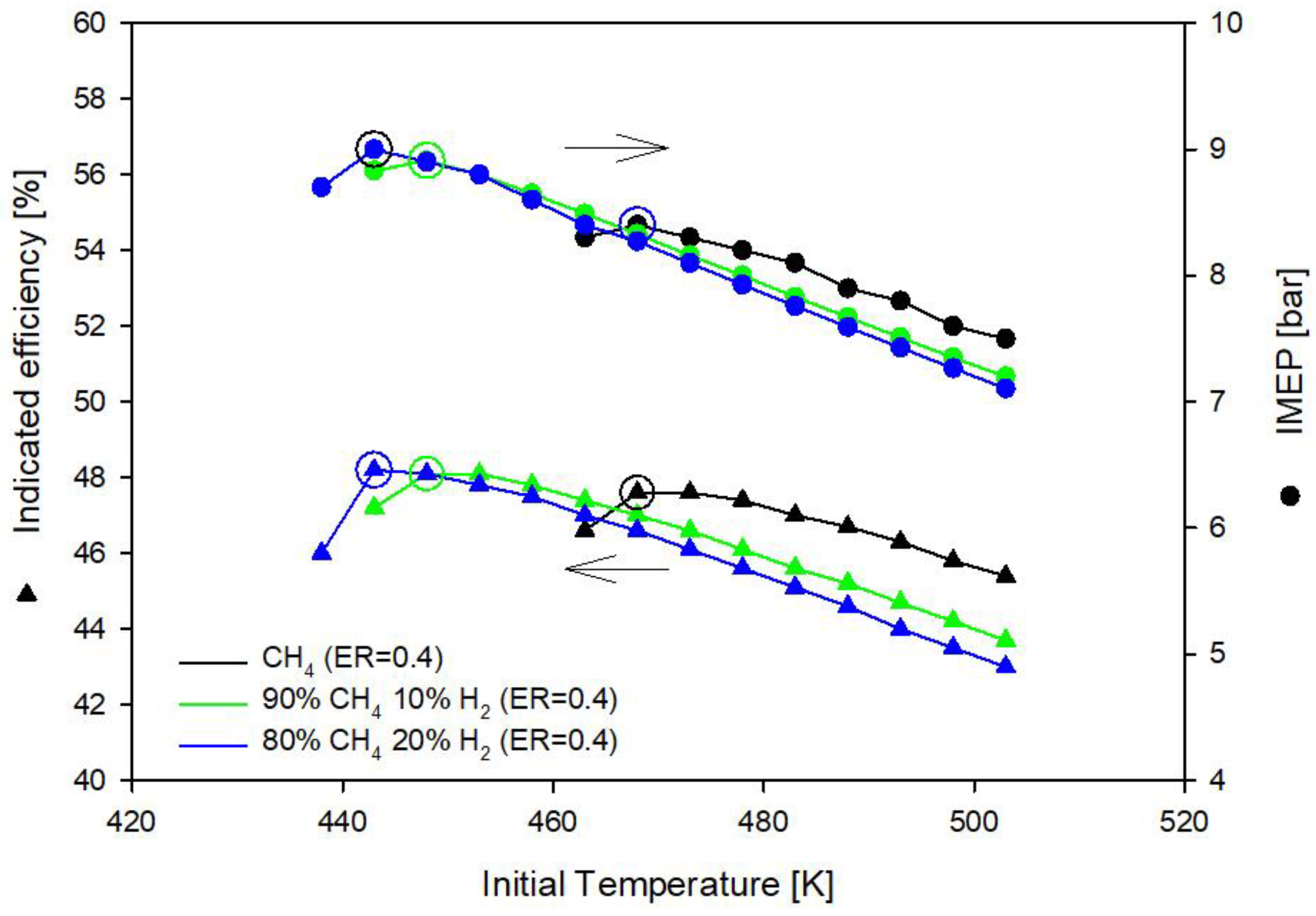
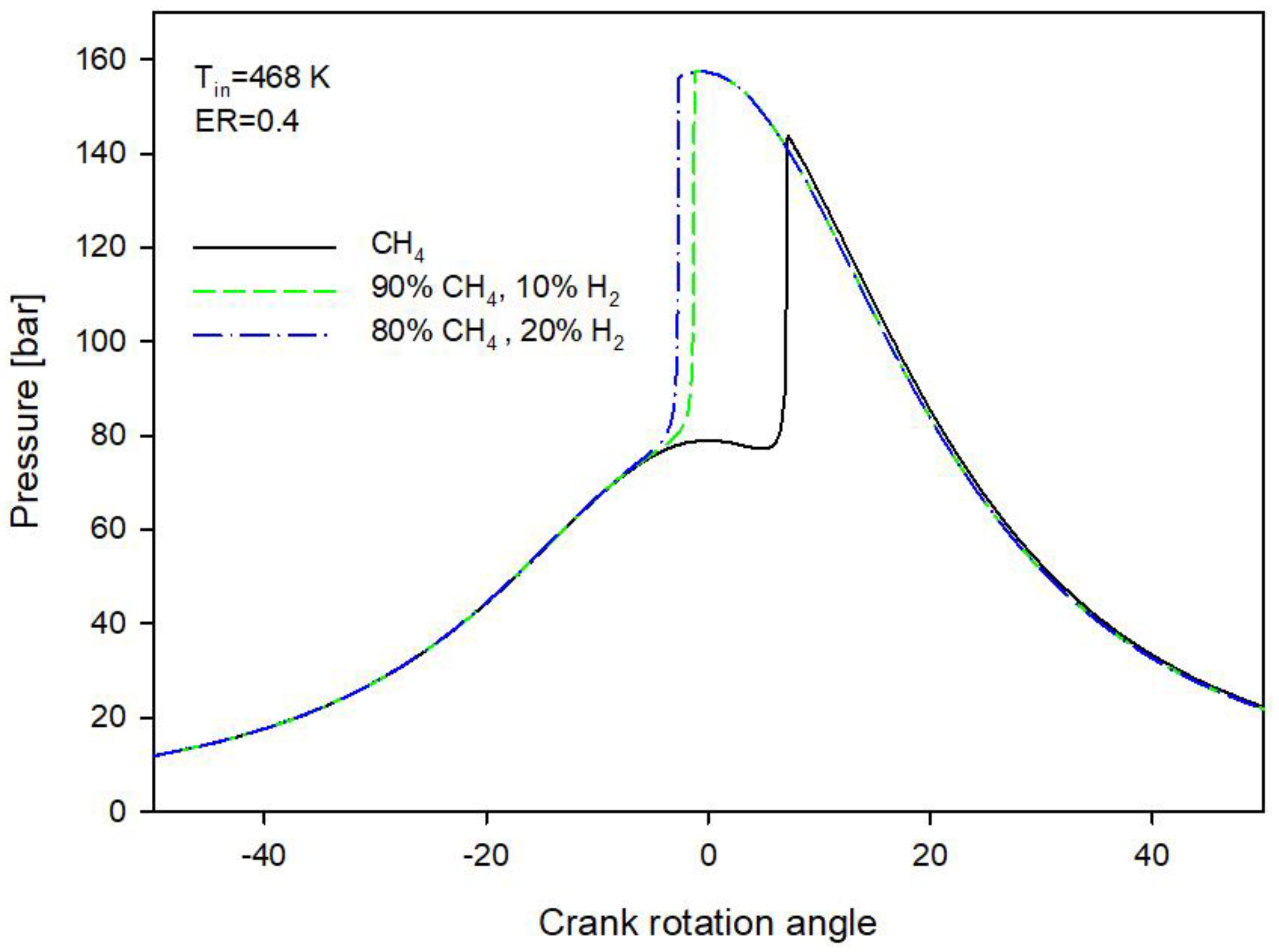
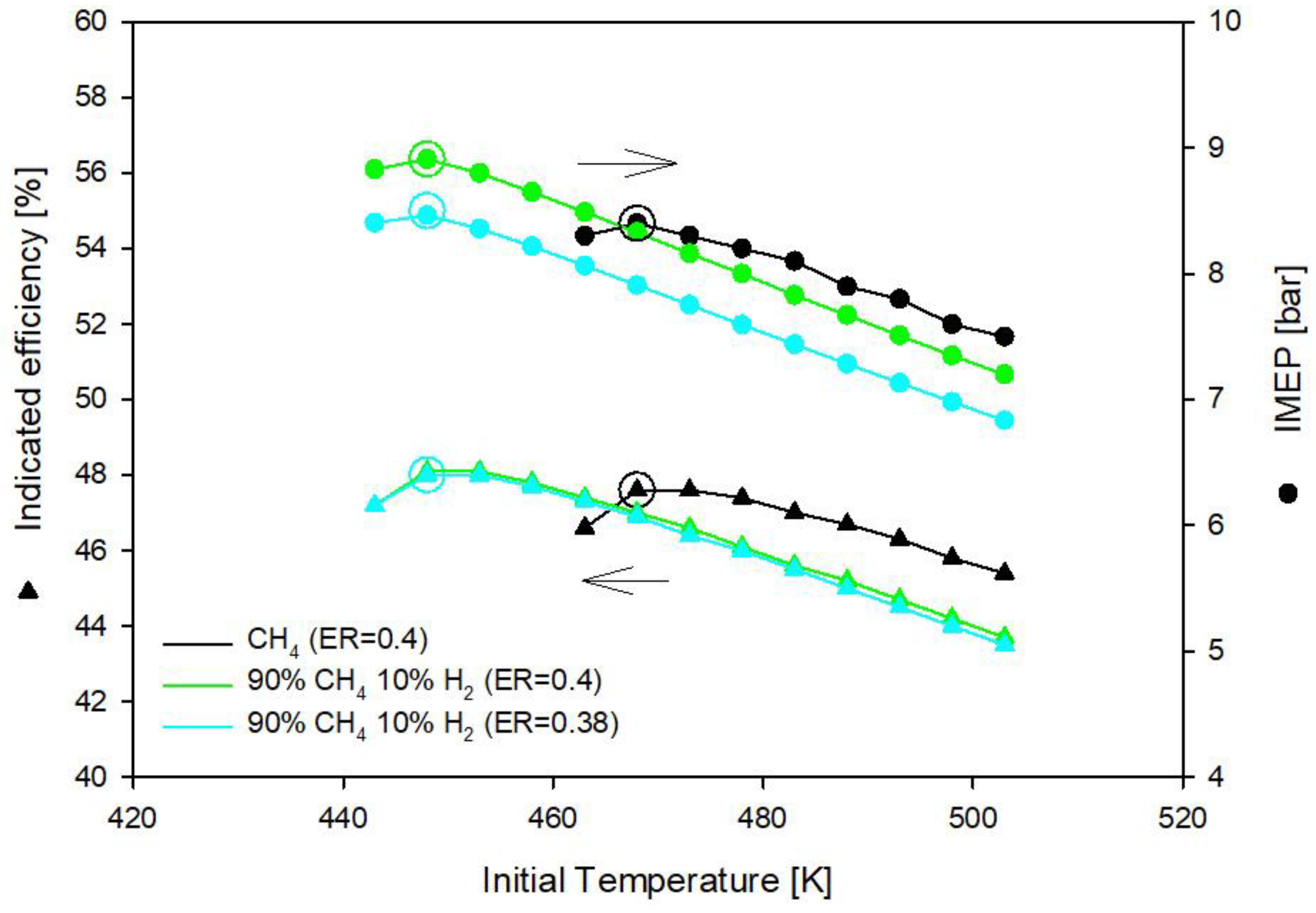
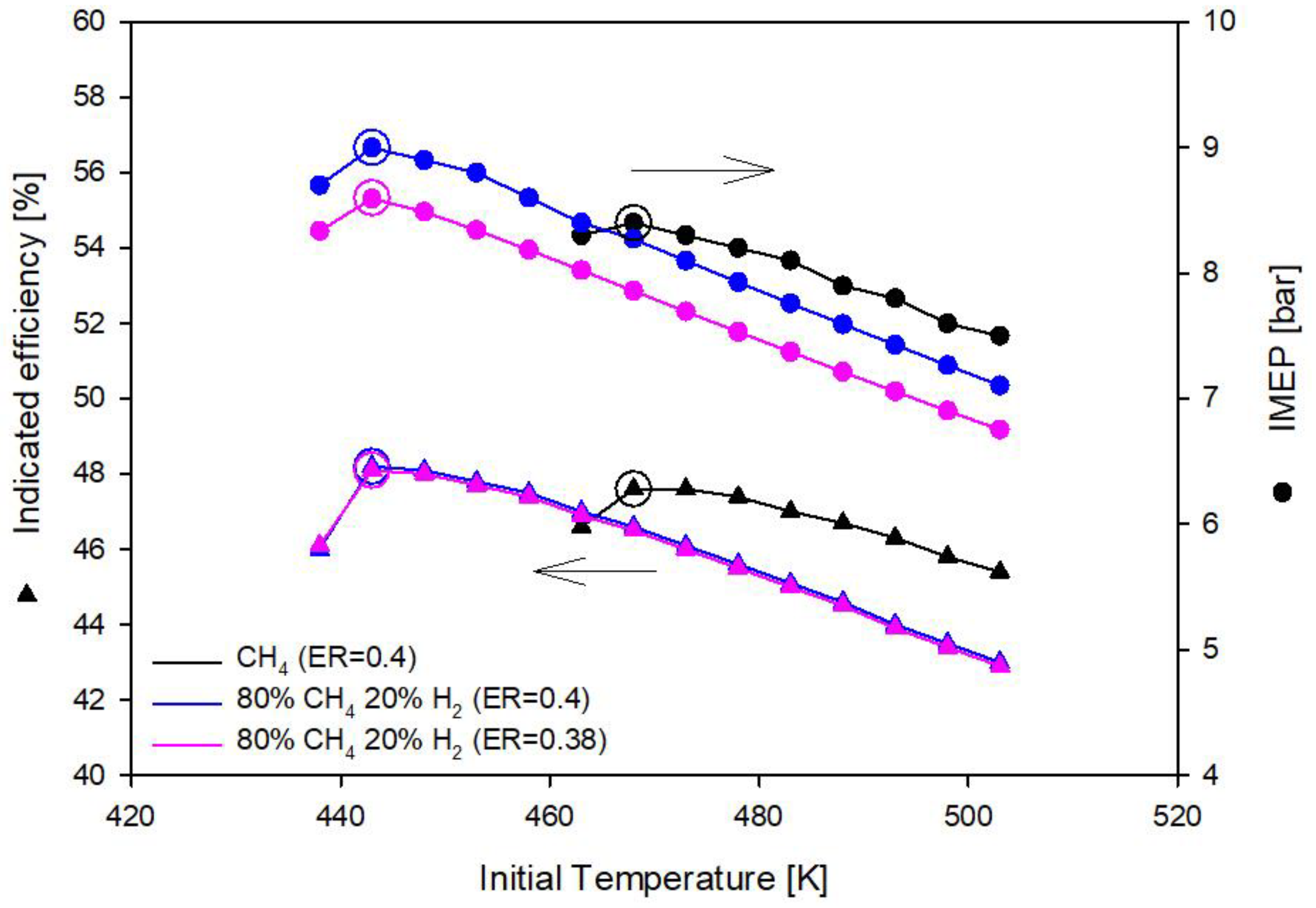
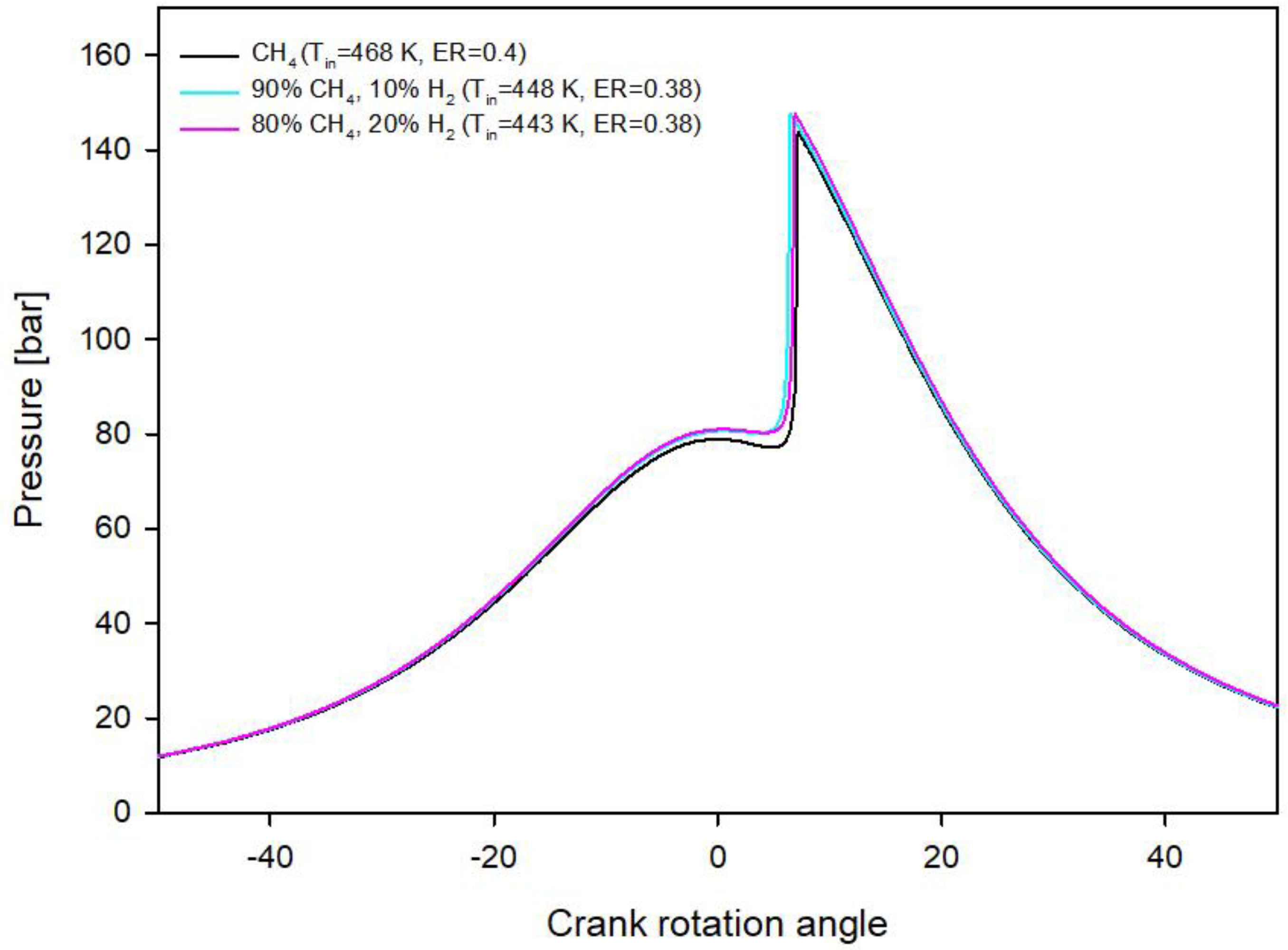
3.1. Methane and Methane-Hydrogen Blends
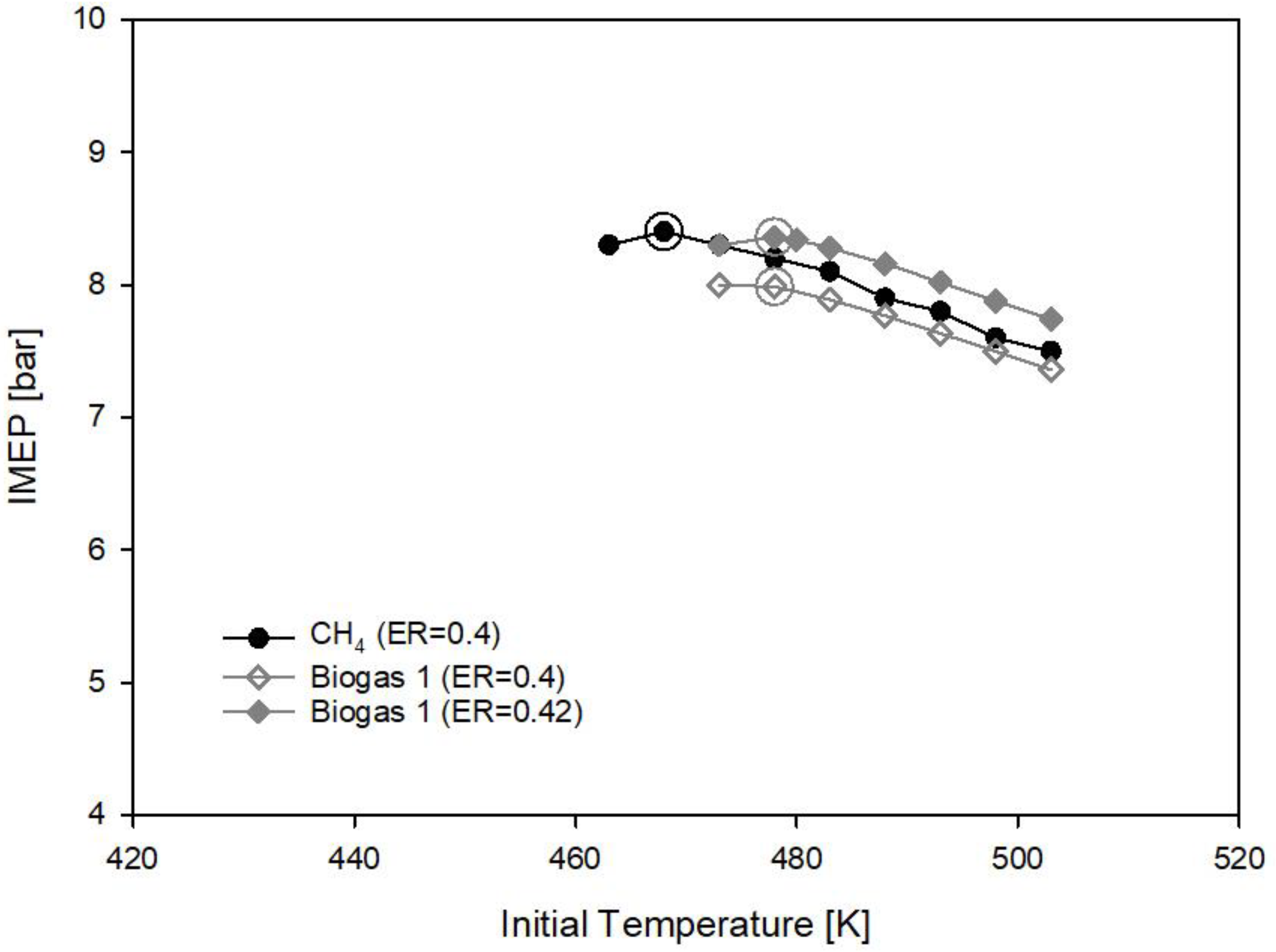
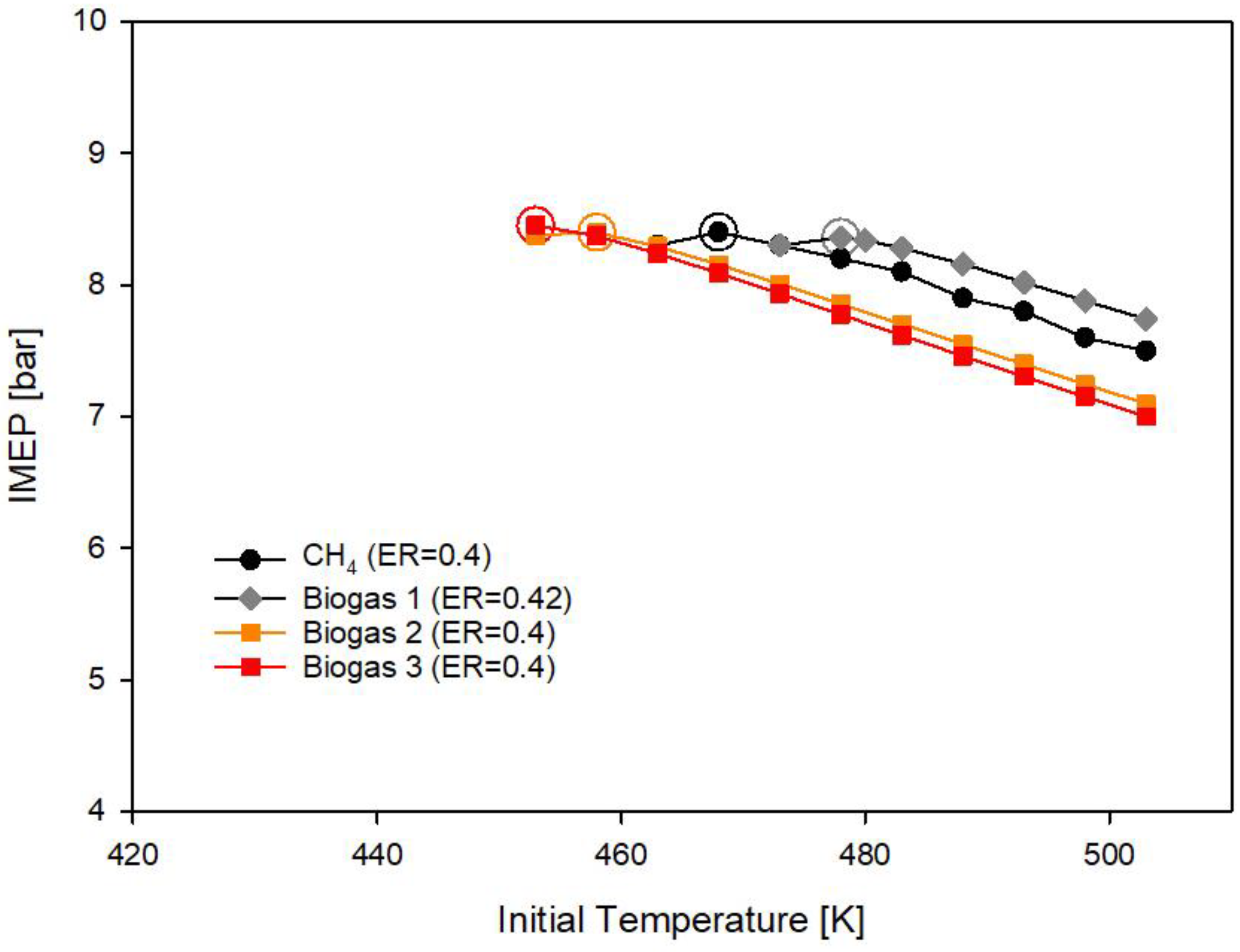
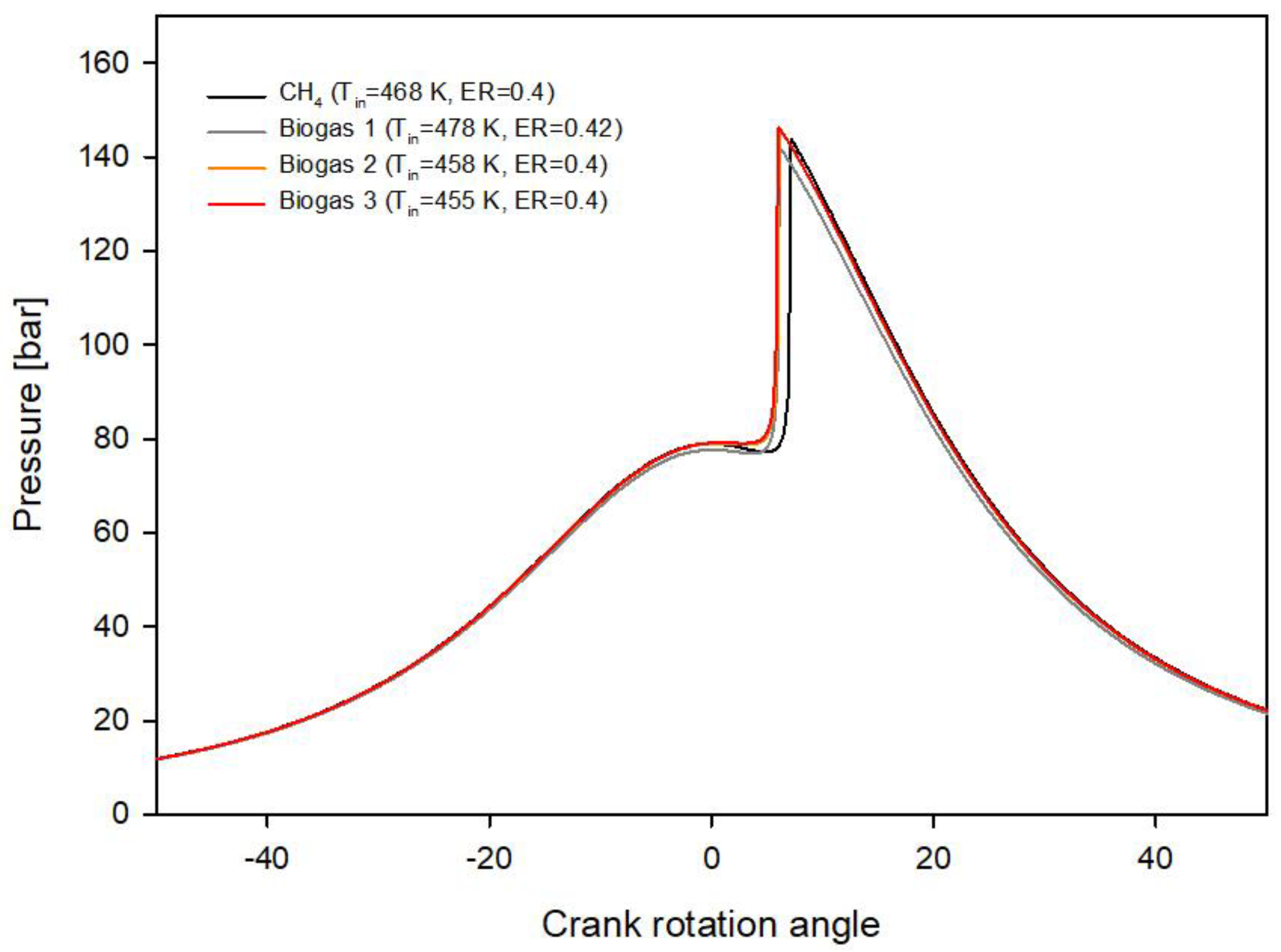
3.2. Biogas and Innovative Biogases
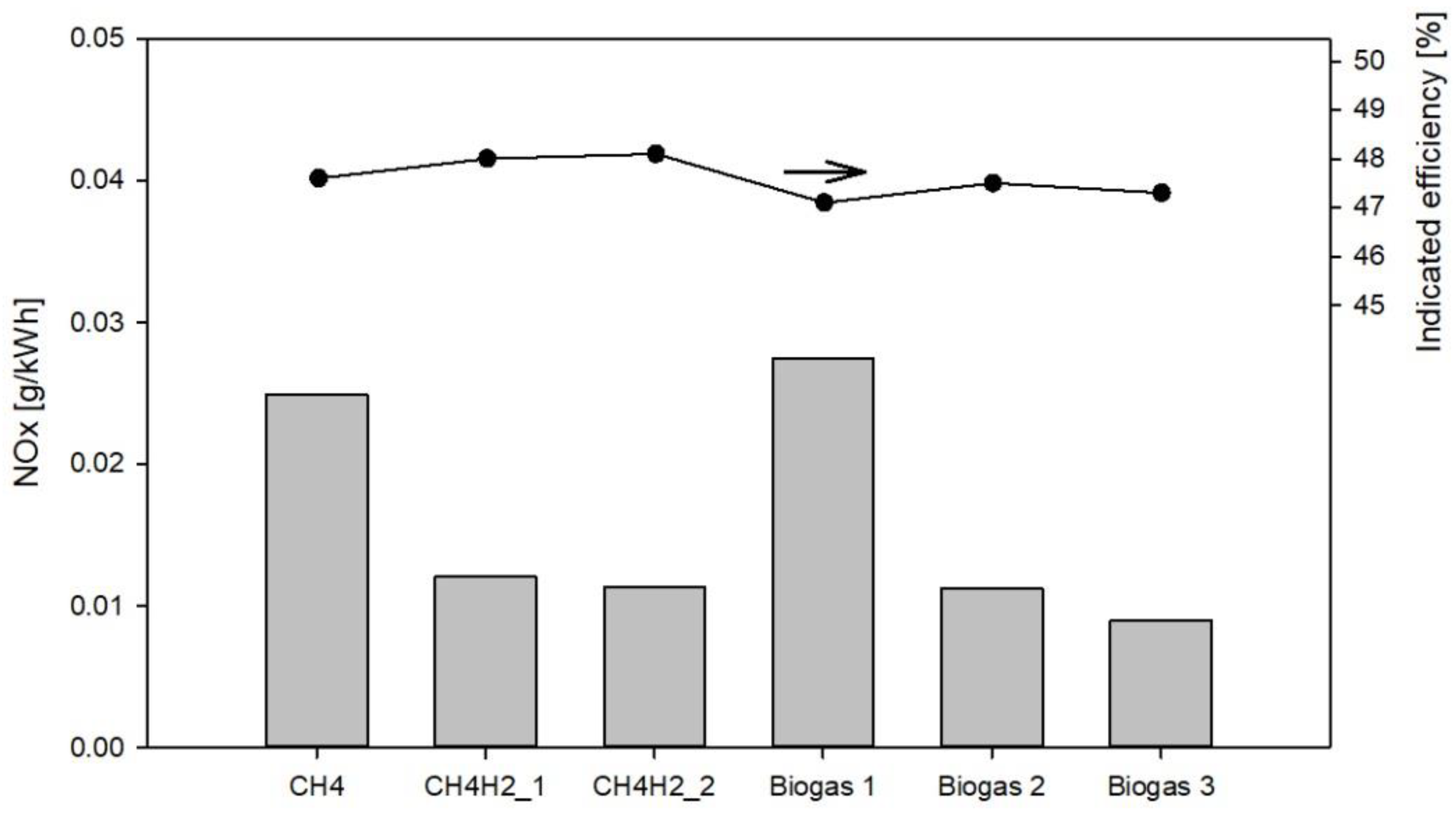
3.3. NOx Emissions
4. Conclusions
- Methane-hydrogen mixtures require lower equivalence ratio (ER = 0.38) than methane (ER = 0.4) to deliver the same imep;
- Hydrogen addition to methane reduces the initial gas temperature requirement: In the optimal operating conditions with methane the initial gas temperature must be 468 K and drops to 448 K with the addition of 10% of hydrogen in CH4H2_1 blend and to 443 K with the addition of 20% of hydrogen in CH4H2_2 blend;
- Biogas 1 made of 66% methane and 33% carbon dioxide requires higher equivalence ratio (ER = 0.42) than methane (ER = 0.4) to deliver the reference imep due to the presence of CO2 in the fuel. The initial gas temperature requirement also increases to 478 K;
- For the innovative biogas 2, with a CH4/H2 ratio equal to 9:1, the required initial temperature decreases to 457 K with an ER = 0.4;
- For the innovative biogas 3, with a CH4/H2 ratio equal to 8:2, the required initial temperature further decreases to 448 K with an ER = 0.4;
- Hydrogen enrichment of methane reduces NOx emissions due to a lower initial gas temperature requirement in comparison with pure methane. The higher the hydrogen molar fraction, the smaller the NOx emissions.
- For the innovative biogases NOx emissions decrease by increasing the amount of H2 in the biogas. Additionally, in this case, this is due to a lower initial gas temperature requirement in comparison with both pure methane and the conventional biogas.
- Though the initial temperature is higher, the innovative biogases show slightly lower NOx emissions than those of the equivalent blends of methane and hydrogen.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Merola, M.C.; Carotenuto, C.; Gargiulo, V.; Stanzione, F.; Ciajolo, A.; Minale, M. Chemical–physical analysis of rheologically different samples of a heavy crude oil. Fuel Process. Technol. 2016, 148, 236–247. [Google Scholar] [CrossRef]
- Minale, M.; Merola, M.C.; Carotenuto, C. Effect of solvents on the microstructure aggregation of a heavy crude oil. Fuel Process. Technol. 2018, 177, 299–308. [Google Scholar] [CrossRef]
- Ahindra, N. Biofuels Refining and Performance; McGraw-Hill: New York, NY, USA, 2008; ISBN 9780071489706. [Google Scholar]
- Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC. Available online: http://data.europa.eu/eli/dir/2009/28/oj (accessed on 10 September 2018).
- Thangavelu, S.K.; Ahmed, A.S.; Ani, F.N. Review on bioethanol as alternative fuel for spark ignition engines. Renew. Sustain. Energy Rev. 2016, 56, 820–835. [Google Scholar] [CrossRef]
- Mata, C.; Gómez, A.; Armas, O. The influence of ethanol-diesel blend on pollutant emissions from different bus fleets under acceleration transitions. Fuel 2017, 209, 322–328. [Google Scholar] [CrossRef]
- Liu, S.; Chen, W.; Zhu, Z.; Jiang, S.; Ren, T.; Guo, H. A Review of the Developed New Model Biodiesels and Their Effects on Engine Combustion and Emissions. Appl. Sci. 2018, 8, 2303. [Google Scholar] [CrossRef]
- De Souza-Santos, M.L.; Ceribeli, K. Technical evaluation of a power generation process consuming municipal solid waste. Fuel 2013, 108, 578–585. [Google Scholar] [CrossRef]
- Rasi, S.; Läntelä, J.; Rintala, J. Upgrading landfill gas using a high pressure water absorption process. Fuel 2014, 115, 539–543. [Google Scholar] [CrossRef]
- Yuan, W.; Bandosz, T.J. Removal of hydrogen sulfide from biogas on sludge-derived adsorbents. Fuel 2007, 86, 2736–2746. [Google Scholar] [CrossRef]
- Chin, M.J.; Poh, P.E.; Tey, B.T.; Chan, E.S.; Chin, K.L. Biogas from palm oil mill effluent (POME): Opportunities and challenges from Malaysia’s perspective. Renew. Sustain. Energy Rev. 2013, 26, 717–726. [Google Scholar] [CrossRef]
- Thien Thu, C.T.; Cuong, P.H.; Hang, L.T.; Chao, N.; Van Anh, L.X.; Trach, N.X.; Sommer, S.G. Manure management practices on biogas and non-biogas pig farms in developing countries using livestock farms in Vietnam as an example. J. Clean. Prod. 2012, 27, 64–71. [Google Scholar] [CrossRef]
- Guarino, G.; Carotenuto, C.; Di Cristofaro, F.; Papa, S.; Morrone, B.; Minale, M. Does the C/N ratio really affect the bio-methane yield? A three years investigation of buffalo manure digestion. Chem. Eng. Trans. 2016, 49, 463–468. [Google Scholar] [CrossRef]
- Luo, D.; Dai, Y. Economic evaluation of coalbed methane production in China. Energy Policy 2009, 37, 3883–3889. [Google Scholar] [CrossRef]
- Yadav, V.K.; Ray, A.; Ravi, M.R. Experimental and computational investigation of the laminar burning velocity of hydrogen-enriched biogas. Fuel 2019, 235, 810–821. [Google Scholar] [CrossRef]
- Hinton, N.; Stone, R. Laminar burning velocity measurements of methane and carbon dioxide mixtures (biogas) over wide ranging temperatures and pressures. Fuel 2014, 116, 743–750. [Google Scholar] [CrossRef]
- Ferguson, C.R.; Kirkpatrick, A.T. Internal Combustion Engines: Applied Thermosciences, 2nd ed.; Wiley & Sons: New York, NY, USA, 2000; ISBN 978-04-7135-617-2. [Google Scholar]
- Hu, Z.; Zhang, X. Experimental study on flame stability of biogas/hydrogen combustion. Int. J. Hydrog. Energy 2018. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill: New York, NY, USA, 1988; ISBN 978-00-7028-637-5. [Google Scholar]
- Ma, F.; Wang, Y.; Liu, H.; Li, Y.; Wang, J.; Zhao, S. Experimental study on thermal efficiency and emission characteristics of a lean burn hydrogen enriched natural gas engine. Int. J. Hydrog. Energy 2007, 32, 5067–5075. [Google Scholar] [CrossRef]
- Mariani, A.; Prati, M.V.; Unich, A.; Morrone, B. Combustion analysis of a spark ignition i. c. engine fuelled alternatively with natural gas and hydrogen-natural gas blends. Int. J. Hydrog. Energy 2013, 38, 1616–1623. [Google Scholar] [CrossRef]
- Gómez Montoya, J.P.; Amell, A.A.; Olsen, D.B.; Amador Diaz, G.J. Strategies to improve the performance of a spark ignition engine using fuel blends of biogas with natural gas, propane and hydrogen. Int. J. Hydrog. Energy 2018, 43, 21592–21602. [Google Scholar] [CrossRef]
- Chung, K.; Chun, K.M.C. Combustion Characteristics and Generating Efficiency Using Biogas with Added Hydrogen. SAE Tech. Pap. 2013. [Google Scholar] [CrossRef]
- Rakopoulos, C.D.; Michos, C.N.; Giakoumis, E.G. Thermodynamic analysis of SI engine operation on variable composition biogas-hydrogen blends using a quasi-dimensional, multi-zone combustion model. SAE J. Automot. Eng. 2009, 2, 880–910. [Google Scholar] [CrossRef]
- Park, C.; Park, S.; Kim, C.; Lee, S. Effects of EGR on performance of engines with spark gap projection and fueled by biogas–hydrogen blends. Int. J. Hydrog. Energy 2012, 37, 14640–14648. [Google Scholar] [CrossRef]
- Park, C.; Park, S.; Lee, Y.; Kim, C.; Lee, S.; Moriyoshi, Y. Performance and emission characteristics of a SI engine fueled by low calorific biogas blended with hydrogen. Int. J. Hydrog. Energy 2011, 36, 10080–10088. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Osaka, Y.; Bai, Y.; Kobayashi, N.; Chen, Y. Combustion and Heat Release Characteristics of Biogas under Hydrogen- and Oxygen-Enriched Condition. Energies 2017, 10, 1200. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H. Investigation of combustion, performance and emission characteristics of 2-stroke and 4-stroke spark ignition and CAI/HCCI operations in a DI gasoline. Appl. Energy 2014, 130, 244–255. [Google Scholar] [CrossRef]
- Cho, G.; Jeong, D.; Moon, G.; Bae, C. Controlled auto-ignition characteristics of methane-air mixture in a rapid intake compression and expansion machine. Energy 2010, 35, 4184–4191. [Google Scholar] [CrossRef]
- Kozarac, D.; Vuilleumier, D.; Saxena, S.; Dibble, R.W. Analysis of benefits of using internal exhaust gas recirculation in biogas-fueled HCCI engines. Energy Convers. Manag. 2014, 87, 1186–1194. [Google Scholar] [CrossRef]
- Yao, M.; Zheng, Z.; Liu, H. Progress and recent trends in homogeneous charge compression ignition (HCCI) engines. Prog. Energy Combust. 2009, 35, 398–497. [Google Scholar] [CrossRef]
- Saxena, S.; Bedoya, I.D. Fundamental phenomena affecting low temperature combustion and HCCI engines, high load limits, and strategies for extending these limits. Prog. Energy Combust. 2013, 39, 457–488. [Google Scholar] [CrossRef]
- Jamsran, N.; Putrasari, Y.; Lim, O. A computational study on the autoignition characteristics of an HCCI engine fueled with natural gas. J. Nat. Gas Sci. Eng. 2016, 29, 469–478. [Google Scholar] [CrossRef]
- Nishi, M.; Kanehara, M.; Iida, N. Assessment for innovative combustion on HCCI engine by controlling EGR ratio and engine speed. Appl. Therm. Eng. 2016, 99, 42–60. [Google Scholar] [CrossRef]
- Mohamed Ibrahim, M.; Ramesh, A. Investigations on the effects of intake temperature and charge dilution in a hydrogen fueled HCCI engine. Int. J. Hydrog. Energy 2014, 39, 14097–14108. [Google Scholar] [CrossRef]
- Chemkin. Theory Manual; Reaction Design: San Diego, CA, USA, 2007. [Google Scholar]
- Gri-Mech. Available online: http://www.me.berkeley.edu/gri_mech/ (accessed on 20 February 2018).
- Buswell, A.M.; Mueller, H.F. Mechanism of Methane Fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- Carotenuto, C.; Guarino, G.; Morrone, B.; Minale, M. Temperature and pH effect on methane production from buffalo manure anaerobic digestion. Int. J. Heat Technol. 2016, 34, S425–S429. [Google Scholar] [CrossRef]
- Carotenuto, C.; Guarino, G.; Minale, M.; Morrone, B. Biogas production from anaerobic digestion of manure at different operative conditions. Int. J. Heat Technol. 2016, 34, 623–629. [Google Scholar] [CrossRef]
- Gilroyed, B.H.; Chang, C.; Chu, A.; Hao, X. Effect of temperature on anaerobic fermentative hydrogen gas production from feedlot cattle manure using mixed microflora. Int. J. Hydrog. Energy 2008, 33, 4301–4308. [Google Scholar] [CrossRef]
- Carillo, P.; Carotenuto, C.; di Cristofaro, F.; Kafantaris, I.; Lubritto, C.; Minale, M.; Morrone, B.; Papa, S.; Woodrow, P. DGGE analysis of buffalo manure eubacteria for hydrogen production: Effect of pH, temperature and pretreatments. Mol. Biol. Rep. 2012, 39, 10193–10200. [Google Scholar] [CrossRef] [PubMed]
- Guarino, G.; di Cristofaro, F.; Carotenuto, C.; Morrone, B.; Minale, M. Effect of thermal and mechanical pre-treatments on the CH 4-H2 production from water buffalo manure in different process conditions. Chem. Eng. Trans. 2014, 38, 205–210. [Google Scholar] [CrossRef]
- Di Cristofaro, F.; Carotenuto, C.; Carillo, P.; Woodrow, P.; Morrone, B.; Minale, M. Evaluation of CH4 and H2 yield with different mixtures of digested and fresh buffalo manure. Chem. Eng. Trans. 2014, 37, 283–288. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D. Perry’s Chemical Engineers’ Handbook; Mc Graw Hill: New York, NY, USA, 1984; ISBN 0-07-Y66482-X. [Google Scholar]


| Engine type | 4-stroke |
| Compression ratio | 16.7:1 |
| Bore [mm] | 135 |
| Stroke [mm] | 170 |
| Engine speed [rpm] | 1500 |
| Initial gas pressure (BDC) [bar] | 2 |
| Fuel | CH4 Molar Fraction | H2 Molar Fraction | CO2 Molar Fraction |
|---|---|---|---|
| CH4 | 1.00 | 0 | 0 |
| CH4H2_1 | 0.90 | 0.10 | 0 |
| CH4H2_2 | 0.80 | 0.20 | 0 |
| Biogas 1 | 0.67 | 0 | 0.33 |
| Biogas 2 | 0.580 | 0.065 | 0.355 |
| Biogas 3 | 0.500 | 0.125 | 0.375 |
| CH4 | H2 | |
|---|---|---|
| Density [kg/Nm3] | 0.7064 | 0.0888 |
| Stoich AFR [mass air/mass fuel] | 17.2 | 34.3 |
| Stoich AFR [vol air/vol fuel] | 9.54 | 2.39 |
| Lower Heating Value [MJ/kg] | 50 | 119.9 |
| Lower Heating Value vol. [MJ/Nm3] | 35.32 | 10.64 |
| Auto-ignition temperature [°C] | 813 | 858 |
| Thermal conductivity at 300 K [W/(m K)] | 0.034 | 0.182 |
| Octane Number | >120 | >130 |
| Laminar flame speed at stoich. conditions [m/s] | 0.38 ÷ 0.40 | 2 ÷ 2.2 |
| Flammability limits in air [%vol.] | 5.3–15 | 4–75 |
| Fuel | ER [-] | Tin [K] | imep [bar] | ηi [%] | NOx [g/kWh] |
|---|---|---|---|---|---|
| CH4 | 0.40 | 468 | 8.4 | 47.6 | 0.0249 |
| CH4H2_1 | 0.38 | 448 | 8.5 | 48.0 | 0.0120 |
| CH4H2_2 | 0.38 | 443 | 8.6 | 48.1 | 0.0113 |
| Biogas 1 | 0.42 | 478 | 8.4 | 47.1 | 0.0275 |
| Biogas 2 | 0.4 | 457 | 8.4 | 47.5 | 0.0112 |
| Biogas 3 | 0.4 | 453 | 8.4 | 47.3 | 0.0089 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariani, A.; Unich, A.; Minale, M. Combustion of Hydrogen Enriched Methane and Biogases Containing Hydrogen in a Controlled Auto-Ignition Engine. Appl. Sci. 2018, 8, 2667. https://doi.org/10.3390/app8122667
Mariani A, Unich A, Minale M. Combustion of Hydrogen Enriched Methane and Biogases Containing Hydrogen in a Controlled Auto-Ignition Engine. Applied Sciences. 2018; 8(12):2667. https://doi.org/10.3390/app8122667
Chicago/Turabian StyleMariani, Antonio, Andrea Unich, and Mario Minale. 2018. "Combustion of Hydrogen Enriched Methane and Biogases Containing Hydrogen in a Controlled Auto-Ignition Engine" Applied Sciences 8, no. 12: 2667. https://doi.org/10.3390/app8122667
APA StyleMariani, A., Unich, A., & Minale, M. (2018). Combustion of Hydrogen Enriched Methane and Biogases Containing Hydrogen in a Controlled Auto-Ignition Engine. Applied Sciences, 8(12), 2667. https://doi.org/10.3390/app8122667








