The Effect of Different Mixed Organic Solvents on the Properties of p(OPal-MMA) Gel Electrolyte Membrane for Lithium Ion Batteries
Abstract
1. Introduction
2. Methods
2.1. Materials
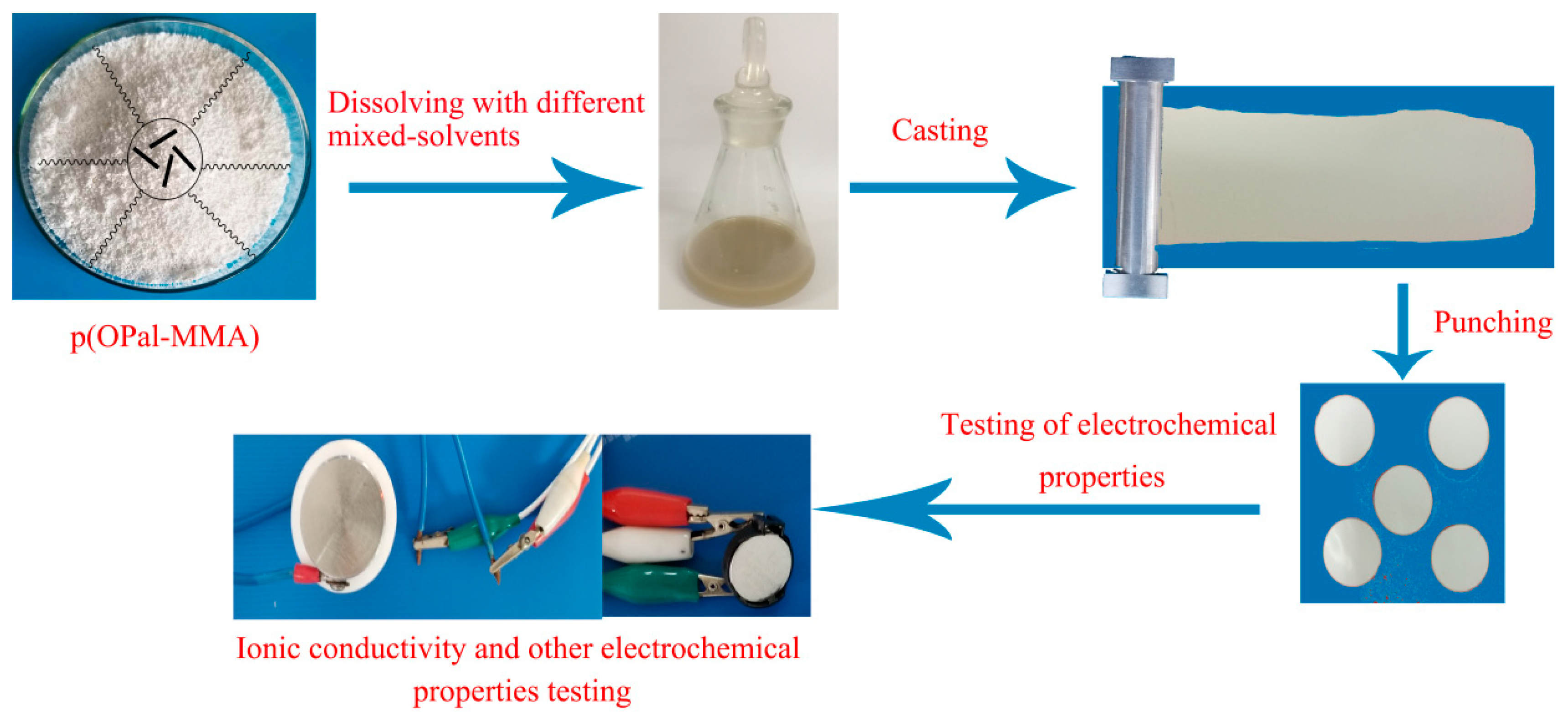
2.2. Preparation Process of the p(OPal-MMA) Polymer Membranes and GPE
2.3. Characterization of Physical Properties
2.4. Electrochemical Testing
3. Result
3.1. SEM Images of p(OPal-MMA) Membranes
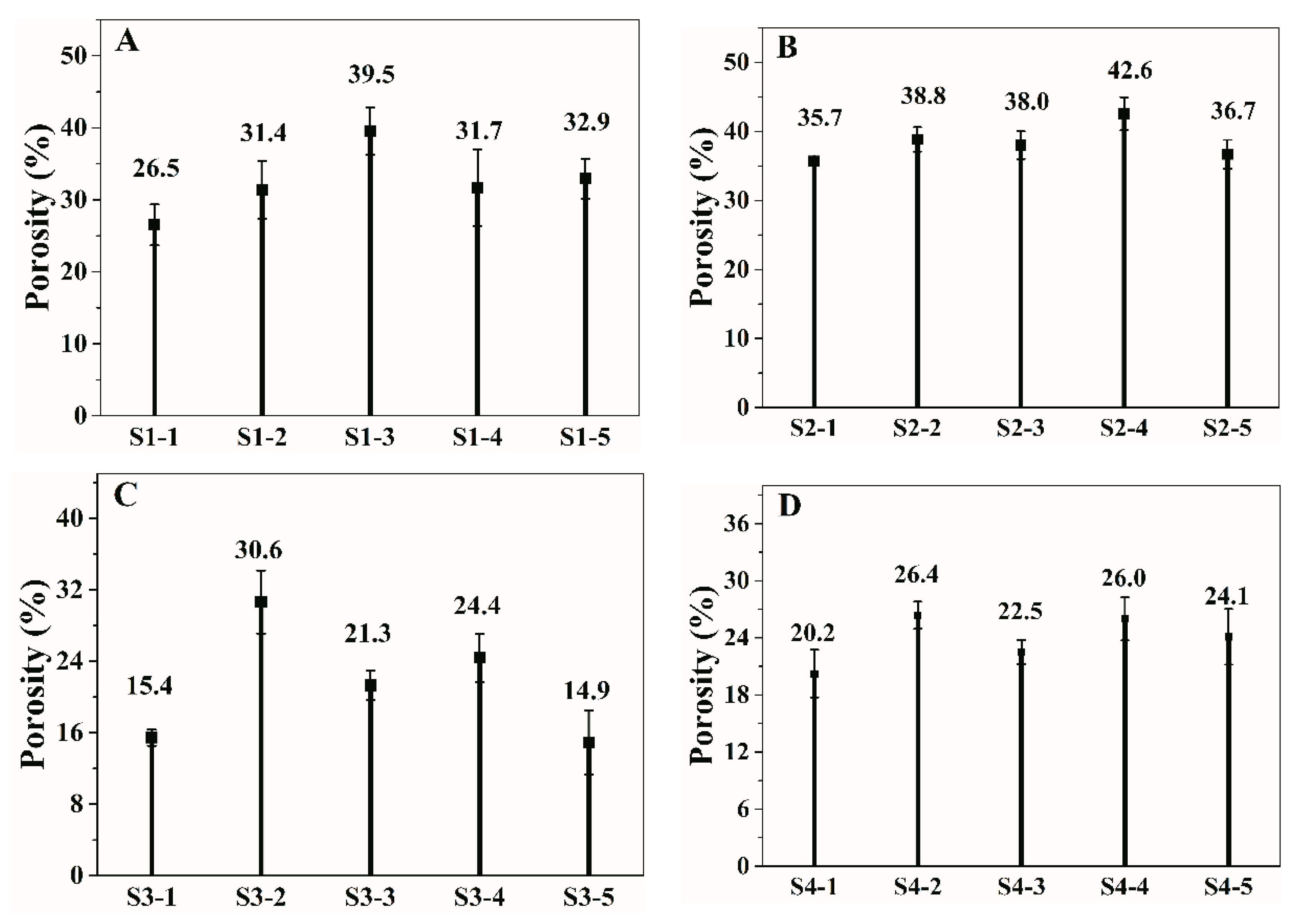
3.2. Porosity of the p(OPal-MMA) Membranes
3.3. The Ionic Conductivity of Gel p(OPal-MMA) Electrolyte
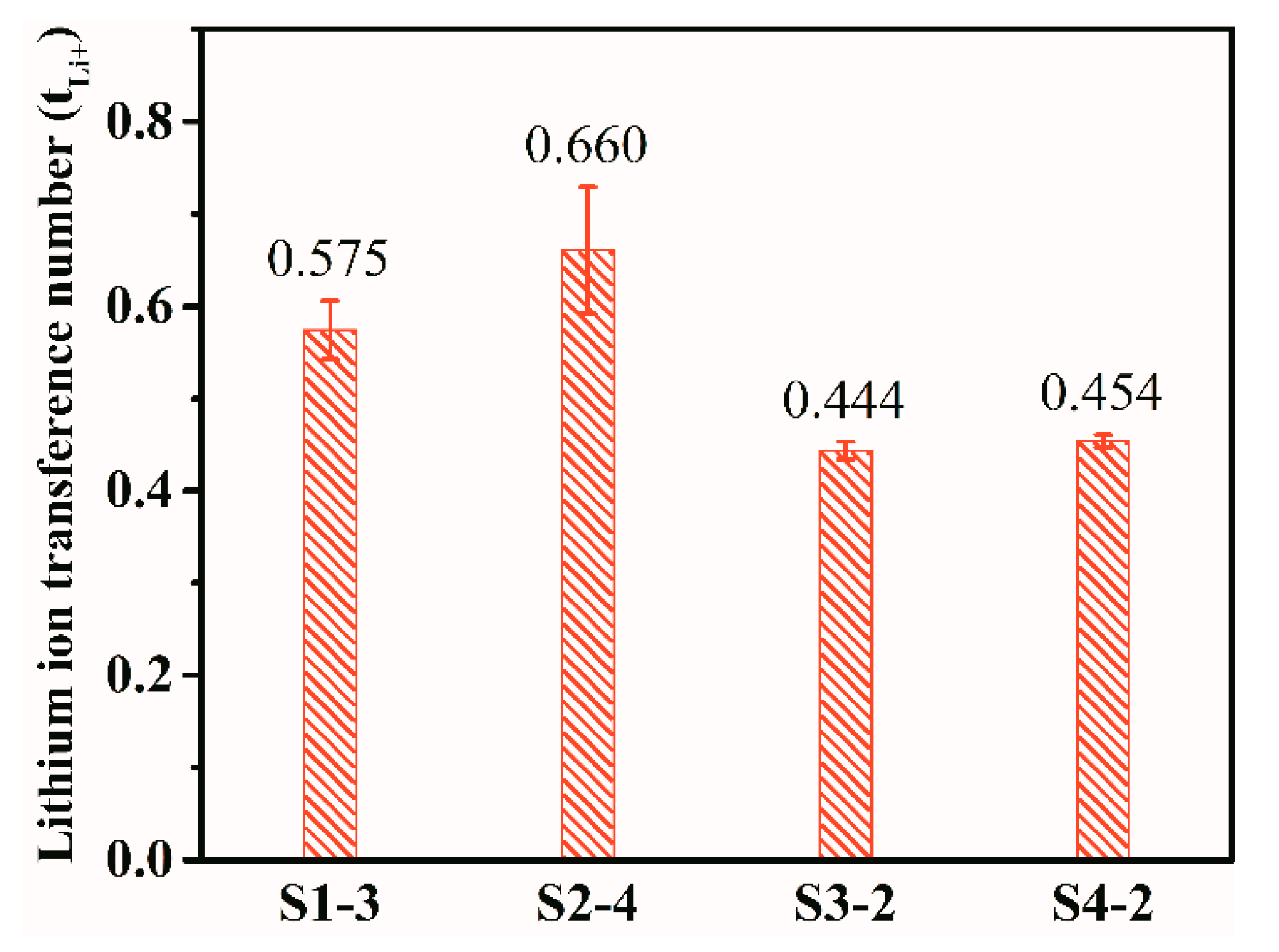
3.4. The Ion Transference Number of Gel p(OPal-MMA) Electrolyte
3.5. The Electrochemical Stability Window of Gel p(OPal-MMA) Electrolyte
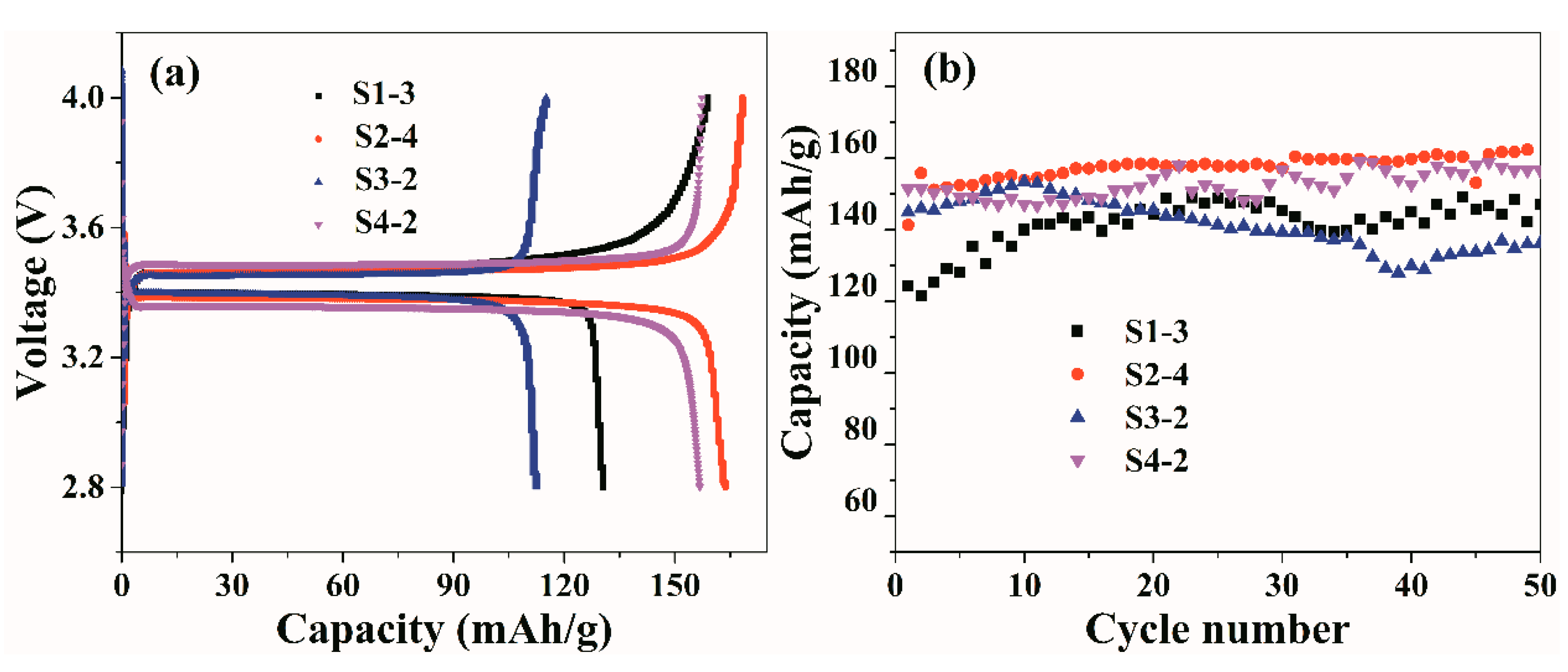
3.6. Electrochemical Performance of Gel p(OPal-MMA) Electrolyte
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, Y.S.; Lee, J.H.; Choi, J.A.; Yoon, W.Y.; Kim, D.W. Cycling Characteristics of Lithium Powder Polymer Batteries Assembled with Composite Gel Polymer Electrolytes and Lithium Powder Anode. Adv. Funct. Mater. 2013, 23, 1019–1027. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.G.; Moon, H.C. Mechanically Robust, Highly Ionic Conductive Gels Based on Random Copolymers for Bending Durable Electrochemical Devices. Adv. Funct. Mater. 2018, 28, 1706948. [Google Scholar]
- Ma, T.; Cui, Z.; Wu, Y.; Qin, S.; Wang, H.; Yan, F.; Han, N.; Li, J. Preparation of PVDF based blend microporous membranes for lithium ion batteries by thermally induced phase separation: I. Effect of PMMA on the membrane formation process and the properties. J. Membr. Sci. 2013, 444, 213–222. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, F.; Zhang, L.; Zhang, T.; Huang, Y.; Chen, Y. A High-Performance Graphene Oxide-Doped Ion Gel as Gel Polymer Electrolyte for All-Solid-State Supercapacitor Applications. Adv. Funct. Mater. 2013, 23, 3353–3360. [Google Scholar]
- Arora, P.; Zhang, Z.J. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Seol, W.H.; Lee, Y.M.; Park, J.K. Preparation and characterization of new microporous stretched membrane for lithium rechargeable battery. J. Power Sources 2006, 163, 247–251. [Google Scholar]
- Yang, C.L.; Li, Z.H.; Li, W.J.; Liu, H.Y.; Xiao, Q.Z.; Lei, G.T.; Ding, Y.H. Batwing-like polymer membrane consisting of PMMA-grafted electrospun PVdF–SiO2 nanocomposite fibers for lithium-ion batteries. J. Membrane Sci. 2015, 495, 341–350. [Google Scholar] [CrossRef]
- Moon, H.C.; Lodge, T.P.; Frisbie, C.D. Electrochemiluminescent displays based on ion gels: Correlation between device performance and choice of electrolyte. J. Mater. Chem. C 2016, 4, 8448–8453. [Google Scholar] [CrossRef]
- Oh, H.; Seo, D.G.; Yun, T.Y.; Kim, C.Y.; Moon, H.C. Voltage-Tunable Multicolor, Sub-1.5 V, Flexible Electrochromic Devices Based on Ion Gels. ACS Appl. Mater. Interfaces 2017, 9, 7658–7665. [Google Scholar]
- Moon, H.C.; Lodge, T.P.; Frisbie, C.D. Solution Processable, Electrochromic Ion Gels for Sub-1 V, Flexible Displays on Plastic. Chem. Mater. 2015, 27, 1420–1425. [Google Scholar] [CrossRef]
- Moon, H.C.; Lodge, T.P.; Frisbie, C.D. Solution-processable electrochemiluminescent ion gels for flexible, low-voltage, emissive displays on plastic. J. Am. Chem. Soc. 2014, 136, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Deka, M.; Kumar, A. Enhanced electrical and electrochemical properties of PMMA–clay nanocomposite gel polymer electrolytes. Electrochim. Acta 2010, 55, 1836–1842. [Google Scholar] [CrossRef]
- Shubha, N.; Prasanth, R.; Hoon, H.H.; Srinivasan, M. Dual phase polymer gel electrolyte based on non-woven poly (vinylidenefluoride-co-hexafluoropropylene)–layered clay nanocomposite fibrous membranes for lithium ion batteries. Mater. Res. Bull. 2013, 48, 526–537. [Google Scholar] [CrossRef]
- Wang, M.; Dong, S. Enhanced electrochemical properties of nanocomposite polymer electrolyte based on copolymer with exfoliated clays. J. Power Sources 2007, 170, 425–432. [Google Scholar] [CrossRef]
- Chen, F.; Lou, D.; Yang, J.; Zhong, M. Mechanical and thermal properties of attapulgite clay reinforced polymethylmethacrylate nanocomposites. Polym. Adv. Technol. 2011, 22, 1912–1918. [Google Scholar] [CrossRef]
- Tian, L.; Xiong, L.; Chen, X.; Guo, H.; Zhang, H.; Chen, X. Enhanced Electrochemical Properties of Gel Polymer Electrolyte with Hybrid Copolymer of Organic Palygorskite and Methyl Methacrylate. Materials 2018, 11, 1814. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Gozdz, A.S.; Schmutz, C.; Shokoohi, F.; Warren, P.C. Performance of Bellcore’s plastic rechargeable Li-ion batteries. Solid State Tonics 1996, 86–88, 49–54. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Pethaiah, S.S.; Rajendran, S. Li-ion conduction in PVAc based polymer blend electrolytes for lithium battery applications. Mater. Chem. Phys. 2011, 129, 471–476. [Google Scholar] [CrossRef]
- Saikia, D.; Kumar, A. Ionic conduction in P(VDF-HFP)/PVDF-(PC + DEC)-LiClO4 polymer gel electrolytes. Electrochim. Acta 2004, 49, 2581–2589. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, Y.S.; Joo, C.W.; Lee, S.G.; Park, J.K.; Han, K.S. Electrospun PVDF nanofiber web as polymer electrolyte or separator. Electrochim. Acta 2004, 50, 339–343. [Google Scholar] [CrossRef]
- Yu, S.; Chen, L.; Chen, Y.; Tong, Y. Microporous gel electrolytes based on amphiphilic poly (vinylidene fluoride-co-hexafluoropropylene) for lithium batteries. Appl. Surf. Sci. 2012, 258, 4983–4989. [Google Scholar] [CrossRef]
- Idris, N.H.; Rahman, M.M.; Wang, J.Z.; Liu, H.K. Microporous gel polymer electrolytes for lithium rechargeable battery application. J. Power Sources 2012, 201, 294–300. [Google Scholar] [CrossRef]
- Jeong, H.S.; Hong, S.C.; Lee, S.Y. Effect of microporous structure on thermal shrinkage and electrochemical performance of Al2O3/poly(vinylidene fluoride-hexafluoropropylene) composite separators for lithium-ion batteries. J. Membr. Sci. 2010, 364, 177–182. [Google Scholar] [CrossRef]
- Li, G.C.; Zhang, P.; Zhang, H.P.; Yang, L.C.; Wu, Y.P. A porous polymer electrolyte based on P (VDF-HFP) prepared by a simple phase separation process. Electrochem. Commun. 2008, 10, 1883–1885. [Google Scholar] [CrossRef]
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Kataoka, H.; Saito, Y.; Sakai, T.; Quartarone, E.; Mustarelli, P. Conduction Mechanisms of PVDF-Type Gel Polymer Electrolytes of Lithium Prepared by a Phase Inversion Process. J. Phys. Chem. B 2000, 104, 11460–11464. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, X.; Li, W.; Li, Z.; Zhang, T.; Zhang, H. Macroporous polymer electrolytes based on PVDF/PEO-b-PMMA block copolymer blends for rechargeable lithium ion battery. J. Membrane Sci. 2009, 334, 117–122. [Google Scholar] [CrossRef]
- Lin, C.W.; Hung, C.L.; Venkateswarlu, M.; Hwang, B.J. Influence of TiO2 nano-particles on the transport properties of composite polymer electrolyte for lithium-ion batteries. J. Power Sources 2005, 146, 397–401. [Google Scholar] [CrossRef]
- Wang, Z.L.; Tang, Z.Y. A novel polymer electrolyte based on PMAML/PVDF-HFP blend. Electrochim. Acta 2004, 49, 1063–1068. [Google Scholar] [CrossRef]
- Pasquier, A.D.; Warren, P.C.; Culver, D.; Gozdz, A.S.; Amatucci, G.G.; Tarascon, J.-M. Plastic PVDF-HFP electrolyte laminates prepared by a phase-inversion process. Solid State Ionics 2000, 135, 249–257. [Google Scholar] [CrossRef]
- Jung, H.R.; Lee, W.J. Electrochemical characteristics of electrospun poly (methyl methacrylate) polyvinyl chloride as gel polymer electrolytes for lithium ion battery. Electrochim. Acta 2011, 58, 674–680. [Google Scholar] [CrossRef]
- Kufian, M.Z.; Aziz, M.F.; Shukur, M.F.; Rahim, A.S.; Ariffin, N.E.; Shuhaimi, N.E.A.; Majid, S.R.; Yahya, R.; Arof, A.K. PMMA-LiBOB gel electrolyte for application in lithium ion batteries. Solid State Ionics 2012, 208, 36–42. [Google Scholar] [CrossRef]
- Liang, B.; Jiang, Q.; Tang, S.; Li, S.; Chen, X. Porous polymer electrolytes with high ionic conductivity and good mechanical property for rechargeable batteries. J. Power Sources 2016, 307, 320–328. [Google Scholar] [CrossRef]
- Evans, J.; Vincent, C.A.; Bruce, P.G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 1987, 28, 2324–2328. [Google Scholar] [CrossRef]
- Zahn, R.; Lagadec, M.F.; Hess, M.; Wood, V. Improving Ionic Conductivity and Lithium-Ion Transference Number in Lithium-Ion Battery Separators. ACS Appl. Mater. Interfaces 2016, 8, 32637–32642. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, S.; Shi, Y.; Yang, Y.; Hou, Y.; Wu, Y. A Composite Gel Polymer Electrolyte with High Performance Based on Poly (Vinylidene Fluoride) and Polyborate for Lithium Ion Batteries. Adv. Energy Mater. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Cao, H.; Song, A.; Lin, Y.; Wang, M.; Li, X. A high-performance and environment-friendly gel polymer electrolyte for lithium ion battery based on composited lignin membrane. J. Solid State Electrochem. 2017, 22, 807–816. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Yang, X.; You, J.; Xu, Y.; Wang, H.; Ma, Y.; Gao, G. Influence of additives in a PVDF-based solid polymer electrolyte on conductivity and Li-ion battery performance. Sustain. Energy Fuels 2018, 2, 492–498. [Google Scholar] [CrossRef]
- Lin, D.; Liu, W.; Liu, Y.; Lee, H.R.; Hsu, P.C.; Liu, K.; Cui, Y. High Ionic Conductivity of Composite Solid Polymer Electrolyte via In Situ Synthesis of Monodispersed SiO2 Nanospheres in Poly(ethylene oxide). Nano Lett. 2016, 16, 459–465. [Google Scholar] [CrossRef]
- Yue, H.; Li, J.; Wang, Q.; Li, C.; Zhang, J.; Li, Q.; Li, X.; Zhang, H.; Yang, S. Sandwich-Like Poly(propylene carbonate)-Based Electrolyte for Ambient-Temperature Solid-State Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2017, 6, 268–274. [Google Scholar] [CrossRef]




| Run | Solvent | p(OPal-MMA) (g) | |||||
|---|---|---|---|---|---|---|---|
| S1 | DMF/g | 5 | 6.25 | 7.5 | 8.75 | 10 | 5 |
| THF/g | 20 | 18.75 | 17.5 | 16.25 | 15 | ||
| Denoted | S1-1 | S1-2 | S1-3 | S1-4 | S1-5 | ||
| S2 | DMF/g | 5 | 6.25 | 7.5 | 8.75 | 10 | |
| Acetone/g | 20 | 18.75 | 17.5 | 16.25 | 15 | ||
| Denoted | S2-1 | S2-2 | S2-3 | S2-4 | S2-5 | ||
| S3 | DMAC/g | 2.5 | 3.75 | 5 | 6.25 | 7.5 | |
| THF/g | 22.5 | 21.25 | 20 | 18.75 | 17.5 | ||
| Denoted | S3-1 | S3-2 | S3-3 | S3-4 | S3-5 | ||
| S4 | DMAC/g | 2.5 | 3.75 | 5 | 6.25 | 7.5 | |
| Acetone/g | 22.5 | 21.25 | 20 | 18.75 | 17.5 | ||
| Denoted | S4-1 | S4-2 | S4-3 | S4-4 | S4-5 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Wang, M.; Xiong, L.; Guo, H.; Huang, C.; Zhang, H.; Chen, X. The Effect of Different Mixed Organic Solvents on the Properties of p(OPal-MMA) Gel Electrolyte Membrane for Lithium Ion Batteries. Appl. Sci. 2018, 8, 2587. https://doi.org/10.3390/app8122587
Tian L, Wang M, Xiong L, Guo H, Huang C, Zhang H, Chen X. The Effect of Different Mixed Organic Solvents on the Properties of p(OPal-MMA) Gel Electrolyte Membrane for Lithium Ion Batteries. Applied Sciences. 2018; 8(12):2587. https://doi.org/10.3390/app8122587
Chicago/Turabian StyleTian, Lanlan, Mengkun Wang, Lian Xiong, Haijun Guo, Chao Huang, Hairong Zhang, and Xinde Chen. 2018. "The Effect of Different Mixed Organic Solvents on the Properties of p(OPal-MMA) Gel Electrolyte Membrane for Lithium Ion Batteries" Applied Sciences 8, no. 12: 2587. https://doi.org/10.3390/app8122587
APA StyleTian, L., Wang, M., Xiong, L., Guo, H., Huang, C., Zhang, H., & Chen, X. (2018). The Effect of Different Mixed Organic Solvents on the Properties of p(OPal-MMA) Gel Electrolyte Membrane for Lithium Ion Batteries. Applied Sciences, 8(12), 2587. https://doi.org/10.3390/app8122587




