A Novel Markerless Lung Tumor-Tracking Method Using Treatment MV Beam Imaging
Abstract
1. Introduction
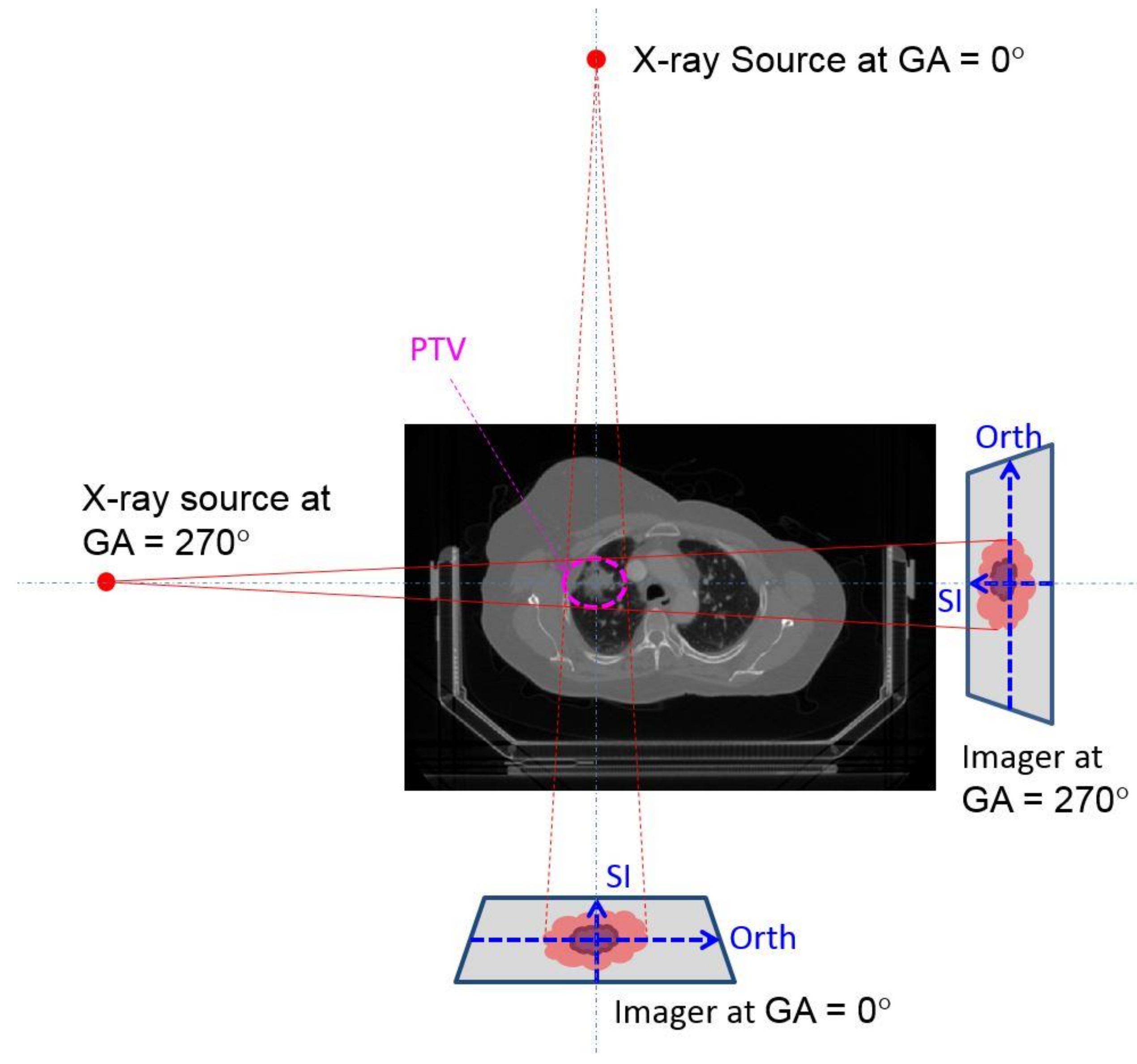
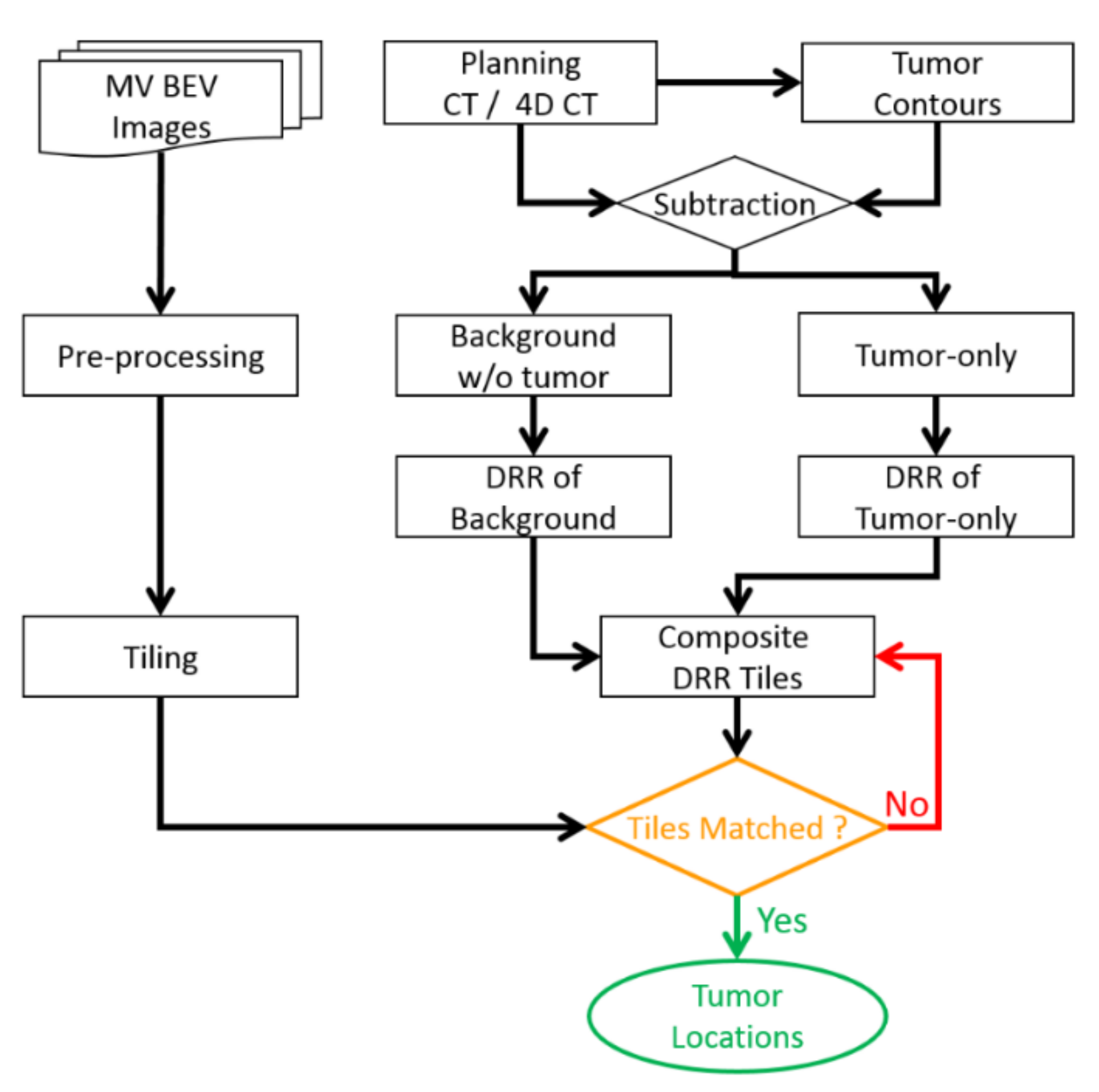
2. Materials and Method
3. Results
3.1. Phantom Study Results
3.2. Patient Data Study Results
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Gantry Angle (°) | Motion Range (mm) | Maximum Deviation (mm) | Average Deviation (mm) | ||||
|---|---|---|---|---|---|---|---|
| SI | Orth | 2D | SI | Orth | 2D | ||
| 30 | 10 | 0.4 | 0.6 | 0.6 | 0.2 | 0.2 | 0.3 |
| 12 | 0.8 | 0.6 | 0.8 | 0.3 | 0.2 | 0.5 | |
| 14 | 0.8 | 0.6 | 0.8 | 0.3 | 0.2 | 0.4 | |
| 16 | 0.8 | 0.6 | 0.8 | 0.3 | 0.3 | 0.5 | |
| 20 | 1.0 | 0.5 | 1.1 | 0.4 | 0.2 | 0.5 | |
| 45 | 10 | 0.7 | 0.7 | 0.7 | 0.2 | 0.3 | 0.4 |
| 12 | 0.7 | 0.6 | 0.9 | 0.2 | 0.3 | 0.4 | |
| 14 | 0.7 | 0.6 | 0.8 | 0.3 | 0.3 | 0.5 | |
| 16 | 0.7 | 0.7 | 0.9 | 0.4 | 0.3 | 0.5 | |
| 20 | 0.8 | 0.5 | 1.0 | 0.3 | 0.2 | 0.4 | |
| 90 | 10 | 0.7 | 0.6 | 0.8 | 0.3 | 0.2 | 0.4 |
| 12 | 0.6 | 0.7 | 0.8 | 0.2 | 0.2 | 0.4 | |
| 14 | 0.5 | 0.8 | 0.8 | 0.2 | 0.3 | 0.4 | |
| 16 | 0.9 | 0.6 | 0.9 | 0.4 | 0.2 | 0.5 | |
| 20 | 0.7 | 0.7 | 0.8 | 0.3 | 0.3 | 0.4 | |
| 150 | 10 | 0.7 | 0.8 | 0.9 | 0.2 | 0.3 | 0.5 |
| 12 | 0.7 | 0.9 | 1.1 | 0.3 | 0.4 | 0.5 | |
| 14 | 0.6 | 1.1 | 1.1 | 0.2 | 0.5 | 0.6 | |
| 16 | 0.6 | 1.3 | 1.3 | 0.3 | 0.4 | 0.6 | |
| 20 | 0.7 | 1.2 | 1.2 | 0.3 | 0.5 | 0.6 | |
| 180 | 10 | 0.4 | 0.8 | 0.9 | 0.2 | 0.3 | 0.4 |
| 12 | 0.6 | 0.6 | 0.7 | 0.2 | 0.2 | 0.3 | |
| 14 | 0.9 | 0.6 | 0.9 | 0.3 | 0.3 | 0.4 | |
| 16 | 0.8 | 0.6 | 0.8 | 0.2 | 0.2 | 0.3 | |
| 20 | 1.2 | 0.8 | 1.3 | 0.5 | 0.3 | 0.7 | |
| 220 | 10 | 0.7 | 0.5 | 0.7 | 0.2 | 0.1 | 0.3 |
| 12 | 0.7 | 0.5 | 0.8 | 0.3 | 0.2 | 0.3 | |
| 14 | 0.6 | 1.0 | 1.0 | 0.2 | 0.4 | 0.5 | |
| 16 | 0.7 | 1.0 | 1.1 | 0.3 | 0.3 | 0.5 | |
| 20 | 0.9 | 0.8 | 0.9 | 0.3 | 0.3 | 0.5 | |
| 270 | 10 | 0.9 | 0.7 | 0.9 | 0.2 | 0.3 | 0.4 |
| 12 | 0.6 | 0.5 | 0.6 | 0.2 | 0.2 | 0.3 | |
| 14 | 0.8 | 0.7 | 0.9 | 0.4 | 0.3 | 0.5 | |
| 16 | 0.8 | 0.6 | 0.9 | 0.3 | 0.2 | 0.4 | |
| 20 | 0.9 | 0.6 | 1.0 | 0.3 | 0.2 | 0.4 | |
| 315 | 10 | 0.7 | 0.7 | 0.8 | 0.3 | 0.3 | 0.5 |
| 12 | 0.8 | 0.9 | 0.9 | 0.3 | 0.4 | 0.5 | |
| 14 | 1.3 | 1.4 | 1.4 | 0.4 | 0.7 | 0.9 | |
| 16 | 1.4 | 1.6 | 1.6 | 0.7 | 0.6 | 1.0 | |
| 20 | 0.7 | 1.1 | 1.1 | 0.3 | 0.4 | 0.6 | |
| 330 | 10 | 1.1 | 1.7 | 1.7 | 0.4 | 0.7 | 0.9 |
| 12 | 0.9 | 1.0 | 1.2 | 0.4 | 0.4 | 0.6 | |
| 14 | 1.0 | 1.0 | 1.2 | 0.4 | 0.4 | 0.6 | |
| 16 | 1.5 | 1.3 | 1.5 | 0.6 | 0.5 | 0.9 | |
| 20 | 1.5 | 1.8 | 1.8 | 0.6 | 0.7 | 1.0 | |
| Gantry Angle (°) | Motion Range (mm) | Maximum Deviation (mm) | Average Deviation (mm) | ||||
|---|---|---|---|---|---|---|---|
| SI | Orth | 2D | SI | Orth | 2D | ||
| 30 | 10 | 0.6 | 0.6 | 0.8 | 0.2 | 0.2 | 0.3 |
| 12 | 0.9 | 0.8 | 0.9 | 0.3 | 0.3 | 0.5 | |
| 14 | 0.9 | 0.9 | 1.0 | 0.3 | 0.3 | 0.5 | |
| 16 | 1.0 | 0.8 | 1.0 | 0.3 | 0.3 | 0.5 | |
| 20 | 1.0 | 1.0 | 1.0 | 0.3 | 0.3 | 0.5 | |
| 45 | 10 | 0.3 | 0.7 | 0.8 | 0.2 | 0.3 | 0.4 |
| 12 | 0.7 | 0.7 | 0.9 | 0.3 | 0.3 | 0.4 | |
| 14 | 0.8 | 0.5 | 0.8 | 0.3 | 0.3 | 0.5 | |
| 16 | 0.7 | 0.5 | 0.8 | 0.2 | 0.3 | 0.4 | |
| 20 | 0.5 | 0.7 | 0.7 | 0.2 | 0.3 | 0.4 | |
| 90 | 10 | 0.7 | 0.9 | 0.9 | 0.2 | 0.2 | 0.4 |
| 12 | 0.5 | 0.9 | 0.9 | 0.2 | 0.3 | 0.4 | |
| 14 | 0.6 | 1.2 | 1.2 | 0.3 | 0.4 | 0.5 | |
| 16 | 0.6 | 1.0 | 1.0 | 0.2 | 0.4 | 0.5 | |
| 20 | 1.1 | 1.3 | 1.3 | 0.5 | 0.5 | 0.8 | |
| 150 | 10 | 0.6 | 0.7 | 0.7 | 0.1 | 0.2 | 0.3 |
| 12 | 0.7 | 0.7 | 0.7 | 0.2 | 0.3 | 0.4 | |
| 14 | 0.6 | 0.8 | 0.9 | 0.2 | 0.3 | 0.4 | |
| 16 | 0.5 | 0.8 | 0.8 | 0.2 | 0.3 | 0.4 | |
| 20 | 0.6 | 0.9 | 0.9 | 0.2 | 0.4 | 0.4 | |
| 180 | 10 | 0.4 | 1.1 | 1.1 | 0.2 | 0.5 | 0.5 |
| 12 | 0.6 | 1.0 | 1.2 | 0.3 | 0.5 | 0.6 | |
| 14 | 0.4 | 1.3 | 1.3 | 0.2 | 0.6 | 0.7 | |
| 16 | 0.8 | 0.9 | 1.3 | 0.3 | 0.4 | 0.5 | |
| 20 | 0.8 | 1.4 | 1.4 | 0.2 | 0.5 | 0.8 | |
| 220 | 10 | 0.6 | 0.9 | 1.1 | 0.3 | 0.3 | 0.5 |
| 12 | 0.6 | 1.0 | 1.0 | 0.2 | 0.3 | 0.4 | |
| 14 | 0.6 | 1.0 | 1.0 | 0.2 | 0.3 | 0.5 | |
| 16 | 0.7 | 1.0 | 1.1 | 0.3 | 0.3 | 0.5 | |
| 20 | 0.9 | 0.8 | 0.9 | 0.3 | 0.3 | 0.5 | |
| 270 | 10 | 0.6 | 0.8 | 0.8 | 0.2 | 0.3 | 0.4 |
| 12 | 0.5 | 0.7 | 0.8 | 0.2 | 0.3 | 0.3 | |
| 14 | 0.9 | 0.9 | 0.9 | 0.3 | 0.3 | 0.4 | |
| 16 | 0.7 | 0.6 | 0.8 | 0.2 | 0.2 | 0.3 | |
| 20 | 1.2 | 0.9 | 1.2 | 0.4 | 0.3 | 0.5 | |
| 315 | 10 | 0.6 | 0.6 | 0.8 | 0.2 | 0.2 | 0.3 |
| 12 | 0.8 | 0.8 | 0.9 | 0.3 | 0.3 | 0.5 | |
| 14 | 1.0 | 1.6 | 1.8 | 0.3 | 0.7 | 0.9 | |
| 16 | 1.4 | 1.6 | 1.6 | 0.7 | 0.6 | 1.0 | |
| 20 | 0.8 | 1.2 | 1.3 | 0.3 | 0.5 | 0.6 | |
| 330 | 10 | 0.6 | 0.6 | 0.6 | 0.2 | 0.2 | 0.3 |
| 12 | 0.8 | 0.7 | 0.8 | 0.3 | 0.3 | 0.4 | |
| 14 | 0.8 | 1.1 | 1.1 | 0.2 | 0.5 | 0.6 | |
| 16 | 0.7 | 1.0 | 1.1 | 0.3 | 0.4 | 0.6 | |
| 20 | 0.9 | 1.2 | 1.3 | 0.3 | 0.5 | 0.7 | |
| Patient # | Fx # | Treatment Field Gantry Angle (°) | Average Position Deviations (mm) | CP 5 mm | CP 10 mm | CP 15 mm | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 180 | 1.0 | −6.4 | 6.7 | 18% | 100% | 100% |
| 270 | −1.2 | −2.6 | 3.2 | 75% | 100% | 100% | ||
| 2 | 180 | 0.4 | −4.2 | 4.6 | 57% | 100% | 100% | |
| 270 | −1.2 | −2.6 | 3.3 | 88% | 100% | 100% | ||
| 2 | 1 | 180 | −2.6 | 7.3 | 7.9 | 0% | 97% | 100% |
| 210 | 3.6 | −3.2 | 4.9 | 61% | 100% | 100% | ||
| 2 | 180 | −1.8 | 7.1 | 7.5 | 0% | 88% | 100% | |
| 210 | 4.5 | −4.2 | 6.1 | 0% | 100% | 100% | ||
| 3 | 1 | 20 | 3.2 | −6.3 | 7.2 | 0% | 100% | 100% |
| 60 | −0.2 | −7.7 | 7.7 | 8% | 84% | 100% | ||
| 120 | 0.4 | 0.6 | 1.2 | 100% | 100% | 100% | ||
| 220 | −0.3 | 7.6 | 7.6 | 0% | 97% | 100% | ||
| 270 | −0.6 | 0.3 | 3.9 | 69% | 100% | 100% | ||
| 2 | 20 | 1.9 | 3.2 | 3.8 | 82% | 100% | 100% | |
| 60 | 1.6 | 1.6 | 3.1 | 94% | 100% | 100% | ||
| 120 | 0.3 | −0.5 | 0.7 | 100% | 100% | 100% | ||
| 220 | −0.3 | −2.7 | 3.8 | 65% | 100% | 100% | ||
| 270 | −2.0 | 0.7 | 3.3 | 100% | 100% | 100% | ||
| 3 | 20 | −1.7 | −0.4 | 2.1 | 100% | 100% | 100% | |
| 60 | 0.0 | −8.4 | 8.4 | 4% | 76% | 100% | ||
| 120 | −1.0 | 3.3 | 3.8 | 66% | 100% | 100% | ||
| 220 | −0.8 | 6.9 | 7.1 | 12% | 95% | 100% | ||
| 270 | 1.7 | −0.7 | 4.0 | 73% | 100% | 100% | ||
| 4 | 1 | 80 | 1.0 | 9.8 | 9.9 | 80% | 100% | 100% |
| 115 | −3.1 | −5.1 | 6.0 | 28% | 100% | 100% | ||
| 155 | −8.1 | 0.5 | 8.2 | 100% | 100% | 100% | ||
| 295 | −4.3 | 0.5 | 4.3 | 76% | 100% | 100% | ||
| 325 | −5.3 | 0.1 | 5.4 | 17% | 100% | 100% | ||
| 2 | 80 | 0.7 | −3.3 | 3.4 | 80% | 100% | 100% | |
| 115 | 0.1 | −1.4 | 2.3 | 98% | 100% | 100% | ||
| 155 | −2.5 | −0.2 | 2.7 | 100% | 100% | 100% | ||
| 295 | −0.8 | 1.7 | 1.8 | 100% | 100% | 100% | ||
| 325 | 1.1 | −1.4 | 2.5 | 92% | 100% | 100% | ||
| 5 | 1 | 25 | 1.4 | 2.4 | 5.2 | 55% | 100% | 100% |
| 55 | −0.1 | −2.6 | 3.4 | 78% | 100% | 100% | ||
| 180 | 3.7 | 0.8 | 4.6 | 59% | 100% | 100% | ||
| 220 | −2.8 | −0.7 | 3.8 | 71% | 97% | 100% | ||
| 270 | 0.5 | −8.2 | 9.0 | 0% | 75% | 100% | ||
| 340 | −0.4 | −2.0 | 3.6 | 81% | 100% | 100% | ||
| 2 | 25 | −2.6 | 6.8 | 8.4 | 1% | 85% | 100% | |
| 55 | 4.5 | −3.8 | 7.0 | 20% | 92% | 100% | ||
| 180 | −2.5 | −6.4 | 7.5 | 20% | 90% | 100% | ||
| 270 | −6.1 | −8.2 | 10.3 | 2% | 43% | 98% | ||
| 340 | 0.7 | 2.5 | 3.9 | 80% | 100% | 100% | ||
| 3 | 25 | 1.3 | 6.3 | 7.1 | 2% | 97% | 100% | |
| 55 | 4.8 | −7.4 | 9.4 | 2% | 67% | 100% | ||
| 180 | 5.3 | −0.6 | 5.5 | 43% | 100% | 100% | ||
| 220 | −1.6 | −5.1 | 6.1 | 44% | 86% | 99% | ||
| 270 | 6.8 | −5.8 | 9.6 | 4% | 54% | 100% | ||
References
- Seppenwoolde, Y.; Shirato, H.; Kitamura, K.; Shimizu, S.; van Herk, M.; Lebesque, J.V.; Miyasaka, K. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 822–834. [Google Scholar] [CrossRef]
- Shirato, H.; Suzuki, K.; Sharp, G.C.; Fujita, K.; Onimaru, R.; Fujino, M.; Kato, N.; Osaka, Y.; Kinoshita, R.; Taguchi, H.; et al. Speed and amplitude of lung tumor motion precisely detected in four-dimensional setup and in real-time tumor-tracking radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.B. Radiotherapy of mobile tumors. Semin. Radiat. Oncol. 2006, 16, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.J. Tracking moving organs in real time. Semin. Radiat. Oncol. 2004, 14, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Keall, P.J.; Mageras, G.S.; Balter, J.M.; Emery, R.S.; Forster, K.M.; Jiang, S.B.; Kapatoes, J.M.; Low, D.A.; Murphy, M.J.; Murray, B.R.; et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med. Phys. 2006, 33, 3874–3900. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Richter, A.; Baier, K.; Wilbert, J.; Guckenberger, M.; Flentje, M. Tracking moving objects with megavoltage portal imaging: A feasibility study. Med. Phys. 2006, 33, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Arimura, H.; Egashira, Y.; Shioyama, Y.; Nakamura, K.; Yoshidome, S.; Anai, S.; Nomoto, S.; Honda, H.; Toyofuku, F.; Higashida, Y.; et al. Computerized method for estimation of the location of a lung tumor on EPID cine images without implanted markers in stereotactic body radiotherapy. Phys. Med. Biol. 2009, 54, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Poels, K.; Verellen, D.; Van de Vondel, I.; El Mazghari, R.; Depuydt, T.; De Ridder, M. Fiducial marker and marker-less soft-tissue detection using fast MV fluoroscopy on a new generation EPID: Investigating the influence of pulsing artifacts and artifact suppression techniques. Med. Phys. 2014, 41, 101911. [Google Scholar] [CrossRef] [PubMed]
- Rottmann, J.; Aristophanous, M.; Chen, A.; Court, L.; Berbeco, R. A multi-region algorithm for markerless beam’s-eye view lung tumor tracking. Phys. Med. Biol. 2010, 55, 5585–5598. [Google Scholar] [CrossRef] [PubMed]
- Rottmann, J.; Keall, P.; Yue, Y.; Berbeco, R. Real-Time Markerless Tumor Tracking with MV Imaging and a Dynamic Multi-Leaf Collimator (DMLC). Med. Phys. 2012, 39, 3890. [Google Scholar] [CrossRef]
- Bryant, J.H.; Rottmann, J.; Lewis, J.H.; Keall, P.J.; Berbeco, R.I. Registration of Clinical Volumes to Beams-Eye-View Images for Real-Time Tracking. Med. Phys. 2013, 40, 471. [Google Scholar] [CrossRef]
- Rottmann, J.; Berbeco, R. Predictor Model Training for Real-Time Motion Management of Lung Tumors. Med. Phys. 2013, 40, 410–411. [Google Scholar] [CrossRef]
- Rottmann, J.; Keall, P.; Berbeco, R. Real-time soft tissue motion estimation for lung tumors during radiotherapy delivery. Med. Phys. 2013, 40, 091713. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Li, R.J.; Mak, R.H.; Rottmann, J.; Bryant, J.H.; Williams, C.L. An initial study on the estimation of time-varying volumetric treatment images and 3D tumor localization from single MV cine EPID images. Med. Phys. 2014, 41, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.; Rottmann, J.; Berbeco, R. The impact of cine EPID image acquisition frame rate on markerless soft-tissue tracking. Med. Phys. 2014, 41, 061702. [Google Scholar] [CrossRef]
- Yip, S.; Rottmann, I.; Chen, H.; Morf, D.; Fueglistaller, R.; Star-Lack, J. Combination of Multiple EPID Imager Layers Improves Image Quality and Tracking Performance of Low Contrast Objects. Med. Phys. 2015, 42, 3742. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Homma, N.; Ichiji, K.; Takai, Y.; Yoshizawa, M. Tracking tumor boundary in MV-EPID images without implanted markers: A feasibility study. Med. Phys. 2015, 42, 2510–2523. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; Bereg, S.; Yan, Y.L.; Chiu, T.C.; Liu, H.H.; Kearney, V. An accurate algorithm to match imperfectly matched images for lung tumor detection without markers. J. Appl. Clin. Med. Phys. 2015, 16, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Zuiderveld, K. Contrast Limited Adaptive Histogram Equalization; Heckbert, P.S., Ed.; Morgan Kaufmann: Burlington, MA, USA, 1994; pp. 474–485. [Google Scholar]
- Yang, Y.; Zhong, Z.C.; Guo, X.H.; Wang, J.; Anderson, J.; Solberg, T. A Novel Markerless Technique to Evaluate Daily Lung Tumor Motion Based on Conventional Cone-Beam CT Projection Data. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, E749–E756. [Google Scholar] [CrossRef] [PubMed]



| Patient # | GTV Size (cm3) | Tumor Location | Abdomen Compression | Motion Range (mm) |
|---|---|---|---|---|
| 1 | 5.1 | right upper lobe | No | 13.8 |
| 2 | 32.0 | left upper lobe | No | 6.1 |
| 3 | 17.6 | left lower lobe | Yes | 8.5 |
| 4 | 86.4 | left lower lobe | Yes | 3.7 |
| 5 | 93.7 | left upper lobe | Yes | 15.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozario, T.; Chiu, T.D.; Chen, M.; Jia, X.; Lu, W.; Bereg, S.; Mao, W. A Novel Markerless Lung Tumor-Tracking Method Using Treatment MV Beam Imaging. Appl. Sci. 2018, 8, 2525. https://doi.org/10.3390/app8122525
Rozario T, Chiu TD, Chen M, Jia X, Lu W, Bereg S, Mao W. A Novel Markerless Lung Tumor-Tracking Method Using Treatment MV Beam Imaging. Applied Sciences. 2018; 8(12):2525. https://doi.org/10.3390/app8122525
Chicago/Turabian StyleRozario, Timothy, Tsuicheng D. Chiu, Mingli Chen, Xun Jia, Weiguo Lu, Sergey Bereg, and Weihua Mao. 2018. "A Novel Markerless Lung Tumor-Tracking Method Using Treatment MV Beam Imaging" Applied Sciences 8, no. 12: 2525. https://doi.org/10.3390/app8122525
APA StyleRozario, T., Chiu, T. D., Chen, M., Jia, X., Lu, W., Bereg, S., & Mao, W. (2018). A Novel Markerless Lung Tumor-Tracking Method Using Treatment MV Beam Imaging. Applied Sciences, 8(12), 2525. https://doi.org/10.3390/app8122525





