Online Determination of Boron Isotope Ratio in Boron Trifluoride by Infrared Spectroscopy
Abstract
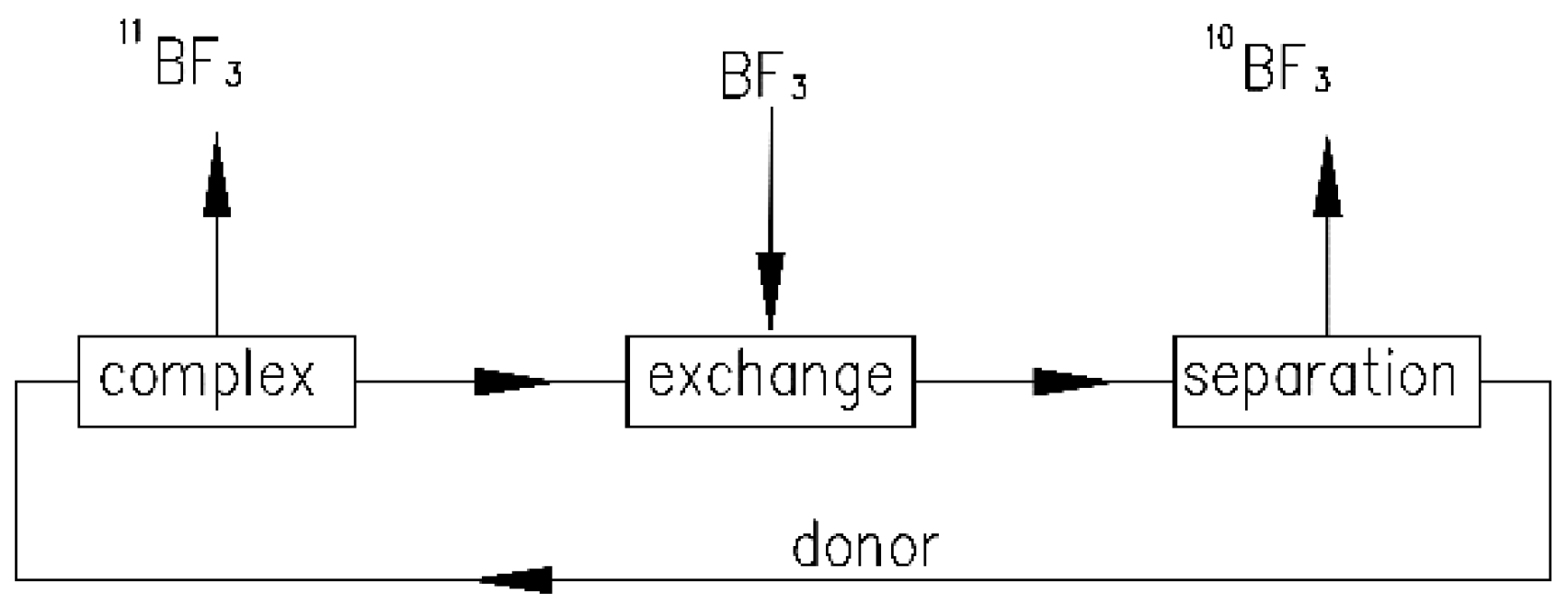
1. Introduction
2. Experimental Method
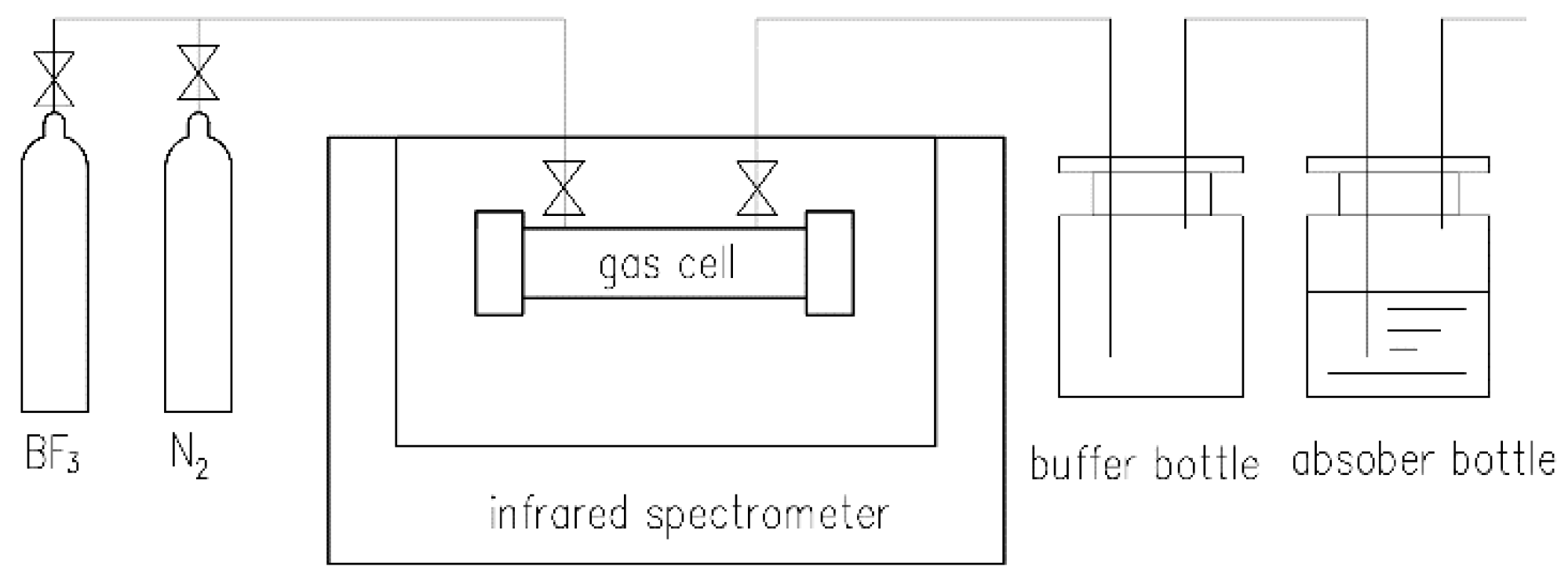
2.1. Instruments and Reagents
2.2. Procedure for Offline Measurement
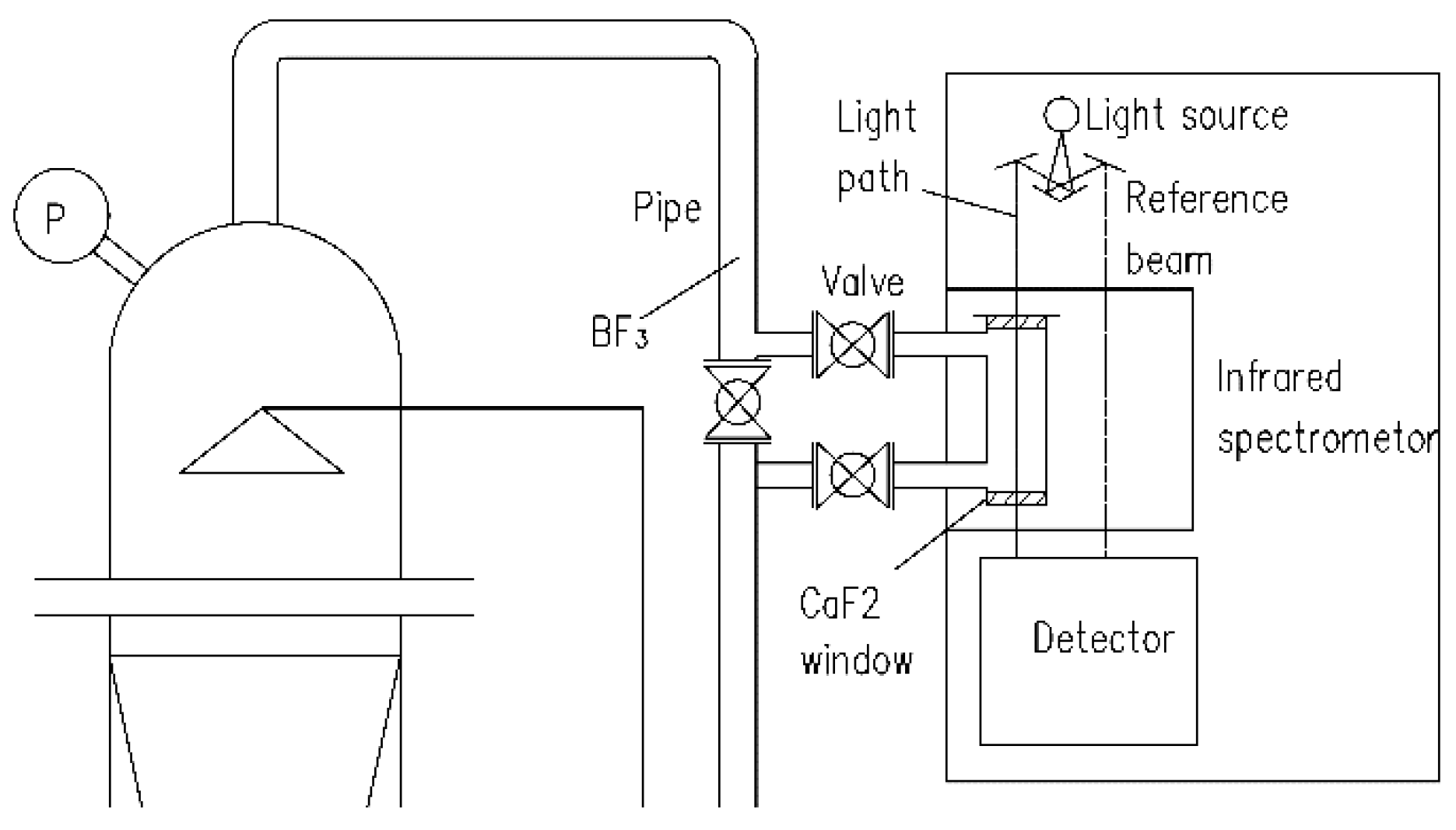
2.3. Procedure for Online Measurement
2.4. Testing method of ICP-MS
3. Results and Discussion
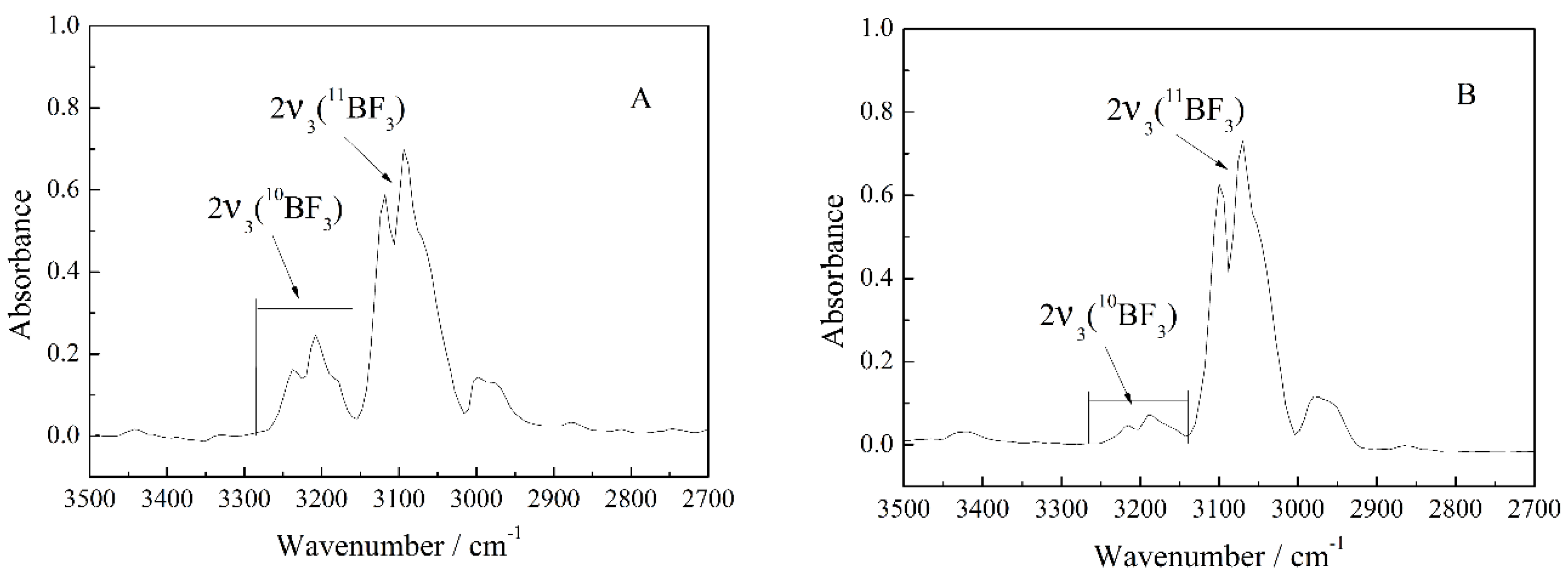
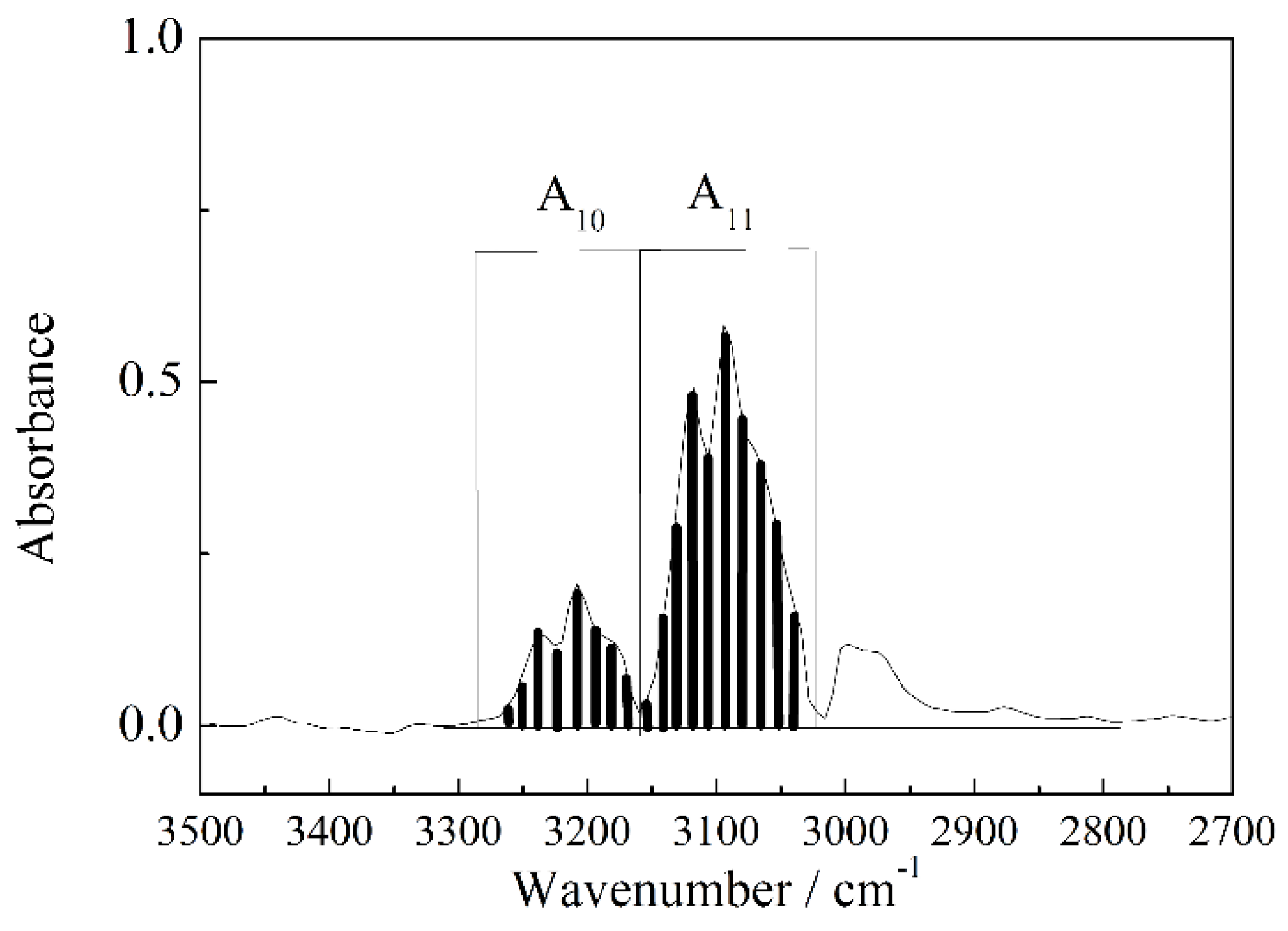
3.1. Calculation and Correction of the Isotope Ratio
3.2. Precision Experiment for the IR Method
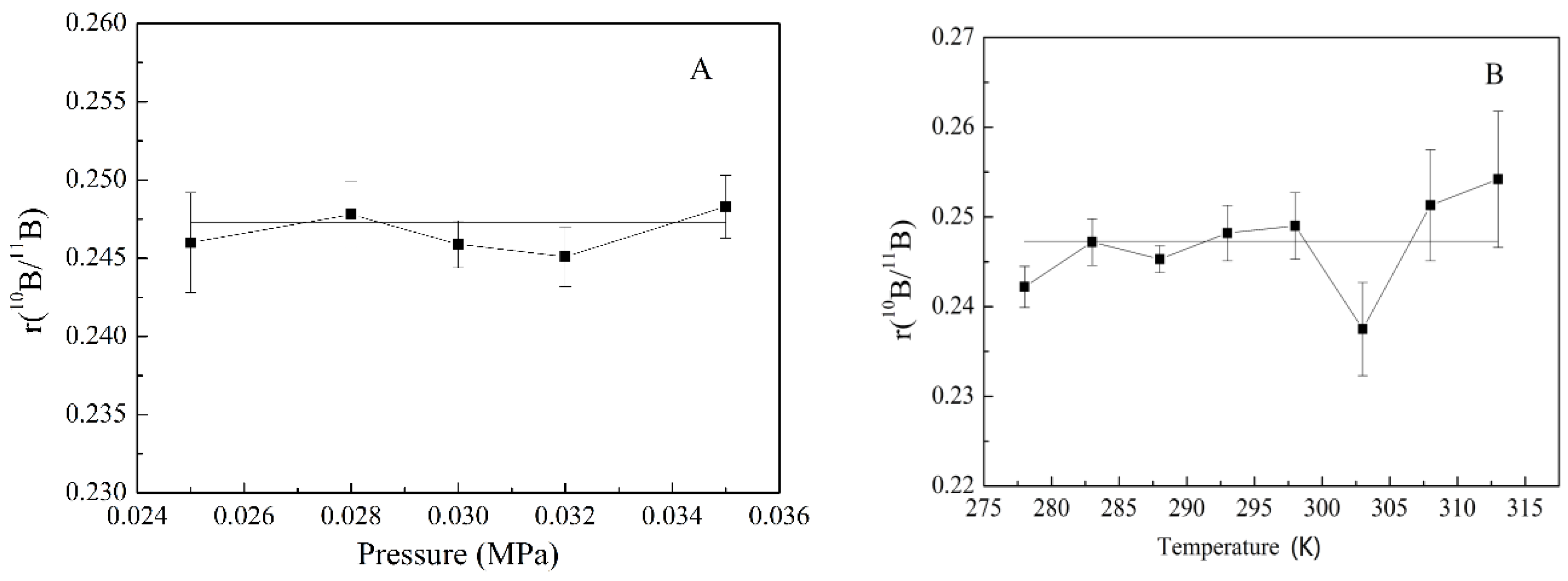
3.3. Influence of the Pressure and Temperature
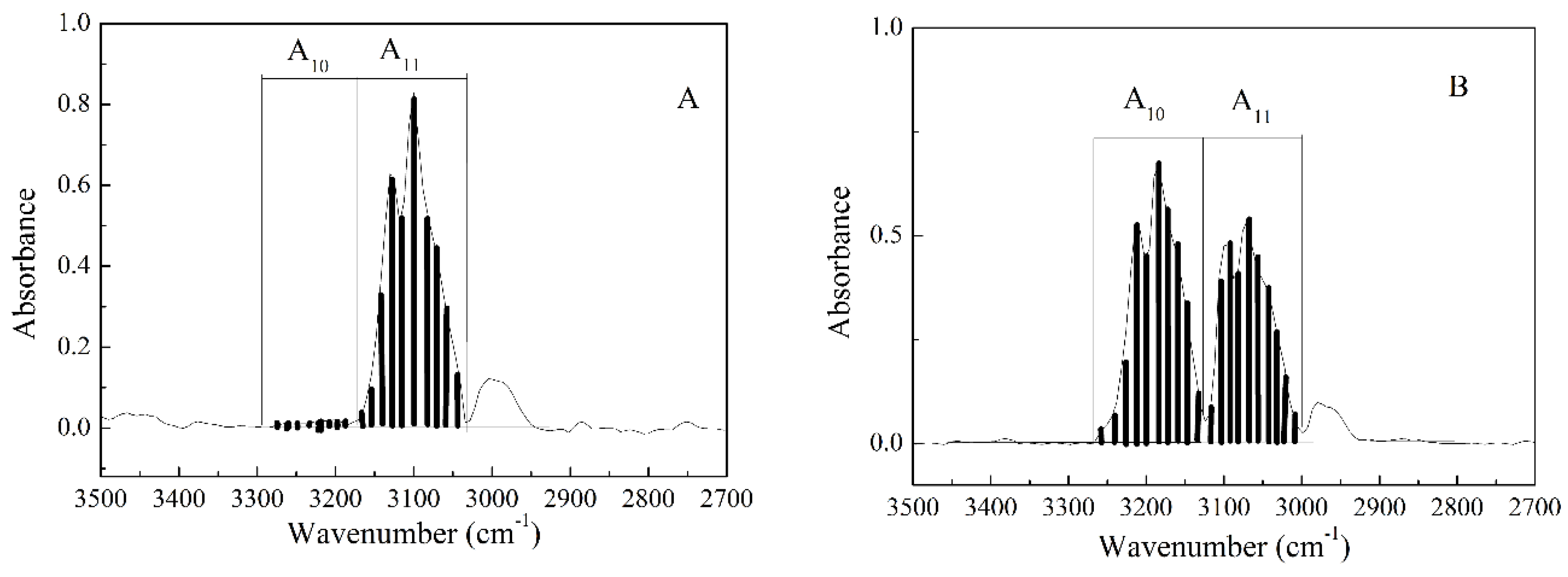
3.4. Investigation of the Enriched BF3 Gas by IR
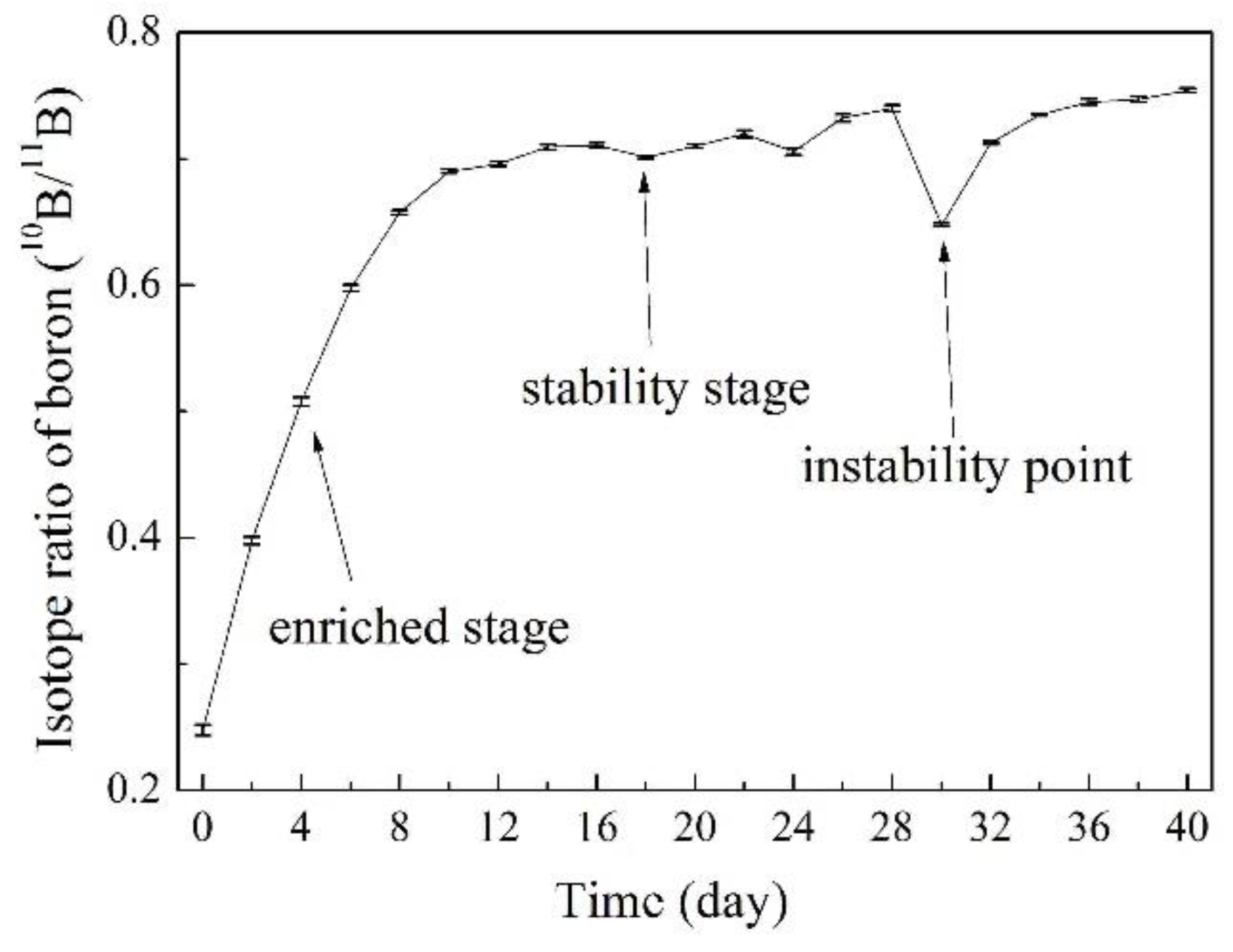
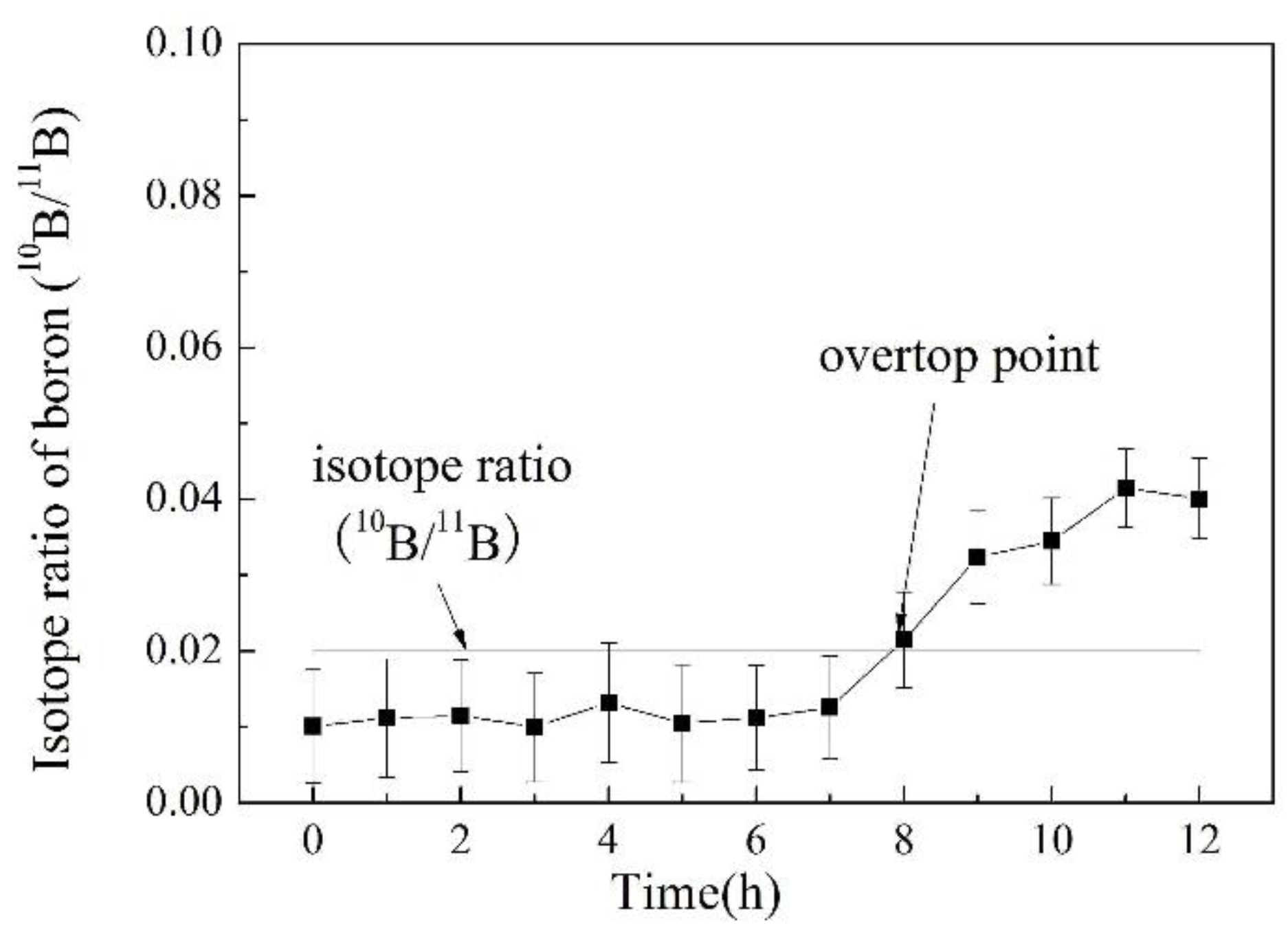
3.5. Online Determination of Boron Isotope Ratio
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.; Zhang, W.J.; Xu, J.; Ren, X. Synthesis of enriched 10B boric acid of nuclear grade. Trans. Tianjin Univ. 2014, 6, 458–462. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, T.Y.; Xu, J. Preparation and characterization of 10B boric acid with high purity for nuclear industry. Springerplus 2016, 1, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.H.; Miranda, M.C.; Rocha, Z.; Leal, A.S.; Comes, D.A.; Sousa, E.M.B. An Assessment of the Potential Use of BNNTs for Boron Neutron Capture Therapy. Nanomaterials 2017, 4, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Conn, A.L.; Wolf, J.E. Large-scale separation of boron isotopes. Ind. Eng. Chem. 1958, 9, 1231–1234. [Google Scholar] [CrossRef]
- Sevryugova, N.N.; Uvarov, O.V.; Zhavoronkov, N.M. Determination of the separation coefficients of the isotopes of boron in the equilibrium evaporation of BCl3. Sov. J. Atomic Energy 1956, 4, 567–572. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.J.; Xu, J.; Li, B. Preparation of boric-10 acid applied in nuclear industry. Trans. Tianjin Univ. 2015, 2, 172–177. [Google Scholar] [CrossRef]
- Ivanov, V.A.; Katalnikov, S.G. Physico-Chemical and engineering principles of boron isotopes separation by using BF3-Anisole•BF3 system. Sep. Sci. Technol. 2001, 8–9, 1737–1768. [Google Scholar] [CrossRef]
- Yoneda, Y.; Uchijima, T.; Makishima, S. Separation of boron isotopes by ion exchange. J. Phys. Chem. 1959, 12, 2057–2058. [Google Scholar] [CrossRef]
- Oi, T.; Tsukamoto, T.; Akai, H.; Kakihana, H.; Hosoe, M. Boron isotope separation by ion-exchange chromatography using an anion-exchange resin in halide forms: Separation factors at 25 °C. J. Chromatogr. A 1988, 3, 343–352. [Google Scholar] [CrossRef]
- Rockwood, S.; Rabideau, S. Boron isotope separation by laser-induced photochemistry. IEEE J. Quantum Electron. 1974, 9, 789–790. [Google Scholar] [CrossRef]
- Lyman, J.L.; Rockwood, S.D. Enrichment of boron, carbon, and silicon isotopes by multiple-photon absorption of doi:10.6-μm laser radiation. J. Appl. Phys. 1976, 2, 595–601. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, W.J.; Han, M.; Han, L.G.; Zhang, X.M.; Zhang, S.F. Operational policy of the boron isotopes separation by chemical exchange reaction and distillation. Chem. Eng. Process. 2008, 1, 17–21. [Google Scholar] [CrossRef]
- Katal’nikov, S.G.; Paramonov, P.M.; Nedzvetskii, V.S. Separation of boron isotopes using isotopic exchange between BF3 and a complex of BF3 with phenetole. Sov. J. Atomic Energy 1967, 4, 372–377. [Google Scholar] [CrossRef]
- Lecuyer, C.; Grandjean, P.; Reynard, B.; Albarede, C.; Telouk, P. 11B/10B analysis of geological materials by ICP–MS Plasma 54: Application to the boron fractionation between brachiopod calcite and seawater. Chem. Geol. 2002, 1, 45–55. [Google Scholar] [CrossRef]
- Porteous, N.C.; Walsh, J.N.; Jarvis, K.E. Measurement of boron isotope ratios in groundwater studies. Analyst 1995, 5, 1397–1400. [Google Scholar] [CrossRef]
- Hepburn, C.A.; Vale, P.; Brown, A.S.; Simms, N.J.; McAdam, E.J. Development of on-line FTIR spectroscopy for siloxane detection in biogas to enhance carbon contactor management. Talanta 2015, 141, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Herrebout, W.A.; Lundell, J.; Van der Veken, B.J. Van der Waals complexes between unsaturated hydrocarbons and boron trifluoride: An infrared and ab initio study of ethyne BF3, propyne BF3 and propyne (BF3)2. J. Mol. Struct. 1999, 480, 489–493. [Google Scholar] [CrossRef]
- Herrebout, W.A.; Van der Veken, B.J. Vibrational spectra and relative stabilities of the van der Waals complexes of boron trifluoride with cis-2-butene, trans-2-butene and 2-methyl propene. J. Mol. Struct. 2000, 550, 389–398. [Google Scholar] [CrossRef]
- Rayón, V.M.; Sordo, J.A. Van der Waals complexes between boron trifluoride and carbon monoxide: A theoretical study. J. Phys. Chem. A 1997, 40, 7414–7419. [Google Scholar] [CrossRef]
| Item | Parameter | Item | Parameter |
|---|---|---|---|
| Nebulizer | Microconcentric Nebulizer | Sensitivity/s−1 (μg·L−1)−1 | 1.759 × 106 CPS |
| Spray chamber temperature/°C | 3 | Scanning mode | Peak-jump |
| Nebulizer gas flow/L·min−1 | 0.93 | Dwell time/ms | 10 |
| Auxiliary gas flow/L·min−1 | 0.9 | Acquisition degree | 10 |
| Cool gas flow/L·min−1 | 10 | Acquisition time/s | 20 |
| Plasma power/W | 1250 | Channels per AMU | 3 |
| Resolution | Standard | Runs/replicates | 3 |
| Sample uptake rate/mL·min−1 | 1 | Sample depth/mm | 104 |
| Ionization lens parameters | L1 3.8; L2 31.8; L3 189.8 | - | - |
| 10B/11B Results of ICP-MS | Mean Value | 10B/11B Value of Standard Material | Relative Error/% | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| 0.2471 | 0.2464 | 0.2469 | 0.2468 | 0.2469 | 0.2475 | 0.2469 | 0.2473 ± 0.0002 | 0.16 |
| A10/A11 (by IR Method) | Mean Value | RSD/% | 10B/11B (by ICP-MS 1) | K | ||
|---|---|---|---|---|---|---|
| 0.4508 | 0.4513 | 0.4634 | 0.4553 | 1.16 | 0.2473 ± 0.0009 | 0.5431 |
| 0.4585 | 0.4601 | 0.4512 | ||||
| 0.4535 | 0.4498 | 0.4515 | ||||
| 0.4626 | ||||||
| 10B/11B (by ICP-MS) | Sample (10B/11B, by IR Method) | Mean Value | S | RSD/% | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
| 1.2351 | 1.2290 | 1.2382 | 1.2401 | 1.2320 | 1.2355 | 1.2413 | 1.2360 | 0.0048 | 0.39 |
| 0.2473 | 0.2405 | 0.2428 | 0.2451 | 0.2422 | 0.2506 | 0.2472 | 0.2447 | 0.0037 | 1.52 |
| 0.0506 | 0.0559 | 0.0532 | 0.0485 | 0.0511 | 0.0463 | 0.0509 | 0.0510 | 0.0034 | 6.64 |
| Series No. | Isotope Ratio of 10B/11B | |
|---|---|---|
| Results of IR Method | Results of ICP-MS | |
| 1 | 1.6385 ± 0.0043 | 1.6390 ± 0.0006 |
| 2 | 0.7536 ± 0.0045 | 0.7546 ± 0.0010 |
| 3 | 0.4815 ± 0.0038 | 0.4832 ± 0.0009 |
| 4 | 0.0915 ± 0.0059 | 0.0905 ± 0.0010 |
| 5 | 0.0504 ± 0.0063 | 0.0488 ± 0.0012 |
| 6 | 0.0120 ± 0.0079 | 0.0102 ± 0.0007 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Tang, Y.; Xu, J. Online Determination of Boron Isotope Ratio in Boron Trifluoride by Infrared Spectroscopy. Appl. Sci. 2018, 8, 2509. https://doi.org/10.3390/app8122509
Zhang W, Tang Y, Xu J. Online Determination of Boron Isotope Ratio in Boron Trifluoride by Infrared Spectroscopy. Applied Sciences. 2018; 8(12):2509. https://doi.org/10.3390/app8122509
Chicago/Turabian StyleZhang, Weijiang, Yin Tang, and Jiao Xu. 2018. "Online Determination of Boron Isotope Ratio in Boron Trifluoride by Infrared Spectroscopy" Applied Sciences 8, no. 12: 2509. https://doi.org/10.3390/app8122509
APA StyleZhang, W., Tang, Y., & Xu, J. (2018). Online Determination of Boron Isotope Ratio in Boron Trifluoride by Infrared Spectroscopy. Applied Sciences, 8(12), 2509. https://doi.org/10.3390/app8122509




