Composite Hydrogels with the Simultaneous Release of VEGF and MCP-1 for Enhancing Angiogenesis for Bone Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microspheres Preparation
2.3. Composite Hydrogels Preparation
2.4. In Vitro Cytocompatibility
2.5. In Vivo Studies
2.5.1. Animal Experiments
2.5.2. X-Ray Scanning
2.5.3. Histological Analysis
2.6. Statistical Analysis
3. Results
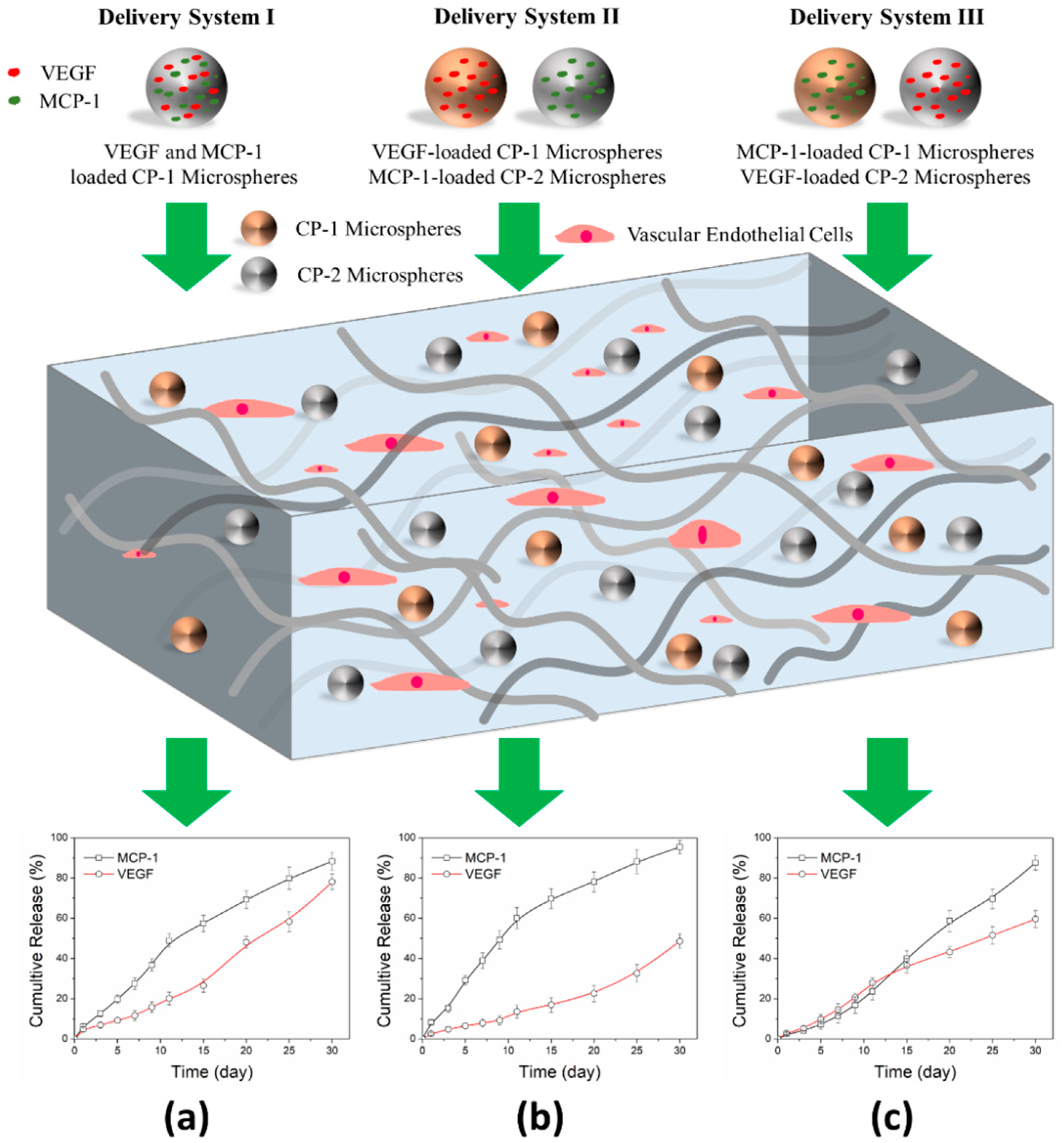
3.1. Release Delivery of VEGF and MCP-1
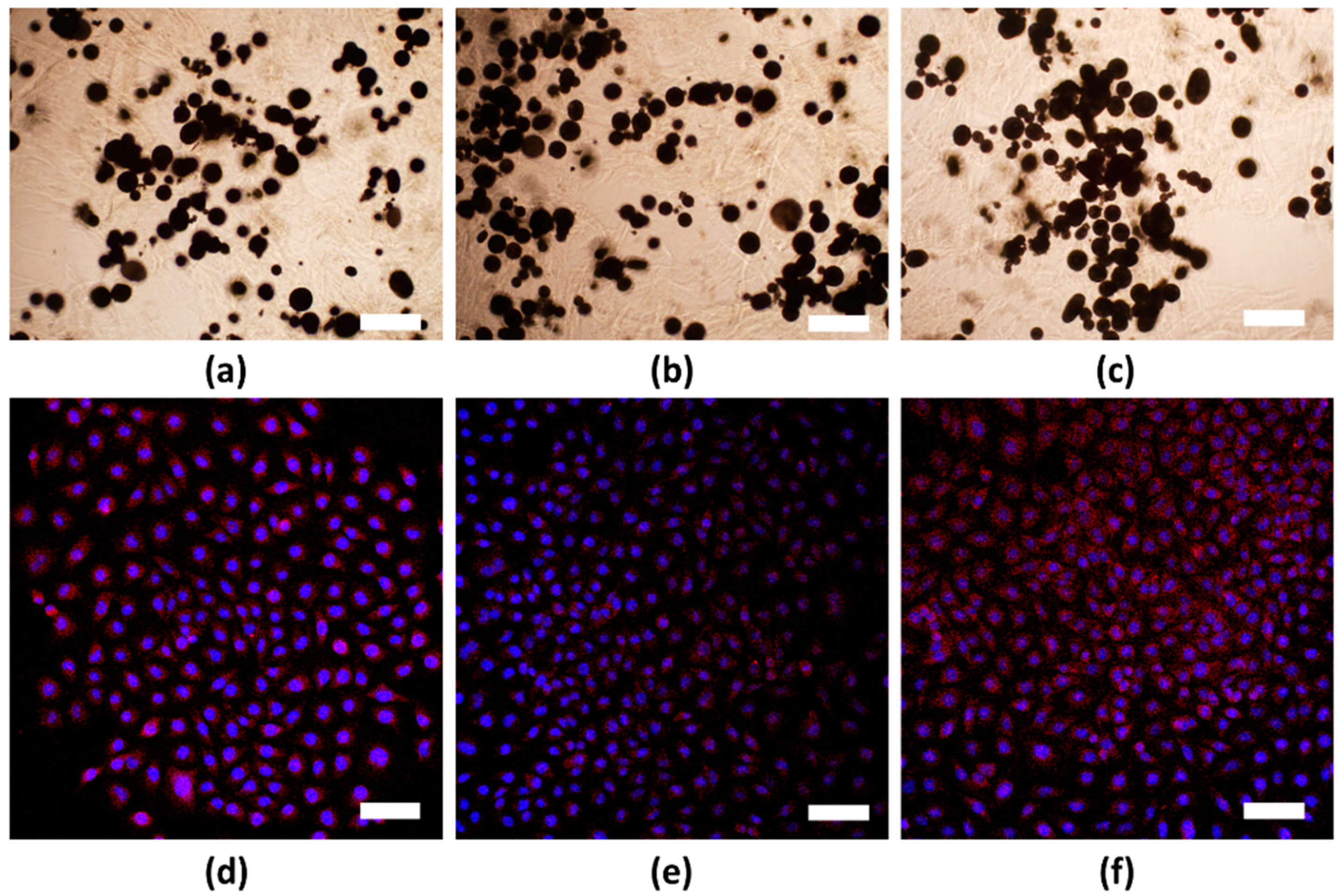
3.2. In Vitro Cell Viability
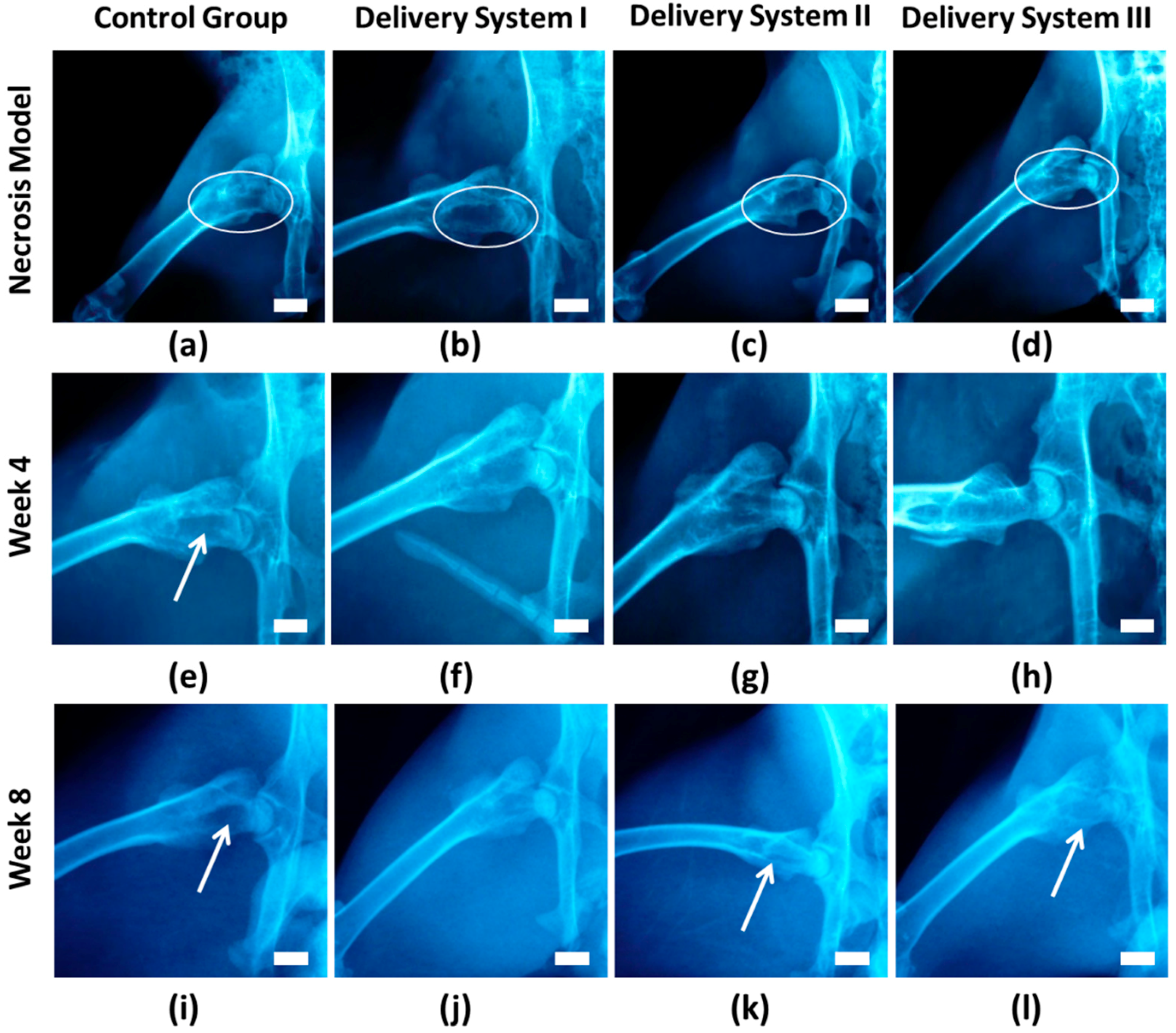
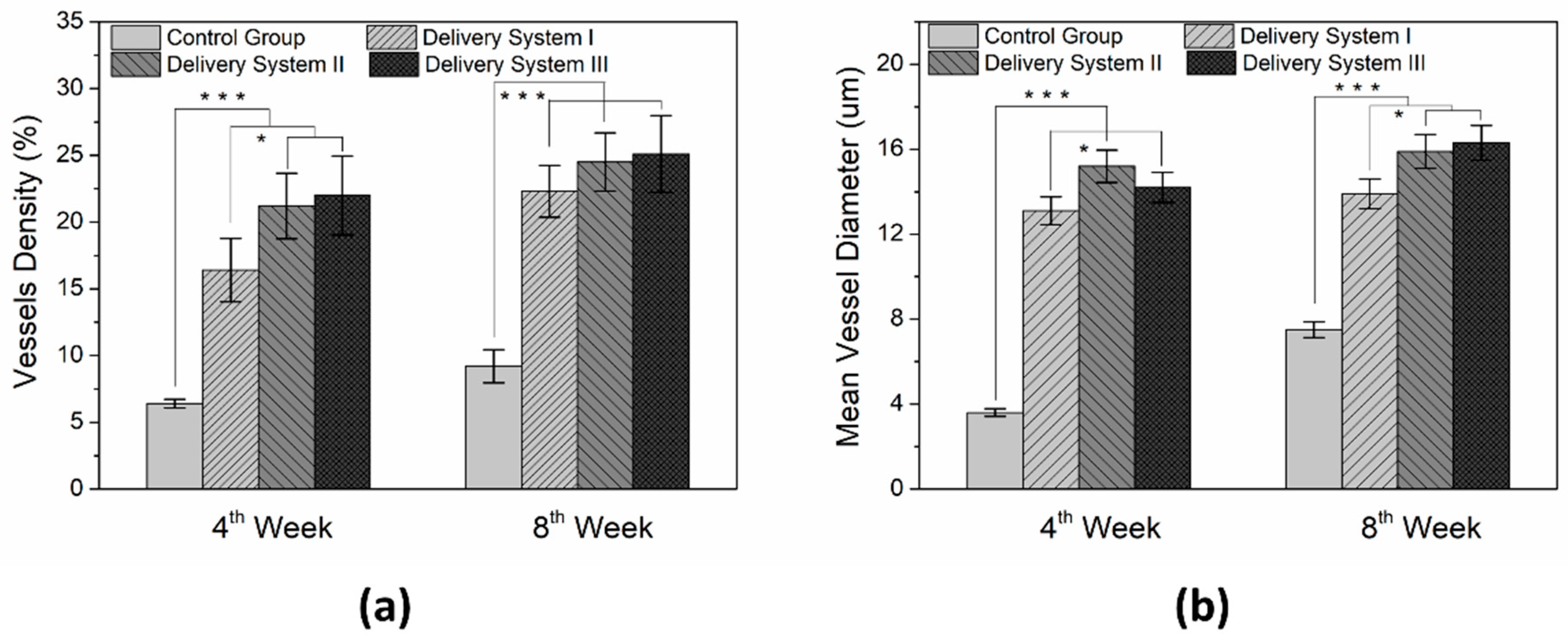
3.3. In Vivo Animal Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rezaeeyazdi, M.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable hyaluronic Acid-co-Gelatin cryogels for tissue-engineering applications. Materials 2018, 11, 1374. [Google Scholar] [CrossRef] [PubMed]
- Shrivats, A.R.; McDermott, M.C.; Hollinger, J.O. Bone tissue engineering: State of the union. Drug Discov. Today 2014, 19, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Ferreira, A.M.; Callaghan, J.T.; Miller, C.A.; Atkinson, J.; Freeman, C.; Hatton, P.V. Multilayer nanoscale encapsulation of biofunctional peptides to enhance bone tissue regeneration in vivo. Adv. Healthc. Mater. 2017, 6, 1601182. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; EI-Fiqi, A.; Kim, H.W. Synergetic cues of bioactive nanoparticles and nanofibrous structure in bone scaffolds to stimulate osteogenesis and angiogenesis. ACS Appl. Mater. Interfaces 2017, 9, 2059–2073. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Jay, S.M.; Shepherd, B.R.; Andrejecsk, J.W.; Kyriakides, T.R.; Pober, J.S.; Saltzman, W.M. Dual delivery of VEGF and MCP-1 to support endothelial cell transplantation for therapeutic vascularization. Biomaterials 2010, 31, 3054–3062. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Mooney, D.J. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Adv. Drug Deliver. Rev. 2007, 59, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliver. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.; Riedemann, L.; Amoozgar, Z.; Seano, G.; Susek, K.; Yu, V.; Dalvie, N.; Amelung, R.L.; Datta, M.; Song, J.W.; et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc. Natl. Acad. Sci. USA 2016, 201525360. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Storkebaum, E.; de Almodóvar, C.R.; Dewerchin, M.; Carmeliet, P. Vascular endothelial growth factor: A neurovascular target in neurological diseases. Nat. Rev. Neurol. 2016, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Dor, Y.; Djonov, V.; Abramovitch, R.; Itin, A.; Fishman, G.I.; Carmeliet, P.; Goelman, G.; Keshet, E. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 2002, 21, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Beamer, B.; Hettrich, C.; Lane, J. Vascular endothelial growth factors: An essential component of angiogenesis and fracture healing. HSS J. 2010, 6, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Klontzas, M.E.; Kenanidis, E.I.; MacFarlane, R.J.; Michail, T.; Potoupnis, M.E.; Heliotis, M.; Mantalaris, A.; Tsiridis, E. Investigational drugs for fracture healing: Preclinical & clinical data. Expert Opin. Inv. Drug. 2016, 25, 585–596. [Google Scholar] [CrossRef]
- Barati, D.; Shariati, S.R.P.; Moeinzadeh, S.; Melero-Martin, J.M.; Khademhosseini, A.; Jabbari, E. Spatiotemporal release of BMP-2 and VEGF enhances osteogenic and vasculogenic differentiation of human mesenchymal stem cells and endothelial colony-forming cells co-encapsulated in a patterned hydrogel. J. Control. Release 2016, 223, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001, 19, 1029. [Google Scholar] [CrossRef] [PubMed]
- Amirian, J.; Linh, N.T.B.; Min, Y.K.; Lee, B.T. Bone formation of a porous Gelatin-Pectin-biphasic calcium phosphate composite in presence of BMP-2 and VEGF. Int. J. Biol. Macromol. 2015, 76, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Awada, H.K.; Johnson, N.R.; Wang, Y. Sequential delivery of angiogenic growth factors improves revascularization and heart function after myocardical infarction. J. Control. Release 2015, 207, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Nie, L.; Zou, P.; Suo, J. Effects of drug and polymer molecular weight on drug release from PLGA-mPEG microspheres. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Nie, L.; Zhang, G.; Hou, R.; Xu, H.; Li, Y.; Fu, J. Controllable promotion of chondrocyte adhesion and growth on PVA hydrogels by controlled release of TGF-β1 from porous PLGA microspheres. Colloid Surface B 2015, 125, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Ou, K.L.; Mao, C.; Hosseinkhani, H. Importance of dual delivery systems for bone tissue engineering. J. Control. Release 2016, 225, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Kim, J.H.; Singh, R.K.; Jang, J.H.; Kim, H.W. Therapeutic-designed electrospun bone scaffolds: Mesoporous bioactive nanocarriers in hollow fiber composites to sequentially deliver dual growth factors. Acta Biomater. 2015, 16, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, C.; Huo, H.; Ji, C.; Sun, M.; Nie, L. Injectable temperature-sensitive hydrogel with VEGF loaded microspheres for vascularization and bone regeneration of femoral head necrosis. Mater. Lett. 2018, 229, 138–141. [Google Scholar] [CrossRef]
- Nie, L.; Zou, P.; Feng, S.; Suo, J. Temperature-sensitive star-shaped block copolymers hydrogels for an injection application: Phase transition behavior and biocompatibility. J. Mater. Sci. Mater. Med. 2013, 24, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Chen, D.; Suo, J.; Zou, P.; Feng, S.; Yang, Q.; Yang, S.; Ye, S. Physicochemical characterization and biocompatibility in vitro of biphasic calcium phosphate/polyvinyl alcohol scaffolds prepared by freeze-drying method for bone tissue engineering applications. Colloid Surface B 2012, 100, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Chen, D.; Fu, J.; Yang, S.; Hou, R.; Suo, J. Macroporous biphasic calcium phosphate scaffolds reinforced by poly-L-lactic acid/hydroxyapatite nanocomposite coatings for bone regeneration. Biochem. Eng. J. 2015, 98, 29–37. [Google Scholar] [CrossRef]
- Tozzi, G.; De Mori, A.; Oliveira, A.; Roldo, M. Composite hydrogels for bone regeneration. Materials 2016, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Mapara, M.; Thomas, B.S.; Bhat, K.M. Rabbit as an animal model for experimental research. Dent. Res. J. 2012, 9, 111c118. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Kuo, C.Y.; Wong, C.B.; Chien, Y.M.; Chen, H.A.; Chen, J.P. Gelatin/nanohydroxyapatite cryogel embedded poly(lactic-co-glycolic acid)/nanohydroxyapatite microsphere hybrid scaffolds for simultaneous bone regeneration and load-bearing. Polymers 2018, 10, 620. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Moulisová, V.; Gonzalez-García, C.; Cantini, M.; Rodrigo-Navarro, A.; Weaver, J.; Costell, M.; Serra, R.S.; Dalby, M.J.; García, A.J.; Salmerón-Sánchez, M. Engineered microenvionments for synergistic VEGF-integrin signaling during vascularization. Biomaterials 2017, 126, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wang, L.; Niu, L.; Lin, J.; Huang, Q.; Jiang, X.; Li, M. Porous silk fibroin microspheres sustainably releasing bioactive basic fibroblast growth factor. Materials 2018, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Bråkenhielm, E.; Pawliuk, R.; Wariaro, D.; Post, M.J.; Wahlberg, E.; Leboulch, P.; Cao, Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat. Med. 2003, 9, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, S.; Gaudiello, E.; Melly, L.; Cerino, G.; Ricci, D.; Martin, I.; Eckstein, F.; Banfi, A.; Marsano, A. Engineered mesenchymal cell-based patches as controlled VEGF delivery systems to induce extrinsic angiogenesis. Acta Biomater. 2016, 42, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Koh, T.J. Macrophage-based therapeutic strategies in regenerative medicine. Adv. Drug Deliver. Rev. 2017, 122, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Baskin, E.; Dinur, T.; Lebel, E.; Tiomkin, M.; Elstein, D.; Zimran, A. Comparison of bone mineral density by dual-energy X-ray absorptiometry and bone strength by speed-of-sound ultrasonography in adults with Gaucher disease. J. Clin. Densitom. 2016, 19, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Andrejecsk, J.W.; Chang, W.G.; Pober, J.S.; Saltzman, W.M. Controlled protein delivery in the generation of microvascular networks. Drug Deliv. Transl. Res. 2015, 5, 75–88. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, L.; Chang, P.; Sun, M.; Huo, H.; Zhang, C.; Ji, C.; Wei, X.; Zhou, Q.; Guo, P.; Yuan, H. Composite Hydrogels with the Simultaneous Release of VEGF and MCP-1 for Enhancing Angiogenesis for Bone Tissue Engineering Applications. Appl. Sci. 2018, 8, 2438. https://doi.org/10.3390/app8122438
Nie L, Chang P, Sun M, Huo H, Zhang C, Ji C, Wei X, Zhou Q, Guo P, Yuan H. Composite Hydrogels with the Simultaneous Release of VEGF and MCP-1 for Enhancing Angiogenesis for Bone Tissue Engineering Applications. Applied Sciences. 2018; 8(12):2438. https://doi.org/10.3390/app8122438
Chicago/Turabian StyleNie, Lei, Pengbo Chang, Meng Sun, Haojie Huo, Chunxia Zhang, Chingching Ji, Xiaoyan Wei, Qiuju Zhou, Peiyin Guo, and Hongyu Yuan. 2018. "Composite Hydrogels with the Simultaneous Release of VEGF and MCP-1 for Enhancing Angiogenesis for Bone Tissue Engineering Applications" Applied Sciences 8, no. 12: 2438. https://doi.org/10.3390/app8122438
APA StyleNie, L., Chang, P., Sun, M., Huo, H., Zhang, C., Ji, C., Wei, X., Zhou, Q., Guo, P., & Yuan, H. (2018). Composite Hydrogels with the Simultaneous Release of VEGF and MCP-1 for Enhancing Angiogenesis for Bone Tissue Engineering Applications. Applied Sciences, 8(12), 2438. https://doi.org/10.3390/app8122438




