Ethanol Production from Waste of Cassava Processing
Abstract
:1. Introduction
2. Materials and Methods
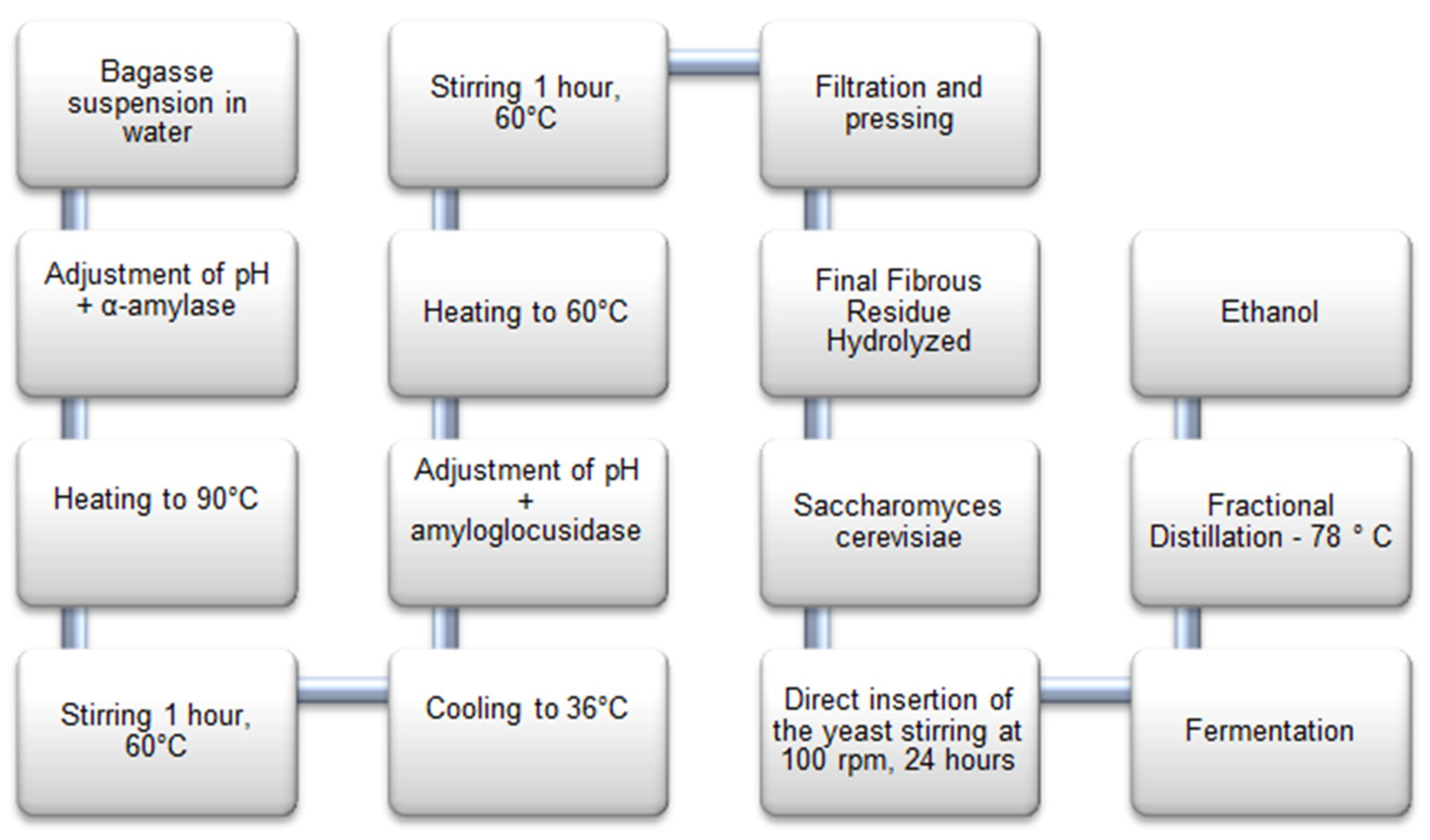
2.1. Materials and Pre-Treatment
2.2. Enzymatic Hydrolysis
2.3. Fermentation and Distillation
2.4. Analysis Methods
3. Results and Discussion
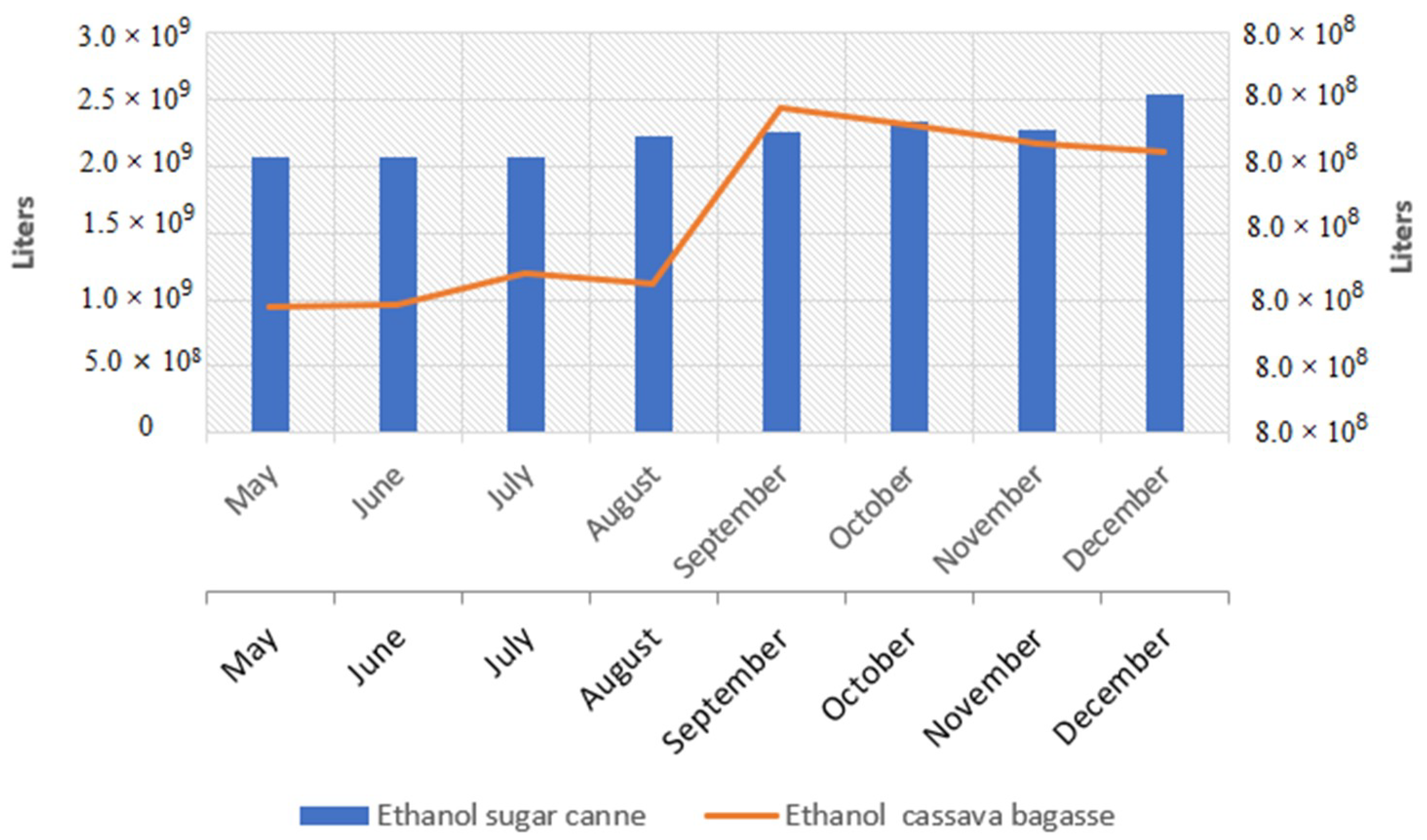
3.1. Potential for Ethanol Production
3.2. Second Generation Ethanol from Brazil
4. Conclusions
- Within the experimental conditions, the results showed that cassava bagasse showed starch content with an average of 64%, making it as an efficient raw material for second-generation ethanol production process.
- There was an average ethanol production potential of 0.25 m³/ton of cassava bagasse.
- This material has potential features that make it an interesting biomass for energy matrix enlargement.
- It is important to note that using cassava bagasse to produce second generation ethanol can prevent future competition for land use between ethanol production and food production, thereby enabling an increase in ethanol production without causing an increase in cassava.
Author Contributions
Funding
Conflicts of Interest
References
- Teixeira, E.M.; Curvelo, A.A.S.; Corrêa, A.C.; Marconcini, J.M.; Glenn, G.M.; Mattoso, L.H.C. Properties of thermoplastic starch from cassava bagasse and cassava starch and their blends with poly (lactic acid). Industrial crops and products. Ind. Crops Prod. 2012, 37, 61–68. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T.; Vandenberge, L.P.S.; Mohan, R. Biotechnological potential of agro-industrial residues II: Cassava bagasse. Bioresour. Technol. 2000, 74, 81–87. [Google Scholar] [CrossRef]
- Escaramboni, B.; Núnez, E.G.F.; Carvalho, A.F.A.; Neto, P.O. Ethanol biosynthesis by fast hydrolysis of cassava bagasse using fungal amylases produced in optimized conditions. Ind. Crops Prod. 2018, 112, 368–377. [Google Scholar] [CrossRef]
- Carpio, L.G.T.; Souza, F.S. Optimal allocation of sugarcane bagasse for producing bioelectricity and second generation ethanol in Brazil: Scenarios of cost reductions. Renew. Energy 2017, 111, 771–780. [Google Scholar] [CrossRef]
- Cardona, C.A.; Quintero, J.A.; Paz, I.C. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2010, 101, 4754–4766. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.O.S.; Cunha, M.P.; Jesus, C.D.F.; Rocha, G.J.M.; Pradella, J.G.C.; Rossell, C.E.V.; Filho, R.M.; Bonomi, A. Second Generation ethanol in Brazil: Can it compete with electricity production? Bioresour. Technol. 2011, 102, 8964–8971. [Google Scholar] [CrossRef] [PubMed]
- UNICA. União dos Produtores de Cana de Açúcar. Available online: www.unica.com.br/unicadata (accessed on 12 August 2018).
- Cavalett, O.; Chagas, M.F.; Junqueira, T.L.; Watanabe, M.D.; Bonomi, A. Environmental impacts of technology learning curve for cellulosic ethanol in Brazil. Ind. Crops Prod. 2017, 106, 31–39. [Google Scholar] [CrossRef]
- Cereda, M.P. Caracterização, usos e tratamentos de resíduos da industrialização da mandioca.Centro de raízes tropicais. Botucatu Centro de Raízes Tropicais 1996, 5, 56. [Google Scholar]
- Cranshak, J.; Andrade, J. Caracterização físico-química de bagaço de mandioca in natura e após tratamento hidrolítico. Rev. Bras. Tecnol. Agroind. 2011, 5, 427–441. [Google Scholar]
- Raupp, D.; Moreira, S.; Banzatto, D.; Sgarbieri, V. Composição e propriedades fisiológico—Nutritivas de uma farinha rica em fibra insolúvel obtida do resíduo fibroso de uma fecularia de mandioca. Ciênc. Tecnol. Aliment. 1999, 19, 205–210. [Google Scholar] [CrossRef]
- Saitto, I.; Cabello, C. Produção de etanol a partir de hidrolisado obtido por tratamento hidrotérmico de farelo de mandioca. Rev. Energ. Agric. 2006, 21, 34–44. [Google Scholar]
- Zuh, F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar]
- Srinnorakutara, T.; Kaewvimol, L.; Saengow, L. Apprach of cassava waste pretreatments for fuel etanol production in Thailand. J. Sci. Res. 2006, 31, 77–84. [Google Scholar]
- Wosiacki, G.; Cereda, M. Valorização de resíduos do processamento de mandioca. Agrar. Sci. Eng. 2002, 8, 27–43. [Google Scholar]
- Camili, E.A. Parâmetros Operacionais do Processo de Produção de Etanol a Partir da Polpa de Mandioca. Ph.D. Thesis, Universidade Estadual Paulista “Julio de Mesquita Filho”, São Paulo, Brazil, 2010; pp. 1–131. [Google Scholar]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy. 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Wang, M.; Han, J.; Dunn, J.B.; Cai, H.; Elgowainy, A. Well-to-wheels energy use and Greenhouse gas emissions of etanol from corn, sugarcane and cellulosic biomass for US use. Environ. Res. Lett. 2012, 7, 1–13. [Google Scholar] [CrossRef]
- Dias, M.O.S.; Cunha, M.P.; Maciel Filho, R.; Bonomi, A.; Jesus, C.D.F.; Rossell, C.E.V. Simulation of integrated first and second generation bioethanol production from sugarcane: comparison between different biomass pretreatment methods. J. Ind. Microbiol. Biotechnol. 2011, 38, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Kadam, K.L.; McMillan, J.D. Availability of corn stover as a sustainable feed-stock for bioethanol production. Bioresour. Technol. 2003, 88, 17–25. [Google Scholar] [CrossRef]
- Emeka, E.E.; Chales, O.O.; Anthony, O.C. Utilization of cellulosic cassava waste for bio-ethanol production. J. Environ. Chem. Eng. 2015, 3, 2797–2800. [Google Scholar]
- Ajibola, F.O.; Edema, M.O.; Oyewole, O.B. Enzymatic Production of Ethanol from Cassava Starch Using Two Strains of Saccharomyces Cerevisiae. Niger. Food J. 2012, 30, 114–121. [Google Scholar] [CrossRef]
- Energy Research Company—EPE. Report of Ethanol Production in Brazil. Available online: http://www.epe.gov.br (accessed on 5 July 2018).
- Dias, M.O.S.; Junqueira, T.L.; Cavallet, O.; Cunha, M.P.; Jesus, C.D.F.; Mantelatto, P.E.; Rossell, C.E.V.; Filho, R.M.; Bonomi, A. Cogeneration in integrated first and second generation ethanol from sugarcane. Chem. Eng. Res. Des. 2013, 91, 1411–1417. [Google Scholar] [CrossRef]
- Raele, R.; Boaventura, J.M.G.; Fischmann, A.A.; Sarturi, G. Scenarios for the second generation ethanol in Brazil. Technol. Forecast. 2014, 87, 205–223. [Google Scholar] [CrossRef]
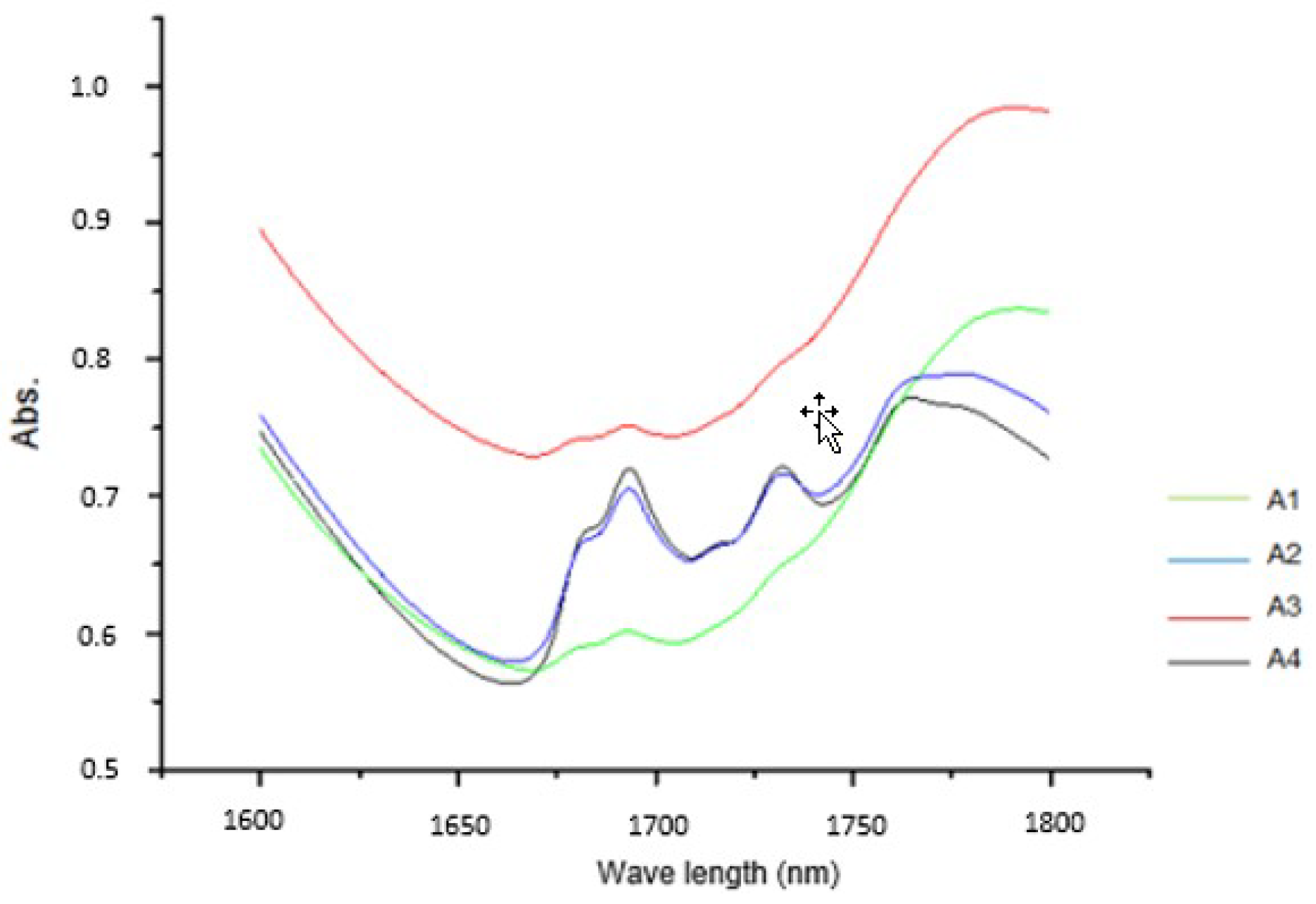
| Samples | Starch (%) | Raw Fiber (g/100 g) | Protein (g/100 g) | Sugars (g/100 g) | Grease Matter (g/100 g) | pH | Humidity (%) | Ash (g/100 g) |
|---|---|---|---|---|---|---|---|---|
| A1 | 64.80 | 3.42 | 0.49 | 8.30 | 0.21 | 4.31 | 87.14 | 0.23 |
| A2 | 59.90 | 3.85 | 0.53 | 6.61 | 0.20 | 4.45 | 89.29 | 0.29 |
| A3 | 64.10 | 6.07 | 0.90 | 8.04 | 0.20 | 4.50 | 88.70 | 0.12 |
| A4 | 68.30 | 3.94 | 0.52 | 8.24 | 0.01 | 4.91 | 86.75 | 0.23 |
| Mean | 64.27 | 4.32 | 0.61 | 7.79 | 0.15 | 4.54 | 87.97 | 0.21 |
| Sample | Humidity (%) | % Total Solids | g/Total Solids | Initial pH | Final pH | Mass (%) | Volume before Hydrolysis (mL) | Volume after Hydrolysis (mL) |
|---|---|---|---|---|---|---|---|---|
| A1 | 64.19 | 35.81 | 7.16 | 6.15 | 5.14 | 27.13 | 150 | 130.67 |
| A2 | 68.88 | 31.12 | 6.22 | 5.98 | 5.23 | 29.52 | 150 | 124.00 |
| A3 | 68.59 | 31.41 | 6.28 | 5.94 | 5.37 | 29.24 | 150 | 132.67 |
| A4 | 75.90 | 24.10 | 4.82 | 6.22 | 5.39 | 32.52 | 150 | 124.17 |
| Treatment | Starch (%) | Ethanol (%) | Ethanol (mL/kg) | Ethanol Yield (%) | Fibrous Residue (%) | Reducing Sugars (g/100 g) | Reducing Sugars (%) |
|---|---|---|---|---|---|---|---|
| A1 | 64.80 | 12.60 | 103.48 | 12.06 | 27.13 | 2.43 | 0.02 |
| A2 | 59.90 | 48.50 | 368.21 | 48.50 | 29.52 | 2.27 | 0.02 |
| A3 | 64.10 | 12.00 | 97.49 | 12.00 | 29.24 | 2.35 | 0.02 |
| A4 | 68.30 | 53.30 | 461.39 | 53.00 | 32.52 | 3.65 | 0.04 |
| Mean | 64.28 | 31.60 | 251.45 | 31.00 | 29.60 | 2.68 | 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, D.G.; Feiden, A.; Bariccatti, R.; De Freitas Zara, K.R. Ethanol Production from Waste of Cassava Processing. Appl. Sci. 2018, 8, 2158. https://doi.org/10.3390/app8112158
Martinez DG, Feiden A, Bariccatti R, De Freitas Zara KR. Ethanol Production from Waste of Cassava Processing. Applied Sciences. 2018; 8(11):2158. https://doi.org/10.3390/app8112158
Chicago/Turabian StyleMartinez, Daiana G., Armin Feiden, Reinaldo Bariccatti, and Katya Regina De Freitas Zara. 2018. "Ethanol Production from Waste of Cassava Processing" Applied Sciences 8, no. 11: 2158. https://doi.org/10.3390/app8112158
APA StyleMartinez, D. G., Feiden, A., Bariccatti, R., & De Freitas Zara, K. R. (2018). Ethanol Production from Waste of Cassava Processing. Applied Sciences, 8(11), 2158. https://doi.org/10.3390/app8112158





