Nanocomposite of Si/C Anode Material Prepared by Hybrid Process of High-Energy Mechanical Milling and Carbonization for Li-Ion Secondary Batteries
Abstract
1. Introduction
2. Experimental Details
2.1. Preparation of Si/C Nanocomposite
2.1.1. Preparation of Nano-Scale Si Powder
2.1.2. Fabrication of Si/C Nanocomposite
2.2. Characterization and Electrochemical Test
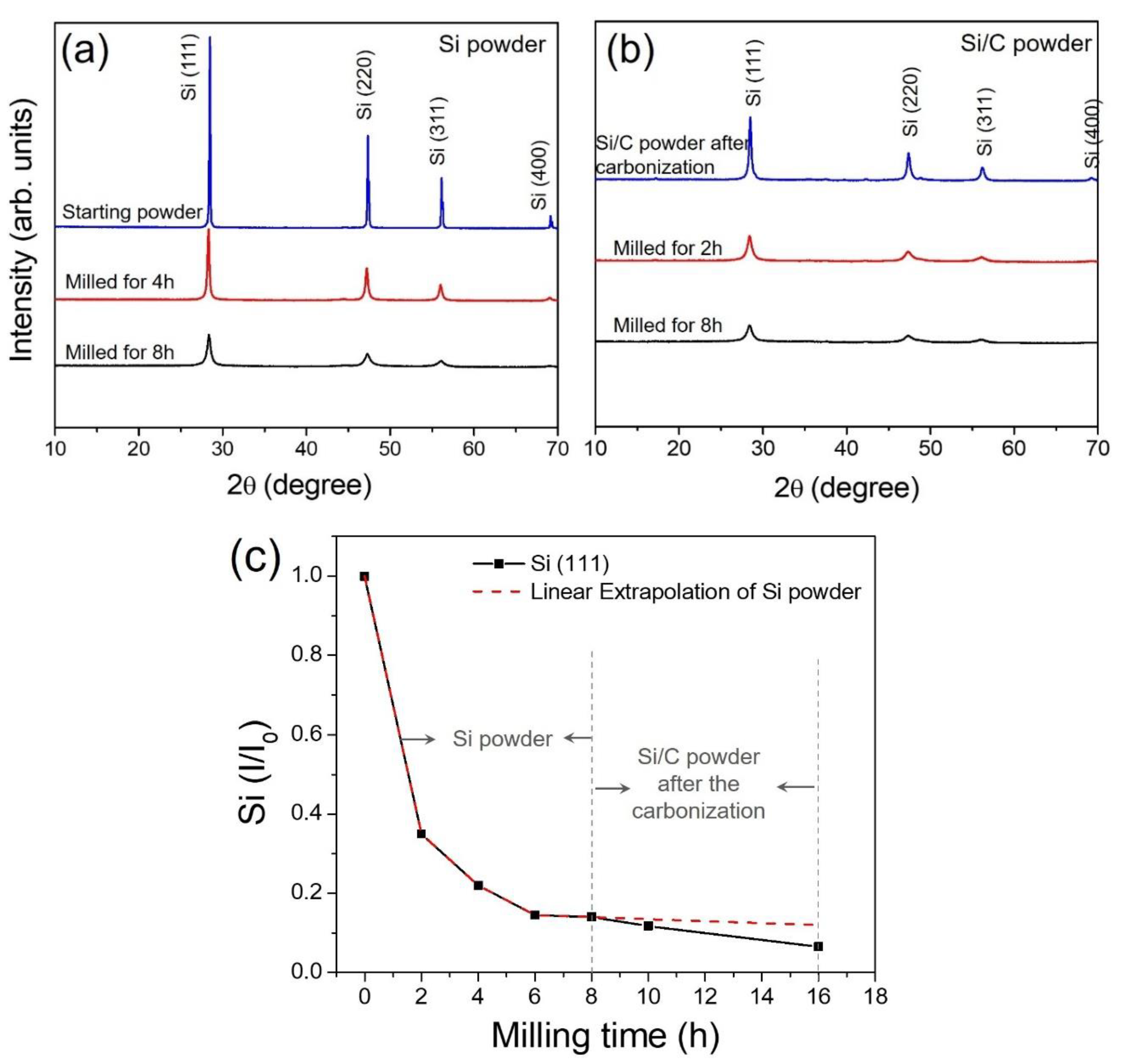
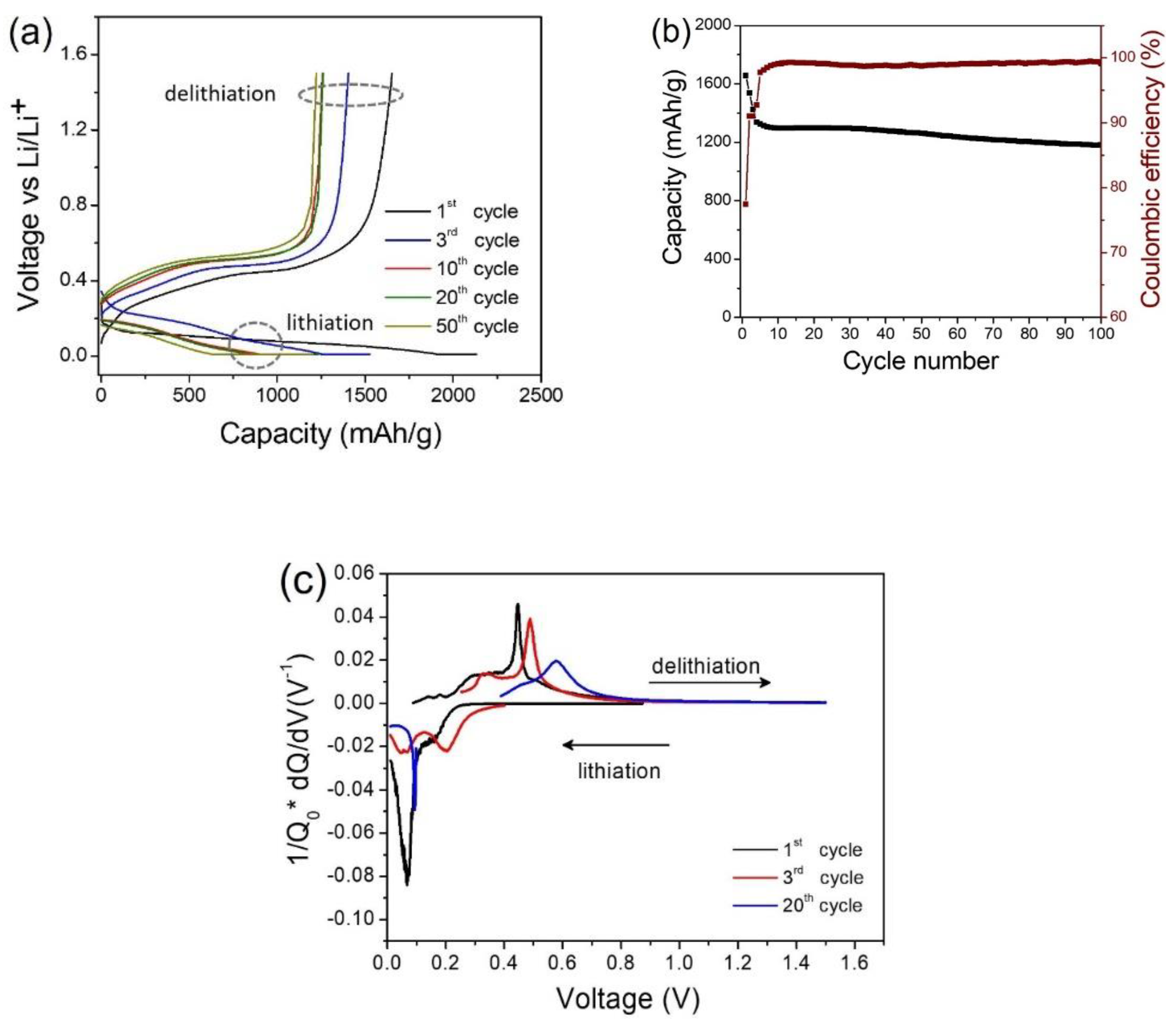
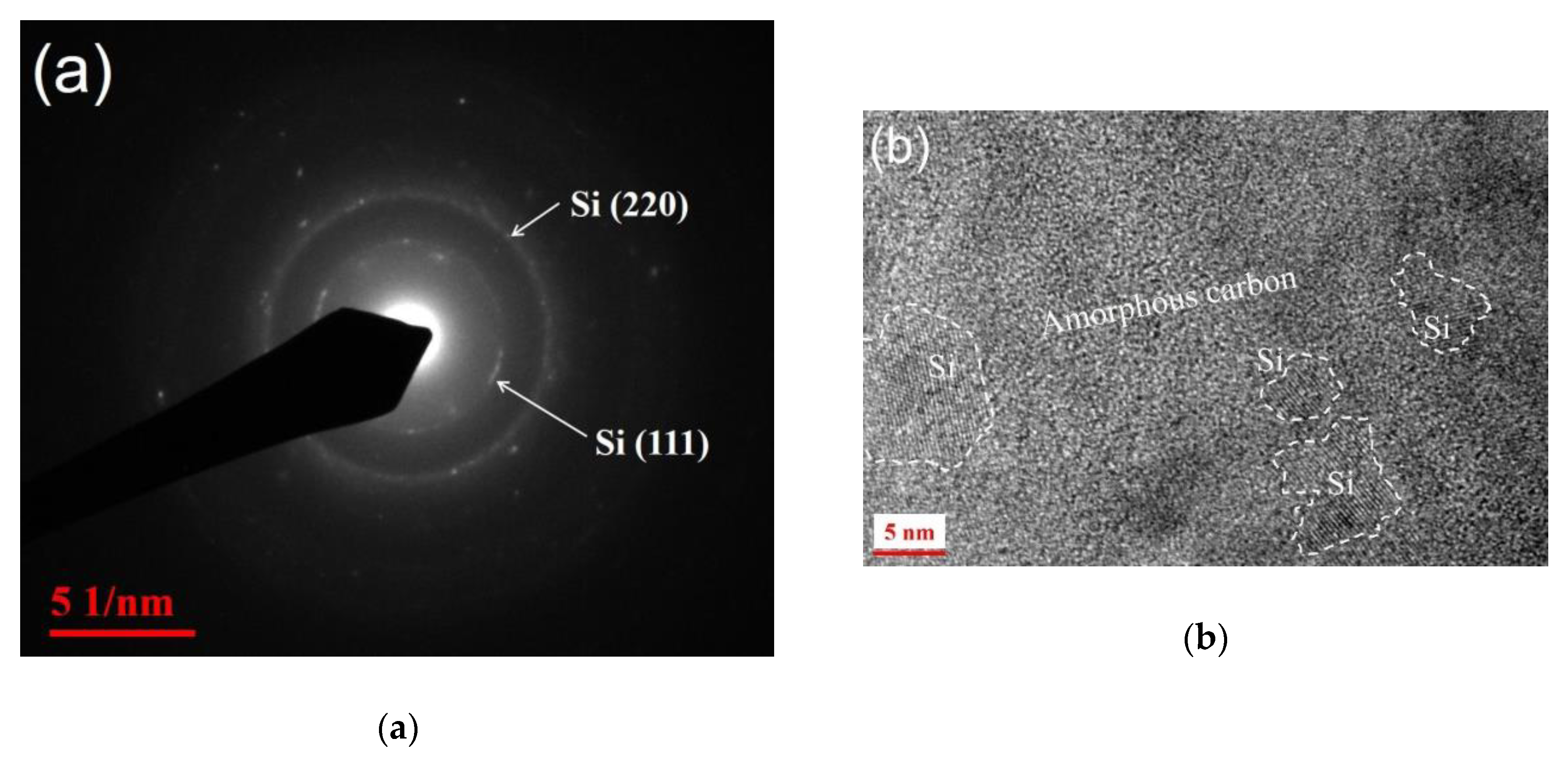
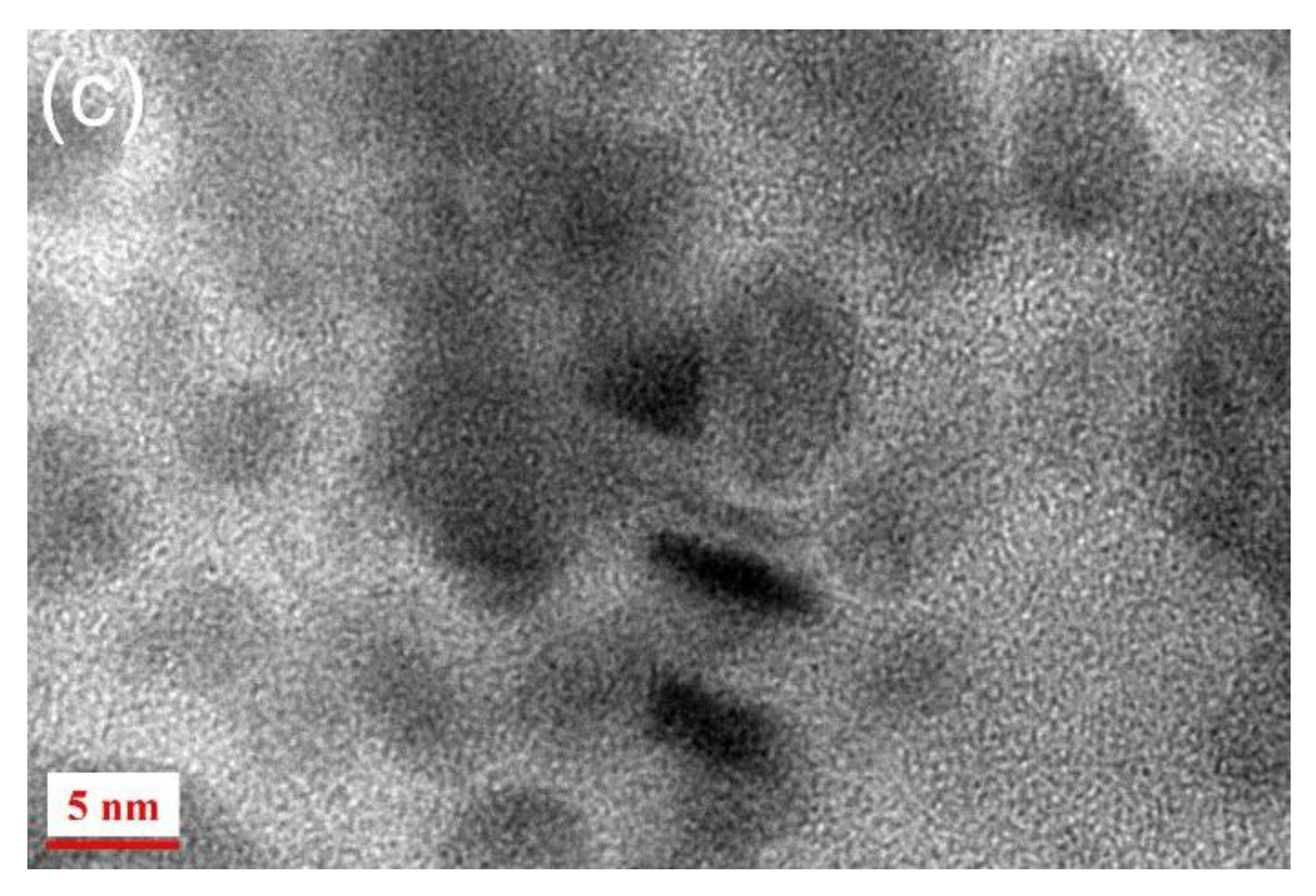
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Blomgren, G.E. The development and future of lithium ion batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Amalraj, S.F.; Hadar, S.; Yuliya, S.; Rao, P.T.; Ravikumar, R.; Satyanarayana, M.; Sandipan, M.; Halalay, I.C.; Shalom, L.; Boris, M.; et al. Horizons for Li-Ion batteries relevant to electro-mobility: High-specific-energy cathodes and chemically active separators. Adv. Mater. 2018, 30, 1801348. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, S.M. Graphite–FeSi alloy composites as anode materials for rechargeable lithium batteries. J. Power Sources 2002, 112, 649–654. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Nix, W.D.; Cui, Y. Understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv. Mater. 2013, 25, 4966–4985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J. Lithium insertion/extraction mechanism in alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 877–885. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, Y.; Kushima, A.; Zhang, S.; Zhu, T.; Li, J.; Huang, J.Y. Insitu TEM experiments of electrochemical lithiation and delithiation of individual nanostructures. Adv. Energy Mater. 2012, 2, 722–741. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; Mcdowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, N.; Lee, H.W.; Zhao, J.; Li, W.; Li, Y.; Cui, Y. Nonfilling carbon coating of porous silicon micrometer-sized particles for high-performance lithium battery anodes. ACS Nano 2015, 9, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Obrovac, M.N.; Chevrier, V.L. Alloy negative electrodes for Li-ion batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef] [PubMed]
- Holtstiege, F.; Wilken, A.; Winter, M.; Placke, T. Running out of lithium? A route to differentiate between capacity losses and active lithium losses in lithium-ion batteries. Phys. Chem. Chem. Phys. 2017, 19, 25905–25918. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Jin, Y.; Tan, Y.; Zong, L.; Hu, Y.; Chen, L.; Chen, Y.; Zhang, Q.; Zhu, J. Scalable production of si nanoparticles directly from low grade sources for lithium-ion battery anode. Nano Lett. 2015, 15, 5750–5754. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, X.; Qiu, T.; Luo, B.; Ning, J.; Li, J.; Zhang, X.; Liang, M.; Zhi, L. High volumetric capacity silicon-based lithium battery anodes by nanoscale system engineering. Nano Lett. 2013, 13, 5578–5584. [Google Scholar] [CrossRef] [PubMed]
- Karki, K.; Zhu, Y.; Liu, Y.; Sun, C.F.; Hu, L.; Wang, Y.; Wang, C.; Cumings, J. Hoop-Strong Nanotubes for Battery Electrodes. ACS Nano. 2013, 7, 8295–8302. [Google Scholar] [CrossRef] [PubMed]
- Abel, P.R.; Lin, Y.M.; Celio, H.; Heller, A.; Mullins, C.B. Improving the stability of nanostructured silicon thin film lithium-ion battery anodes through their controlled oxidation. ACS Nano 2012, 6, 2506–2516. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Liu, Y.; Ma, T.; Zhu, W.; Qiu, X. Hollow structured silicon anode with stabilized solid electrolyte interphase film for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 23501–23506. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Hu, R.; Zhang, M.; Liu, J.; Zhu, M. Binding of carbon coated nano-silicon in graphene sheets by wet ball- milling and pyrolysis as high performance anodes for lithium-ion batteries. J. Power Sources 2016, 318, 113–120. [Google Scholar] [CrossRef]
- Lv, P.; Zhao, H.; Wang, J.; Liu, X.; Zhang, T.; Xia, Q. Facile preparation and electrochemical properties of SiO2/C composite as anode material for lithium ion batteries. J. Power Source 2013, 237, 291–294. [Google Scholar] [CrossRef]
- Chen, X.; Gerasopoulos, K.; Guo, J.; Brown, A.; Wang, C.; Ghodssi, R.; Culver, J.N. Virus-enabled silicon anode for lithium-ion batteries. ACS Nano 2010, 4, 5366–5372. [Google Scholar] [CrossRef] [PubMed]
- Kummer, M.; Badillo, J.P.; Schmitz, A.; Bremes, H.G.; Winter, M.; Schulz, C.; Wiggers, H. Silicon/polyaniline nanocomposites as anode material for lithium ion batteries. J. Electrochem. Soc. 2013, 161, A40–A45. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward efficient binders for Li-ion battery Si-based anodes: Polyacrylic acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lindgren, F.; Philippe, B.; Gorgoi, M.; Bjorefors, F.; Edstrom, K.; Gustafsson, T. Improved performance of the silicon anode for li-ion batteries: Understanding the surface modification mechanism of fluoroethylene carbonate as an effective electrolyte additive. Chem. Mater. 2015, 27, 2591–2599. [Google Scholar] [CrossRef]
- Fan, X.Y.; Ke, F.S.; Wei, G.Z.; Huang, L.; Sun, S.G. Sn-Co alloy anode using porous Cu as current collector for lithium ion battery. J. Alloys Compd. 2009, 476, 70–73. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, J.; Fu, L.J.; Yang, L.C.; Wu, Y.P.; Wu, H.Q. Natural graphite coated by Si nanoparticles as anode materials for lithium ion batteries. J. Mater. Chem. 2007, 17, 1321–1325. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, K.; Ji, G.; Lee, J.Y.; Zou, C.; Chen, X.; Wu, J. Graphene/nanosized silicon composites for lithium battery anodes with improved cycling stability. Carbon 2011, 49, 1787–1796. [Google Scholar] [CrossRef]
- Fan, X.; Zou, L.; Zheng, Y.P.; Kang, F.Y.; Shen, W.C. Electrospinning preparation of nanosilicon/disordered carbon composite as anode materials in li-ion battery. Electrochem. Solid-State Lett. 2009, 12, A199–A201. [Google Scholar] [CrossRef]
- Zhou, Z.B.; Xu, Y.H.; Liu, W.G.; Niu, L.B. High capacity Si/DC/MWCNTs nanocomposite anode materials for lithium ion batteries. J. Alloys Compd. 2010, 493, 636–639. [Google Scholar] [CrossRef]
- Yen, Y.C.; Chao, S.C.; Wu, H.C.; Wu, N.L. Study on Solid-Electrolyte-Interphase of Si and C-Coated Si Electrodes in Lithium Cells. J. Electrochem. Soc. 2009, 156, A95–A102. [Google Scholar] [CrossRef]
- Holzapfel, M.; Buqa, H.; Scheifele, W.; Novak, P.; Petrat, F.M. A new type of nano-sized silicon/carbon composite electrode for reversible lithium insertion. Chem. Commun. 2005, 12, 1566–1568. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.M.; Reimers, J.N.; Fuller, E.W.; Dahn, J.R. Lithium insertion in pyrolyzed siloxane polymers. Solid State Ion. 1994, 74, 249–254. [Google Scholar] [CrossRef]
- Liu, Y.; Hanai, K.; Horikawa, K.; Imanishi, N.; Hirano, A.; Takeda, Y. Electrochemical characterization of a novel Si–graphite–Li2.6Co0.4N composite as anode material for lithium secondary batteries. Mater. Chem. Phys. 2004, 89, 80–84. [Google Scholar] [CrossRef]
- Guo, Z.P.; Milin, E.; Wang, J.Z.; Chen, J.; Liu, H.K. Silicon/disordered carbon nanocomposites for lithium-ion battery anodes. J. Electrochem. Soc. 2005, 152, A2211–A2216. [Google Scholar] [CrossRef]
- Hasegawa, T.; Mukai, S.R.; Shirato, Y.; Tamon, H. Preparation of carbon gel microspheres containing silicon powder for lithium ion battery anodes. Carbon 2004, 42, 2573–2579. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Yang, X.L.; Huang, S.H. Composite anode materials for Li-ion batteries. J. Power Sources 2007, 174, 1041–1045. [Google Scholar] [CrossRef]
- Patel, P.; Kim, I.S.; Kumta, P.N. Nanocomposites of silicon/titanium carbide synthesized using high-energy mechanical milling for use as anodes in lithium-ion batteries. Mater. Sci. Eng. B 2005, 116, 347–352. [Google Scholar] [CrossRef]
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-based anodes for lithium-ion batteries: Effectiveness of materials synthesis and electrode preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Chan, C.K.; Ruffo, R.; Hong, S.S.; Cui, Y. Surface chemistry and morphology of the solid electrolyte interphase on silicon nanowire lithium-ion battery anodes. J. Power Sources 2009, 189, 1132–1140. [Google Scholar] [CrossRef]
- McDowell, M.T.; Ryu, I.; Lee, S.W.; Wang, C.; Nix, W.D.; Cui, Y. Studying the kinetics of crystalline silicon nanoparticle lithiation with in situ transmission electron microscopy. Adv. Mater. 2012, 24, 6034–6041. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, M.; Kugino, S.; Dimov, N. Electrochemical behaviors of silicon based anode material. J. Power Sources 2006, 153, 375–379. [Google Scholar] [CrossRef]
- Ng, S.H.; Wang, J.; Wexler, D.; Chew, S.Y.; Liu, H.K. Amorphous carbon-coated silicon nanocomposites: A low-temperature synthesis via spray pyrolysis and their application as high-capacity anodes for lithium-ion batteries. J. Phys. Chem. C 2007, 111, 11131–11138. [Google Scholar] [CrossRef]
- Gu, P.; Cai, R.; Zhou, Y.; Shao, Z. Si/C composite lithium-ion battery anodes synthesized from coarse silicon and citric acid through combined ball milling and thermal pyrolysis. Electrochim. Acta 2010, 55, 3876–3883. [Google Scholar] [CrossRef]
- Datta, M.K.; Maranchi, J.; Chung, S.J.; Epur, R.; Kadakia, K.; Jampani, P.; Kumta, P.N. Amorphous silicon-carbon based nano-scale thin film anode materials for lithium ion batteries. Electrochim. Acta 2011, 56, 4717–4723. [Google Scholar] [CrossRef]
- Cui, L.F.; Ruffo, R.; Chan, C.K.; Peng, H.; Cui, Y. Crystalline-amorphous core−shell silicon nanowires for high capacity and high current battery electrodes. Nano Lett. 2008, 9, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Blomgren, G.E.; Kumta, P.N. Si-SiC nanocomposite anodes synthesized using high-energy mechanical milling. J. Power Sources 2004, 130, 275–280. [Google Scholar] [CrossRef]
- Datta, M.K.; Kumta, P.N. In situ electrochemical synthesis of lithiated silicon-carbon based composites anode materials for lithium ion batteries. J. Power Sources 2009, 194, 1043–1052. [Google Scholar] [CrossRef]
- Wang, X.L.; Han, W.Q. Graphene enhances Li storage capacity of porous single-crystalline silicon nanowires. ACS Appl. Mater. Interfaces 2010, 2, 3709–3713. [Google Scholar] [CrossRef] [PubMed]
- Sourice, J.; Quinsac, A.; Leconte, Y.; Sublemontier, O.; Porcher, W.; Haon, C.; Bordes, A.; De Vito, E.; Boulineau, A.; Jouanneau Si Larbi, S.; et al. One-step synthesis of Si@C nanoparticles by laser pyrolysis: High-capacity anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 6637–6644. [Google Scholar] [CrossRef] [PubMed]
- Haregewoin, A.M.; Leggesse, E.G.; Jiang, J.C.; Wang, F.M.; Hwang, B.J.; Lin, S.D. Comparative study on the solid electrolyte interface formation by the reduction of alkyl carbonates in lithium ion battery. Electrochim. Acta 2014, 136, 274–285. [Google Scholar] [CrossRef]


| Sample Name | Average Particle Size (nm) | Standard Deviation (nm) |
|---|---|---|
| Si-8 h milling | 250 | 79 |
| Si/C composite | 350 | 138 |
| Si/C-8 h milling | 200 | 61 |
| Sample Name | Weight Ratio of Active Material in Electrode | 1st Discharge Capacity | 1st Coulombic Efficiency | Cyclic Stability | Capacity Retention | Ref. |
|---|---|---|---|---|---|---|
| Si/C nanocomposite | 80% | 1658 mAh/g (at 0.1C) | 77.5% | 1181 mAh/g (at 1 C) after 100 cycles | 71.2% at 100th cycle | In this work |
| Si/graphite composite | 80% | 1533 mAh/g (at 0.2 A/g) | 72.4% | 665 mAh/g (at 0.2 A/g) after 100 cycles | 43.3% at 100th cycle | [18] |
| Si/C composite | 80% | 1387 mAh/g (at 0.2 A/g) | 82.5% | 678 mAh/g (at 0.2 A/g) after 100 cycles | 49.2% at 100th cycle | [18] |
| Si/C nanocomposite | 80% | 2600 mAh/g (at 0.1 A/g) | 71% | 1120 mAh/g (at 0.1 A/g) after 100 cycles | 43% at 100th cycle | [42] |
| Si/C nanocomposite | 80% | 1561 mAh/g (at 0.15 mA/cm2) | 61.9% | 626 mAh/g (at 0.15 mA/cm2) after 30 cycles | 40.1% at 30th cycle | [43] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddipatla, R.; Loka, C.; Choi, W.J.; Lee, K.-S. Nanocomposite of Si/C Anode Material Prepared by Hybrid Process of High-Energy Mechanical Milling and Carbonization for Li-Ion Secondary Batteries. Appl. Sci. 2018, 8, 2140. https://doi.org/10.3390/app8112140
Maddipatla R, Loka C, Choi WJ, Lee K-S. Nanocomposite of Si/C Anode Material Prepared by Hybrid Process of High-Energy Mechanical Milling and Carbonization for Li-Ion Secondary Batteries. Applied Sciences. 2018; 8(11):2140. https://doi.org/10.3390/app8112140
Chicago/Turabian StyleMaddipatla, Reddyprakash, Chadrasekhar Loka, Woo Jeong Choi, and Kee-Sun Lee. 2018. "Nanocomposite of Si/C Anode Material Prepared by Hybrid Process of High-Energy Mechanical Milling and Carbonization for Li-Ion Secondary Batteries" Applied Sciences 8, no. 11: 2140. https://doi.org/10.3390/app8112140
APA StyleMaddipatla, R., Loka, C., Choi, W. J., & Lee, K.-S. (2018). Nanocomposite of Si/C Anode Material Prepared by Hybrid Process of High-Energy Mechanical Milling and Carbonization for Li-Ion Secondary Batteries. Applied Sciences, 8(11), 2140. https://doi.org/10.3390/app8112140





