Abstract
The influencing factors of ammonia content and centrifugal speed on ammonium rhenate recovery from waste superalloy were systematically analyzed. It was found that proper ammonia content and centrifugal speed could promote crystal growth and a higher purity of ammonium rhenate was obtained. Moreover, the XRD patterns showed that excessive ammonia content and inappropriate centrifugal speed restrained the growth of (101) crystal plane and (112) crystal plane, which caused the crystal structural regularity and relative crystallinity to attenuate. The microscopic morphology and purity of the crystal also changed.
1. Introduction
Superalloy is widely used in the aerospace, oil, metallurgy, and chemical industries, automobile manufacturing, and so on [1,2]. With the rapid consumption of resources, the recovery and utilization of waste superalloys have become important [3,4]. Rhenium is a kind of rare metal resource that has low global reserves and a stiff price [5]. Therefore, extracting rhenium from waste superalloys is both economically and environmentally significant.
There have been many reports about extraction processes of rhenium in recent years. Zou et al. proposed a process for recovery of molybdenum and rhenium from molybdenite concentrate by optimizing roasting-acid and leaching-ion exchange, and that process has been applied practically [6,7]. Qin et al. contrived a new technology for extracted NH4ReO4 with an ion exchanging method [8,9]. Hyun Soo Kim et al. recovered rhenium from a molybdenite roaster fume as high-purity ammonium perrhenate [10,11,12,13]. However, there are few reports about influencing factors of the morphology, crystallinity, and purity of NH4ReO4 crystals in the recovery process, which leads to different batches of products having large differences in purity and microscopic morphology. Furthermore, it makes optimization of the production process difficult.
According to the industrial process of rhenium recovery from waste superalloy, the electrolytes of waste superalloy are recrystallized to recover ammonium rhenate. However, it is difficult to determine the ammonia content and centrifugal speed as the important process parameters in this process, which leads to different batches of the products having large differences in purity, crystallinity, and microscopic morphology, and the products generally include W, Mo, and other elements with lower purity. This study innovatively analyzed the influence of ammonia content and centrifugal speed on crystal growth direction selectivity, microscopic morphology, aging time, and purity of ammonium rhenate, to provide theoretical experiences for determination of the process parameters in industrial recovery.
2. Instrument and Experiment
Instrument: IRIS Intrepid II XSP inductive coupling plasma emission spectrometer (ICP), American Elements. Quintix224-1CN electronic balance, DL5M low-speed centrifuge, Xiangyi Company, Changsha China. RE-2000A rotary evaporator, Yijie Science Company, Hangzhou, China. SSX-550 scanning electron microscope, Shimadzu, Kyoto Japan. PW3040/60 x-ray diffractometer, Panalytical, Almelo, Netherlands.
Experiment: A certain amount of ammonium rhenium solution from waste superalloy electrolyte was put into the rotary evaporator to evaporate at the same centrifugal temperature, and an appropriate amount of aqueous ammonia was added. Then the residual solution was put into a centrifuge bottle to crystallize at a controlled centrifugal rate, and the sample was filtered and washed to dry, waiting for measurement. The experimental parameters are shown in Table 1.

Table 1.
Experimental parameters at different experimental conditions.
XRD detection: Light tube type was Cu target, ceramic X light tube; λ = 0.15406 nm, scan range 10–90°, scanning speed 2°/min.
3. Results and Discussion
3.1. Influence of NH3 (wt %)
The NH4ReO4 crystals were crystallized at the same centrifugal rate and temperature (25 °C), but different amounts of aqueous ammonia were added. The relationship between purity, aging time, and amount of aqueous ammonia is shown in Figure 1.
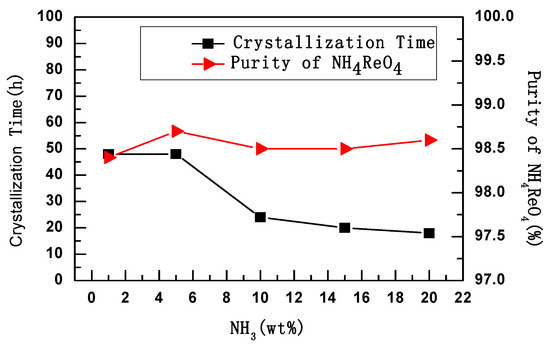
Figure 1.
NH3 (wt %)—conversion relation of crystallization time and purity.
It was found that crystallization time decreased with the increase of aqueous ammonia; however, the amount of aqueous ammonia had a smaller influence on the purity of NH4ReO4, as shown in Figure 1. When the amount of aqueous ammonia was 1–5%, the result did not have research significance, because the crystallization time reached 48 h, which consumed excessive energy.
When the amount of aqueous ammonia was 10%, the NH4ReO4 particles formed many complete rhombic crystals; the surface structure of these crystals was smooth, as shown in Figure 2a. This suggests that the NH4ReO4 particles combined compactly and the surface structure of these crystals grew integrated.
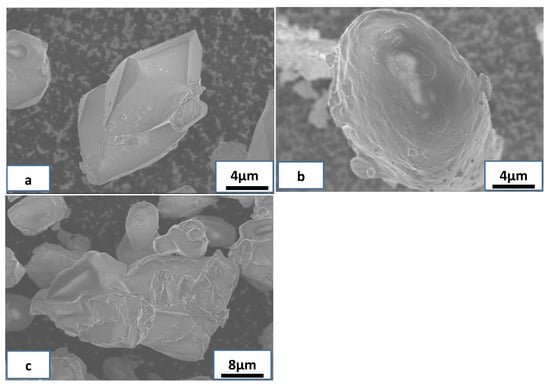
Figure 2.
SEM images of NH4ReO4 particles at different NH3 wt %: (a) NH3 10 wt %, (b) NH3 15 wt %, (c) NH3 20 wt %.
When the amount of aqueous ammonia was 15%, the crystallization time reduced from 24 h to 20 h and many NH4ReO4 particles formed near spherical crystals; the surface structure of these crystals was coarse, as shown in Figure 2b. This suggests that the combination of NH4ReO4 particles was insufficient and the surface structure of these crystals grew half-baked. The reason for that phenomenon is that the increase of ammonia ion promotes reverse dissolution equilibrium, which breaks between crystal nucleus growth and crystal growth. This means that the growth rate of NH4ReO4 crystals is too fast and therefore the growth is incomplete. When the amount of aqueous ammonia was 20%, the crystallization time reduced to 18 h and the NH4ReO4 particles formed many large agglomerated crystals, as shown in Figure 2c. The reason for that phenomenon is that the increase of ammonia ion promotes further reverse dissolution equilibrium. The growth of crystal nucleus is incomplete, while the growth rate of NH4ReO4 crystals is too fast.
The XRD patterns of the samples with different amounts of aqueous ammonia are shown in Figure 3, and the XRD information is given in Table 2. The main diffraction peaks of ammonium rhenate and other crystal items were not observed. When the amount of aqueous ammonia was 10%, characteristic peaks in the XRD patterns were the highest, full width at half maximum (FWHM) was minimal, and relative crystallinity [14] was the ideal. If the relative crystallinity of the samples prepared at NH3 10 wt % is treated as 100%, according to the amorphization formula A = (1 − Areax/Area0) × 100%, the relative crystallinity of the samples at NH3 15 wt % and NH3 20 wt % is 63.9% and 50.5%.
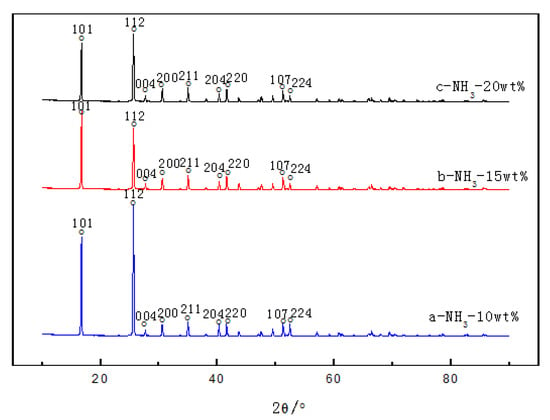
Figure 3.
XRD patterns of NH4ReO4 crystals at different NH3 wt %: (a) 10%, (b) 15%, (c) 20%.

Table 2.
XRD information of NH4ReO4 crystals at different NH3 wt %. FWHM, full width at half maximum.
According to Table 2, the selectivity of crystal growth direction on (101) crystal plane and (112) crystal plane at different aging times can be calculated by the Scherrer formula, D = Kλ/(B cos θ), and the results are given in Table 3. From Table 3 it can be seen that with increased ammonia content, the growth of (101) crystal plane and (112) crystal plane was restrained. However, the growth gates of (101) crystal plane and (112) crystal plane were promoted.

Table 3.
Selectivity of crystal growth direction for NH4ReO4 at different NH3 wt %.
3.2. Influence of Centrifugal Speed
The ammonium rhenate was crystallized with the same amount of aqueous ammonia and at the same centrifugal temperature (25 °C) but at different centrifugal speeds. The relationship between purity, aging time, and centrifugal speed is shown in Figure 4.
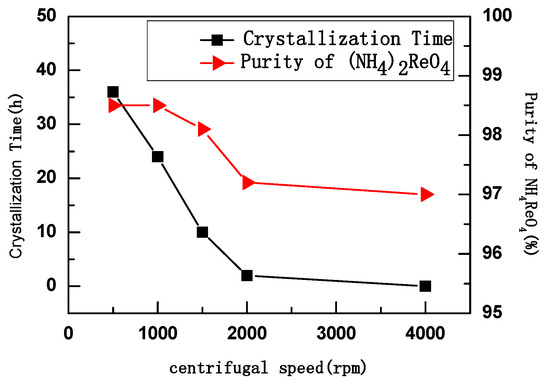
Figure 4.
Centrifugal speed—conversion relation of crystallization time and purity.
It was found that crystallization time decreased with increased centrifugal speed, as shown in Figure 4. Moreover, the purity of NH4ReO4 was 98.5% at centrifugal speeds of 500–1000 rpm; when centrifugal speed was higher than 1000 rpm, the purity of NH4ReO4 began to decrease. Therefore, centrifugal speeds above 1500 rpm have no significance.
When centrifugal speed was 500 rpm, the NH4ReO4 particles formed many large agglomerated crystals, as shown in Figure 5a. Since the NH4ReO4 particles could not collide or break into the secondary nucleation stage at low centrifugal speed, the particles gathered together and formed large agglomerated crystals under Van der Waals force.
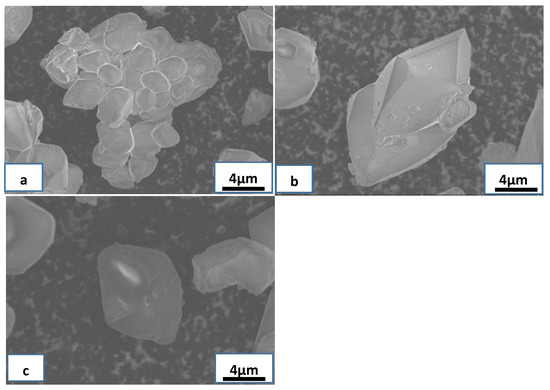
Figure 5.
SEM images of NH4ReO4 particles at different centrifugal speeds: (a) 500 rpm, (b) 1000 rpm, (c) 1500 rpm.
When centrifugal speed was 1000 rpm, the NH4ReO4 particles formed many complete rhombic crystals, as shown in Figure 5b. This suggests that the large particles collided and broke into the secondary nucleation stage at an appropriate centrifugal speed; the particles gathered together and formed integrated crystals. When centrifugal speed was 1500 rpm, the NH4ReO4 particles formed many smaller rhomboid crystals, as shown in Figure 5c. The reason is that the larger particles collided and broke into secondary, and the secondary nucleation became the main source of nucleation in the system at an excessive centrifugal speed. Due to the excessive speed, the crystals broke up into many smaller crystals before they grew, and the collision probability and intensity increased, which enhanced the growth rate of the crystals and caused the formation of many smaller crystals.
The XRD patterns of the samples at different centrifugal speeds are shown in Figure 6, and the XRD information is given in Table 4. It was found that the main diffraction peaks were ammonium rhenate, and other crystal items were not observed. When the centrifugal speed was 1000 rpm, the characteristic peaks in the XRD patterns were highest, FWHM was minimum, and relative crystallinity was the ideal. If the relative crystallinity of the samples prepared at 1000 rpm was treated as 100%, according to the amorphization formula A = (1 − Areax/Area0) × 100%, the relative crystallinity of the samples at 500 rpm and NH3 1500 rpm was 69.2% and 47.8%.
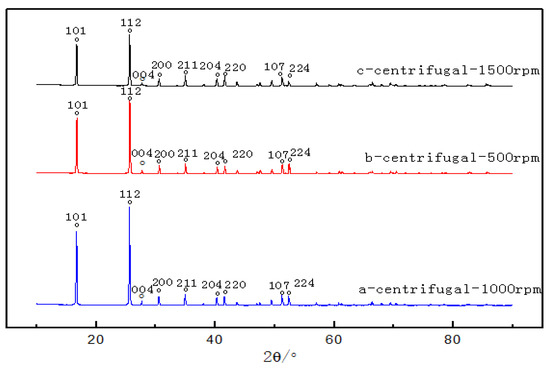
Figure 6.
XRD patterns of NH4ReO4 crystals at different centrifugal speeds: (a) 1000 rpm, (b) 500 rpm, (c) 1500 rpm.

Table 4.
XRD information of NH4ReO4 crystals at different centrifugal speeds.
According to Table 4, the selectivity of crystal growth direction on (101) crystal plane and (112) crystal plane at different centrifugal speeds can be calculated by the Scherrer formula, D = Kλ/(B cos θ), and the results are given in Table 5. From Table 3 it can be seen that the growth of (101) crystal plane and (112) crystal plane was promoted at an appropriate centrifugal speed, and was promoted with increased ammonia content.

Table 5.
Selectivity of crystal growth direction for NH4ReO4 crystals at different centrifugal speeds.
From what has been discussed above, excessive aqueous ammonia and centrifugal speed lead to rapid crystal growth, thus introducing impurities. The appropriate amount of aqueous ammonia and centrifugal speed are favorable for the morphology and relative crystallinity of NH4ReO4 crystal, and higher-purity NH4ReO4 crystals are obtained. The reason is that the appropriate amount of aqueous ammonia causes the dissolution equilibrium to reverse, which reduces the induction period of nucleation, and the formation of crystal nucleus and growth of crystal reach an ideal equilibrium state. The appropriate centrifugal speed makes the larger sedimentary particles collide and break into the secondary crystallization stage, increases the number of crystal nuclei, and speeds up the growth rate of the crystal, which causes the crystals to grow at an ideal rate.
4. Conclusions
(1) The amount of aqueous ammonia has little influence on the purity of NH4ReO4, but centrifugal speed has a great influence on it, and the purity of NH4ReO4 is reduced by excessive centrifugal speed.
(2) Aging time decreases with increased ammonia content and centrifugal speed. The amount of aqueous ammonia and centrifugal speed have a great influence on the morphology of NH4ReO4 crystal, and it is complete rhombic crystal at the appropriate amount of aqueous ammonia and centrifugal speed.
(3) The growth of NH4ReO4 crystal reached the ideal status at 10 wt % NH3, but the growth of (101) and (112) crystal planes was restrained with the increase of ammonia content. However, the growth gates of (101) and (112) crystal planes were amplified.
(4) The growth of NH4ReO4 crystal reached the best status at 1000 rpm centrifugal speed, and the growth of (101) and (112) crystal planes was restrained when the system had excessive or weak centrifugal speed. However, the growth gates of (101) and (112) crystal planes were amplified with increased centrifugal speed.
Author Contributions
J.T. and Y.Z. performed the experiments and tests. Y.S., G.H., and Y.D. analyzed the results. J.T. and Y.Z. designed the experiments. J.T., Y.Z., Y.S., G.H., F.H., and Y.D. wrote the manuscript. All authors gave their final approval for publication.
Funding
This work was financially supported by the National Key R & D Program of China under grant no. 2017YFA0700700 (2017YFA0700704). The PhD Startup Fund was supported by the Liaoning Institute of Science and Technology. Shenyang R & D Platform Special Fund was supported (F16-082-8-00). The authors are grateful for this support.
Acknowledgments
The authors acknowledge the help of Long Wang of the Analytical and Testing Center of Northeastern University for the SEM and XRD analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xian, W.; Qian, W.Y.; Han-Qi, M. The current situation of processing technology of superalloys scrap. China Molybdenum Ind. 2015, 39, 8–11. [Google Scholar] [CrossRef]
- Chen, X.; Tan, Z.; Wu, Y.F.; Liu, B.; Guo, Z.J.; Li, R.B. Rcecnt progress of recovery of scattered metal rhenium from high-temperature alloy scraps. Mod. Chem. Ind. 2017, 1, 60–63. [Google Scholar] [CrossRef]
- Barakat, M.A.; Mahmoud, M.H.H. Recovery of platinum from spent catalyst. Hydrometallurgy 2004, 72, 176–184. [Google Scholar] [CrossRef]
- Jha, M.K.; Lee, J.; Kim, M.; Jeong, J.; Kim, B.; Kumar, V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy 2013, 133, 23–32. [Google Scholar] [CrossRef]
- Kasikov, L.G.; Petrova, A.M. Processing of deactivated platinum-rhenium catalysts. Theor. Found. Chem. Eng. 2009, 43, 544–552. [Google Scholar] [CrossRef]
- Zou, Z.Q.; Zhou, Q.J. Recovery of Molybdenum and Rhenium from Molybdenite Concentratein Dexing Copper Ore by Lime Roasting-N235 Extraction Method. Min. Metall. Eng. 2002, 22, 79–85. [Google Scholar]
- Hai, X.; He-Sheng, L.; Dong, W. Study on CaSO4 crystallization process and its influential factors. Ind. Water Treat. 2011, 31, 67–69. [Google Scholar] [CrossRef]
- Yu-Nan, Q. New Technology for Extracted NH4ReO4 with Ion Exchanging Method. China Molybdenum Ind. 1998, 22, 39–41. [Google Scholar]
- Lin, C.S.; Gao, Q.S. Rescarch on Recovery of Rhenium Form Drip-Washing Liquid by Ion Exchange with 201×7 Resins. China Molybdenum Ind. 2009, 33, 30–31. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, J.S.; Seo, S.Y.; Tran, T.; Kin, M.J. Recovery of rhenium from a molybdenite roaster fume as high purity ammonium perrhenate. Hydrometallurgy 2015, 156, 158–164. [Google Scholar] [CrossRef]
- Seo, S.Y.; Choi, W.S.; Jin, T.; Myong, Y.; Kim, J.; Tran, T. Recovery of rhenium and molybdenum from a roaster fume scrubbing liquor by adsorption using activated carbon. Hydrometallurgy 2012, 129–130, 145–150. [Google Scholar] [CrossRef]
- Lan, X.; Liang, S.; Song, Y. Recovery of rhenium from molybdenite calcine by a resin-in-pulp process. Hydrometallurgy 2006, 82, 133–136. [Google Scholar] [CrossRef]
- Shariat, M.H.; Hassani, M. Rhenium recovery from Sarcheshmeh molybdenite concentrate. J. Mater. Process. Technol. 1998, 74, 243–250. [Google Scholar] [CrossRef]
- Jun-Jie, T.; Yan, L.; Lei, T.; Dong-Xing, W.; Ting-An, Z. The influence of flow field distribution on the crystallization of spherical nickel hydroxide in reactor. Chin. J. Inorg. Chem. 2016, 32, 1127–1134. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).