Reduction in Trace Element Mediated Oxidative Stress towards Cropped Plants via Beneficial Microbes in Irrigated Cropping Systems: A Review
Abstract
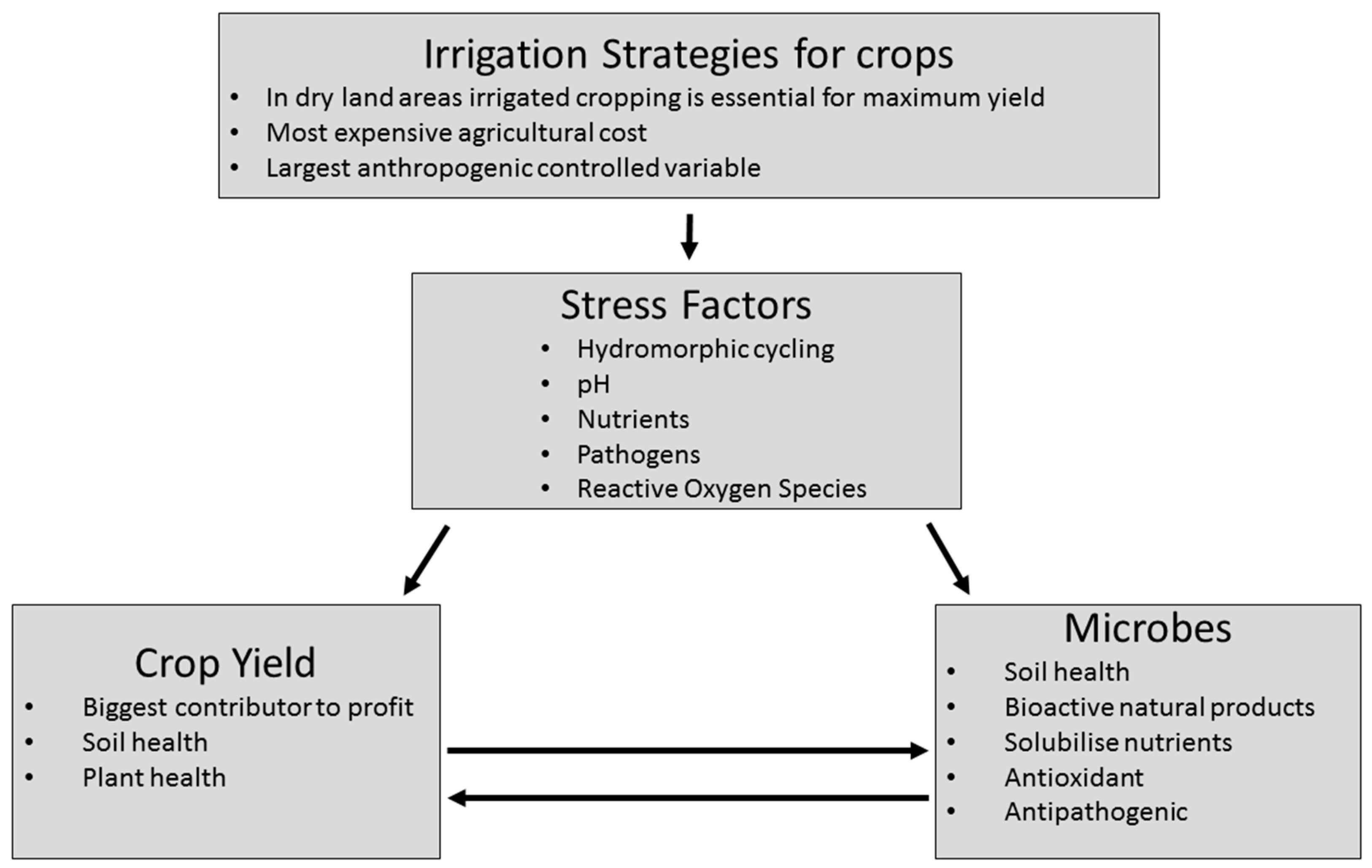
1. Introduction
2. Physiological and Biochemical Impacts of Oxidative Stress towards Cropped Plants
3. The Impact of Trace Element Availability on the Induction of Oxidative Stress in Cropped Plants
4. Plant Antioxidant Defenses against Oxidative Stress Challenges
5. Can Bacterial Antioxidant Activity Reduce Oxidative Stress towards Cropped Plants?
Author Contributions
Funding
Conflicts of Interest
References
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trend Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Cucu, M.A.; Said-Pullicino, D.; Maurino, V.; Bonifacio, E.; Romani, M.; Celi, L. Influence of redox conditions and rice straw incorporation on nitrogen availability in fertilized paddy soils. Biol. Fertil. Soils 2014, 50, 755–764. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, M. Effect of moisture regime on the redistribution of heavy metals in paddy soil. J. Environ. Sci. 2011, 23, 434–443. [Google Scholar] [CrossRef]
- Timsina, J.; Jat, M.L.; Majumdar, K. Rice-maize systems of south Asia: Current status, future prospects and research priorities for nutrient management. Plant Soil 2010, 335, 65–82. [Google Scholar] [CrossRef]
- Pantano, G.; Campanha, M.B.; Moreira, A.B.; Bisinoti, M.C. Occurrence of Cu and Cr in the sedimentary humic substances and pore water from a typical sugar cane cultivation area in São Paulo, Brazil. J. Soils Sediments 2014, 14, 377–384. [Google Scholar] [CrossRef]
- Morrison, M.J.; Stewart, D.W. Heat stress during flowering in summer Brassica (Crop Physiology & Metabolism). Crop Sci. 2002, 42, 797–803. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Zhang, B. Evaluation and selection of reliable reference genes for gene expression under abiotic stress in cotton (Gossypium hirsutum L.). Gene 2013, 530, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Kamal, A.H.M.; Hossain, Z. Wheat proteomics: Proteome modulation and abiotic stress acclimation. Front. Plant Sci. 2014, 5, 684. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar] [CrossRef] [PubMed]
- Gozubuyuk, Z.; Sahin, U.; Adiguzel, M.C.; Ozturk, I.; Celik, A. The influence of different tillage practices on water content of soil and crop yield in vetch–winter wheat rotation compared to fallow–winter wheat rotation in a high altitude and cool climate. Agric. Water Manag. 2015, 160, 84–97. [Google Scholar] [CrossRef]
- Sprague, S.J.; Kirkegaard, J.A.; Graham, J.M.; Dove, H.; Kelman, W.M. Crop and livestock production for dual-purpose winter canola (Brassica napus) in the high-rainfall zone of south-eastern Australia. Field Crop. Res. 2014, 156, 30–39. [Google Scholar] [CrossRef]
- Breidenbach, B.; Blaser, M.B.; Klose, M.; Conrad, R. Crop rotation of flooded rice with upland maize impacts the resident and active methanogenic microbial community. Environ. Microbiol. 2016, 18, 2868–2885. [Google Scholar] [CrossRef] [PubMed]
- Sunrice-Australia. Sunrice Annual Report 2016. Available online: https://www.sunrice.com.au/media/577307/sunrice-annual-report-2016.pdf (accessed on 2 March 2018).
- Canegrowers. Canegrowers Annual Report 2015/16. Available online: http://www.canegrowers.com.au/icms_docs/271105_canegrowers-annual-report-2015-16.pdf (accessed on 2 March 2018).
- Cotton-Australia. Australian Cotton Industry Overview. Available online: http://cottonaustralia.com.au/cotton-library/fact-sheets/cotton-fact-file-the-australian-cotton-industry (accessed on 2 March 2018).
- RuralBank. Australian Crop Update 2016. Available online: https://www.ruralfinance.com.au/uploads/aga_documents/crop-report-2016.pdf (accessed on 2 March 2018).
- Belder, P.; Spiertz, J.H.J.; Bouman, B.A.M.; Lu, G.; Tuong, T.P. Nitrogen economy and water productivity of lowland rice under water-saving irrigation. Field Crop Res. 2005, 93, 169–185. [Google Scholar] [CrossRef]
- Hu, P.; Ouyang, Y.; Wu, L.; Shen, L.; Luo, Y.; Christie, P. Effects of water management on arsenic and cadmium speciation and accumulation in an upland rice cultivar. J. Environ. Sci. 2015, 27, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Eranki, P.L.; El-Shikha, D.; Hunsaker, D.J.; Bronson, K.F.; Landis, A.E. A comparative life cycle assessment of flood and drip irrigation for guayule rubber production using experimental field data. Ind. Crop Prod. 2017, 99, 97–108. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Meharg, A.A.; Smolders, E.; Manzano, R.; Becerra, D.; Sánchez-Llerena, J.; Albarrán, Á.; López-Piñero, A. Sprinkler irrigation of rice fields reduces grain arsenic but enhances cadmium. Sci. Total Environ. 2014, 485–486, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Borin, J.B.M.; Carmona, F.d.C.; Anghinoni, I.; Martins, A.P.; Jaeger, I.R.; Marcolin, E.; Hernandes, G.C.; Camargo, E.S. Soil solution chemical attributes, rice response and water use efficiency under different flood irrigation management methods. Agric. Water Manag. 2016, 176, 9–17. [Google Scholar] [CrossRef]
- Kijne, J.W. Abiotic stress and water scarcity: Identifying and resolving conflicts from plant level to global level. Field Crop Res. 2006, 97, 3–18. [Google Scholar] [CrossRef]
- Bankaji, I.; Sleimi, N.; López-Climent, M.F.; Perez-Clemente, R.M.; Gomez-Cadenas, A. Effects of Combined Abiotic Stresses on Growth, Trace Element Accumulation, and Phytohormone Regulation in Two Halophytic Species. J. Plant Growth Regul. 2014, 33, 632–643. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Bonten, L.T.C.; Koopmans, G.F.; Song, J.; Luo, Y.; Temminghoff, E.J.M.; Comans, R.N.J. Solubility of trace metals in two contaminated paddy soils exposed to alternating flooding and drainage. Geoderma 2016, 261, 59–69. [Google Scholar] [CrossRef]
- Gao, X.; Hoffland, E.; Stomph, T.; Grant, C.A.; Zou, C.; Zhang, F. Improving zinc bioavailability in transition from flooded to aerobic rice. A review. Agron. Sustain. Dev. 2012, 32, 465–478. [Google Scholar] [CrossRef]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M.G. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Soil–plant transfer of trace elements—An environmental issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Halili, J.; Bislimi, K.; Mazreku, I.; Behluli, A.; Osmani, F.; Maloku, A.; Halili, F. Translocation of some heavy metals from soil in fruit-wines of the grape vine vineyards of Rahovec. Int. Multidiscip. Sci. Geoconf. SGEM 2013, 1, 531. [Google Scholar]
- Nessa, F.; Jewel, M.A.H. Analysis of soil nutrient and heavy metal concentration in agricultural land of Zirani industrial area, Savar, Dhaka. Int. J. Innov. Sci. Res. 2014, 10, 90–98. [Google Scholar]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 191–248. [Google Scholar]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Saenen, E.; Horemans, N.; Vanhoudt, N.; Vandenhove, H.; Biermans, G.; Van Hees, M.; Wannijn, J.; Vangronsveld, J.; Cuypers, A. Effects of pH on uranium uptake and oxidative stress responses induced in Arabidopsis thaliana. Environ. Toxicol. Chem. 2013, 32, 2125–2133. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.; Munné-Bosch, S. Production and scavenging of reactive oxygen species and redox signaling during leaf and flower senescence: Similar but different. Plant Physiol. 2016, 171, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Dizdaroglu, M.; Jaruga, P. Mechanisms of free radical-induced damage to DNA. Free Radic. Res. 2012, 46, 382–419. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, U.; Das, D.; Banerjee, R.K. Reactive oxygen species: Oxidative damage and pathogenesis. Curr. Sci. India 1999, 77, 658–666. [Google Scholar]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Halford, N.G.; Curtis, T.Y.; Chen, Z.; Huang, J. Effects of abiotic stress and crop management on cereal grain composition: Implications for food quality and safety. J. Exp. Bot. 2014, 66, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, S.; Cortleven, A.; Iven, T.; Feussner, I.; Havaux, M.; Riefler, M.; Schmülling, T. Circadian Stress Regimes Affect the Circadian Clock and Cause Jasmonic Acid-Dependent Cell Death in Cytokinin-Deficient Arabidopsis Plants. Plant Cell 2016, 28, 1616–1639. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ding, Z.; Tie, W.; Yan, Y.; Liu, Y.; Wu, C.; Liu, J.; Wang, J.; Peng, M.; Xu, B.; et al. Comparative physiological and transcriptomic analyses provide integrated insight into osmotic, cold, and salt stress tolerance mechanisms in banana. Sci. Rep. 2017, 7, 43007. [Google Scholar] [CrossRef] [PubMed]
- Tsabari, O.; Nevo, R.; Meir, S.; Carrillo, L.R.; Kramer, D.M.; Reich, Z. Differential effects of ambient or diminished CO2 and O2 levels on thylakoid membrane structure in light-stressed plants. Plant J. 2015, 81, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Cold stress regulation of gene expression in plants. Trend Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.N.; Ferreira-Silva, S.L.; Fontenele, A.d.V.; Ribeiro, R.V.; Viégas, R.A.; Silveira, J.A.G. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J. Plant Physiol. 2010, 167, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.-B.; Chu, L.-Y.; Jaleel, C.A.; Zhao, C.-X. Water-deficit stress-induced anatomical changes in higher plants. C. R. Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Arinaga, N.; Umezawa, T.; Katsura, S.; Nagamachi, K.; Tanaka, H.; Ohiraki, H.; Yamada, K.; Seo, S.-U.; Abo, M.; et al. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 2013, 25, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Larher, F. Osmotic stress induced changes in lipid composition and peroxidation in leaf discs of Brassica napus L. J. Plant Physiol. 1998, 153, 754–762. [Google Scholar] [CrossRef]
- Grybos, M.; Davranche, M.; Gruau, G.; Petitjean, P. Is trace metal release in wetland soils controlled by organic matter mobility or Fe-oxyhydroxides reduction? J. Colloid Interface Sci. 2007, 314, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Cong, V.H.; Sakakibara, Y. Identification and application of Phyto-Fenton reactions. Chemosphere 2016, 144, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Sitthisak, S.; Sengupta, M.; Johnson, M.; Jayaswal, R.; Morrissey, J.A. Copper stress induces a global stress response in Staphylococcus aureus and represses sae and agr expression and biofilm formation. Appl. Environ. Microbiol. 2010, 76, 150–160. [Google Scholar] [CrossRef]
- Ward, S.K.; Hoye, E.A.; Talaat, A.M. The global responses of Mycobacterium tuberculosis to physiological levels of copper. J. Bacteriol. 2008, 190, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- Teitzel, G.M.; Geddie, A.; Susan, K.; Kirisits, M.J.; Whiteley, M.; Parsek, M.R. Survival and growth in the presence of elevated copper: Transcriptional profiling of copper-stressed Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7242–7256. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, A.G.; McAinsh, M.; Davies, J.M. The hydroxyl radical in plants: From seed to seed. J. Exp. Bot. 2015, 66, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.G.; Cheryl-lynn, Y.O.; Walker, M.J.; Djoko, K.Y.; McEwan, A.G. Transition Metal Homeostasis in Streptococcus pyogenes and Streptococcus pneumoniae. Adv. Microb. Physiol. 2017, 70, 123–191. [Google Scholar] [PubMed]
- Pandey, N.; Pathak, G.C.; Pandey, D.K.; Pandey, R. Heavy metals, Co, Ni, Cu, Zn and Cd, produce oxidative damage and evoke differential antioxidant responses in spinach. Braz. J. Plant Physiol. 2009, 21, 103–111. [Google Scholar] [CrossRef]
- Leskova, A.; Giehl, R.F.H.; Hartmann, A.; Fargasová, A.; von Wirén, N. Heavy metals induce iron-deficiency responses at different hierarchic and regulatory levels. Plant Physiol. 2017, 174, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Soetan, K.; Olaiya, C.; Oyewole, O. The importance of mineral elements for humans, domestic animals and plants—A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Bordo, D.; Djinovic, K.; Bolognesi, M. Conserved Patterns in the Cu, Zn Superoxide Dismutase Family. J. Mol. Biol. 1994, 238, 366–386. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Van Camp, W.; Van Montagu, M.; Inze, D.; Asada, K. Superoxide dismutase in plants. CRC Crit. Rev. Plant Sci. 1994, 13, 199–218. [Google Scholar] [CrossRef]
- Kanematsu, S.; Asada, K. Ferric and manganic superoxide dismutases in Euglena gracilis. Biochem. Biophys. 1979, 195, 535–545. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zamocky, M.; Furtmüller, P.G.; Obinger, C. Evolution of catalases from bacteria to humans. Antioxid. Redox Signal. 2008, 10, 1527–1548. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Suggett, A. The catalase–hydrogen peroxide system. Kinetics of catalatic action at high substrate concentrations. Biochem. J. 1968, 110, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Chandlee, J.M.; Tsaftaris, A.S.; Scandalios, J.G. Purification and partial characterization of three genetically defined catalases of maize. Plant Sci. Lett. 1983, 29, 117–131. [Google Scholar] [CrossRef]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Y.; Wang, P.C.; Chen, J.; Song, C.P. Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J. Integr. Plant Biol. 2008, 50, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Rieuwerts, J.S.; Thornton, I.; Farago, M.E.; Ashmore, M.R. Factors influencing metal bioavailability in soils: Preliminary investigations for the development of a critical loads approach for metals. Chem. Speciat. Bioavailab. 1998, 10, 61–75. [Google Scholar] [CrossRef]
- Brallier, S.; Harrison, R.; Henry, C.; Dongsen, X. Liming effects on availability of Cd, Cu, Ni and Zn in a soil amended with sewage sludge 16 years previously. Water Air Soil Pollut. 1996, 86, 195–206. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharm. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, I.; de Vos, R.C.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trend Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Sánchez-Cañizares, C.; Jorrín, B.; Poole, P.S.; Tkacz, A. Understanding the holobiont: The interdependence of plants and their microbiome. Curr. Opin. Microbiol. 2017, 38, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Penrose, D.M.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, A.; de Souza, R.; Passaglia, L.M. Ecological role of bacterial inoculants and their potential impact on soil microbial diversity. Plant Soil 2016, 400, 193–207. [Google Scholar] [CrossRef]
- Howard, J.B.; Rees, D.C. Structural basis of biological nitrogen fixation. Chem. Rev. 1996, 96, 2965–2982. [Google Scholar] [CrossRef] [PubMed]
- Burd, G.I.; Dixon, D.G.; Glick, B.R. Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can. J. Microbiol. 2000, 46, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef]
- Kuklinsky-Sobral, J.; Araújo, W.L.; Mendes, R.; Geraldi, I.O.; Pizzirani-Kleiner, A.A.; Azevedo, J.L. Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ. Microbiol. 2004, 6, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Heo, Y.-J.; Lee, J.K.; Cho, Y.-H. KatA, the major catalase, is critical for osmoprotection and virulence in Pseudomonas aeruginosa PA14. Infect. Immun. 2005, 73, 4399–4403. [Google Scholar] [CrossRef] [PubMed]
- Jamet, A.; Sigaud, S.; Van de Sype, G.; Puppo, A.; Hérouart, D. Expression of the bacterial catalase genes during Sinorhizobium meliloti-Medicago sativa symbiosis and their crucial role during the infection process. Mol. Plant Microbe Interact. 2003, 16, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Alhasawi, A.; Castonguay, Z.; Appanna, N.D.; Auger, C.; Appanna, V.D. Glycine metabolism and anti-oxidative defence mechanisms in Pseudomonas fluorescens. Microbiol. Res. 2015, 171, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Bihani, S.C.; Chakravarty, D.; Ballal, A. KatB, a cyanobacterial Mn-catalase with unique active site configuration: Implications for enzyme function. Free Radic. Biol. Med. 2016, 93, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Banerjee, M.; Bihani, S.C.; Ballal, A. A salt-inducible Mn-catalase (KatB) protects cyanobacterium from oxidative stress. Plant Physiol. 2015, 175, 1632. [Google Scholar] [CrossRef] [PubMed]
- Jamet, A.; Mandon, K.; Puppo, A.; Hérouart, D. H2O2 is required for optimal establishment of the Medicago sativa/Sinorhizobium meliloti symbiosis. J. Bacteriol. 2007, 189, 8741–8745. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct. Plant Biol. 2008, 35, 141–151. [Google Scholar] [CrossRef]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant–pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Cárdenas, M.L.; Narváez-Vásquez, J.; Ryan, C.A. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 2001, 13, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Tondo, M.L.; Delprato, M.L.; Kraiselburd, I.; Fernández Zenoff, M.V.; Farías, M.E.; Orellano, E.G. KatG, the bifunctional catalase of Xanthomonas citri subsp. citri, responds to hydrogen peroxide and contributes to epiphytic survival on citrus leaves. PLoS ONE 2016, 11, e0151657. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Crowell, S.A.; Harding, C.M.; De Silva, P.M.; Harrison, A.; Fernando, D.M.; Mason, K.M.; Santana, E.; Loewen, P.C.; Kumar, A.; et al. KatG and KatE confer Acinetobacter resistance to hydrogen peroxide but sensitize bacteria to killing by phagocytic respiratory burst. Life Sci. 2016, 148, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Brossier, F.; Boudinet, M.; Jarlier, V.; Petrella, S.; Sougakoff, W. Comparative study of enzymatic activities of new KatG mutants from low- and high-level isoniazid-resistant clinical isolates of Mycobacterium tuberculosis. Tuberculosis 2016, 100, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Behnsen, J.; Raffatellu, M. Siderophores: More than stealing iron. MBio 2016, 7, e01906. [Google Scholar] [CrossRef] [PubMed]
- Premachandra, D.; Hudek, L.; Brau, L. Bacterial modes of action for enhancing of plant growth. J. Biotechnol. Biomater. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Hudek, L.; Rai, S.; Michalczyk, A.; Rai, L.; Neilan, B.; Ackland, M.L. Physiological metal uptake by Nostoc punctiforme. Biometals 2012, 25, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Jacobsen, F.E.; Giedroc, D.P. Coordination chemistry of bacterial metal transport and sensing. Chem. Rev. 2009, 109, 4644–4681. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Singh, R.; Arora, N.K. Alleviation of Heavy Metal Stress in Plants and Remediation of Soil by Rhizosphere Microorganisms. Front. Microbiol. 2017, 8, 1706. [Google Scholar] [CrossRef] [PubMed]
- Mejáre, M.; Bülow, L. Metal-binding proteins and peptides in bioremediation and phytoremediation of heavy metals. Trends Biotechnol. 2001, 19, 67–73. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Ali, Q.; Mubin, M.; Ali, S.; Arif, M.S.; Hussain, S.; Riaz, M.; Abbas, F. Copper-resistant bacteria reduces oxidative stress and uptake of copper in lentil plants: Potential for bacterial bioremediation. Environ. Sci. Pollut. Res. Int. 2016, 23, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Vivas, A.; Biró, B.; Ruíz-Lozano, J.M.; Barea, J.M.; Azcón, R. Two bacterial strains isolated from a Zn-polluted soil enhance plant growth and mycorrhizal efficiency under Zn-toxicity. Chemosphere 2006, 62, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Radzki, W.; Gutierrez Mañero, F.J.; Algar, E.; Lucas García, J.A.; García-Villaraco, A.; Ramos Solano, B. Bacterial siderophores efficiently provide iron to iron-starved tomato plants in hydroponics culture. Antonie Leeuwenhoek 2013, 104, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Johri, B.N. Growth promoting influence of siderophore-producing Pseudomonas strains GRP3A and PRS9 in maize (Zea mays L.) under iron limiting conditions. Microbiol. Res. 2003, 158, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kołodyńska, D. Application of a new generation of complexing agents in removal of heavy metal ions from different wastes. Environ. Sci. Pollut. Res. Int. 2013, 20, 5939–5949. [Google Scholar] [CrossRef] [PubMed]
- Grčman, H.; Velikonja-Bolta, Š.; Vodnik, D.; Kos, B.; Leštan, D. EDTA enhanced heavy metal phytoextraction: Metal accumulation, leaching and toxicity. Plant Soil 2001, 235, 105–114. [Google Scholar] [CrossRef]
- Hudek, L.; Enez, A.; Webster, W.A.J.; Premachandra, D.; Bräu, L. Inoculation of Brassica napus L. (canola) with Pseudomonas fluorescens DUS1-27 leads to inhibition of plant growth due to accumulation of hydrogen peroxide. Plant Soil 2018. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enez, A.; Hudek, L.; Bräu, L. Reduction in Trace Element Mediated Oxidative Stress towards Cropped Plants via Beneficial Microbes in Irrigated Cropping Systems: A Review. Appl. Sci. 2018, 8, 1953. https://doi.org/10.3390/app8101953
Enez A, Hudek L, Bräu L. Reduction in Trace Element Mediated Oxidative Stress towards Cropped Plants via Beneficial Microbes in Irrigated Cropping Systems: A Review. Applied Sciences. 2018; 8(10):1953. https://doi.org/10.3390/app8101953
Chicago/Turabian StyleEnez, Aydin, Lee Hudek, and Lambert Bräu. 2018. "Reduction in Trace Element Mediated Oxidative Stress towards Cropped Plants via Beneficial Microbes in Irrigated Cropping Systems: A Review" Applied Sciences 8, no. 10: 1953. https://doi.org/10.3390/app8101953
APA StyleEnez, A., Hudek, L., & Bräu, L. (2018). Reduction in Trace Element Mediated Oxidative Stress towards Cropped Plants via Beneficial Microbes in Irrigated Cropping Systems: A Review. Applied Sciences, 8(10), 1953. https://doi.org/10.3390/app8101953




