New Generation of Electrochemical Sensors Based on Multi-Walled Carbon Nanotubes
Abstract
1. Introduction
2. MWCNT Synthesis Methods
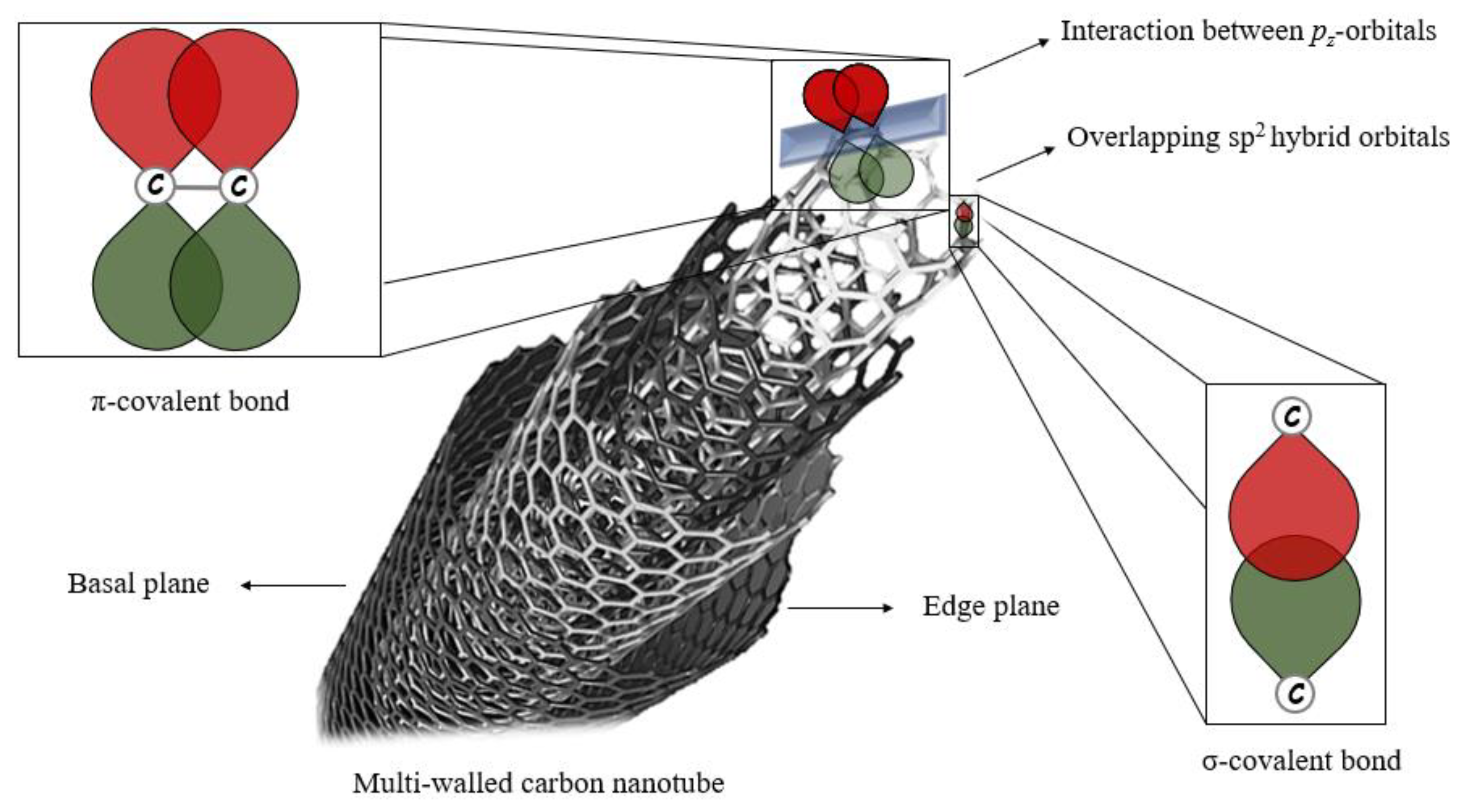
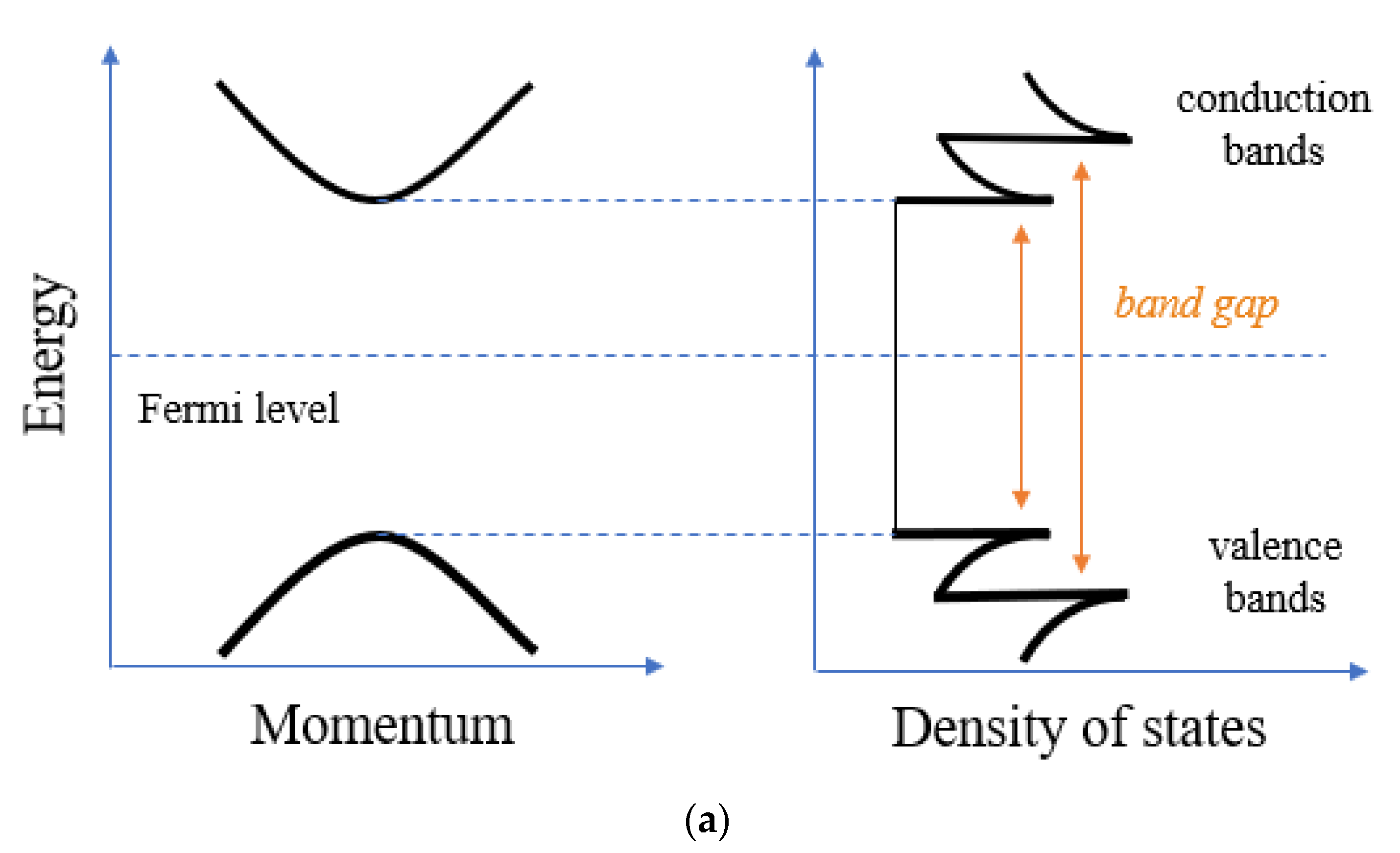
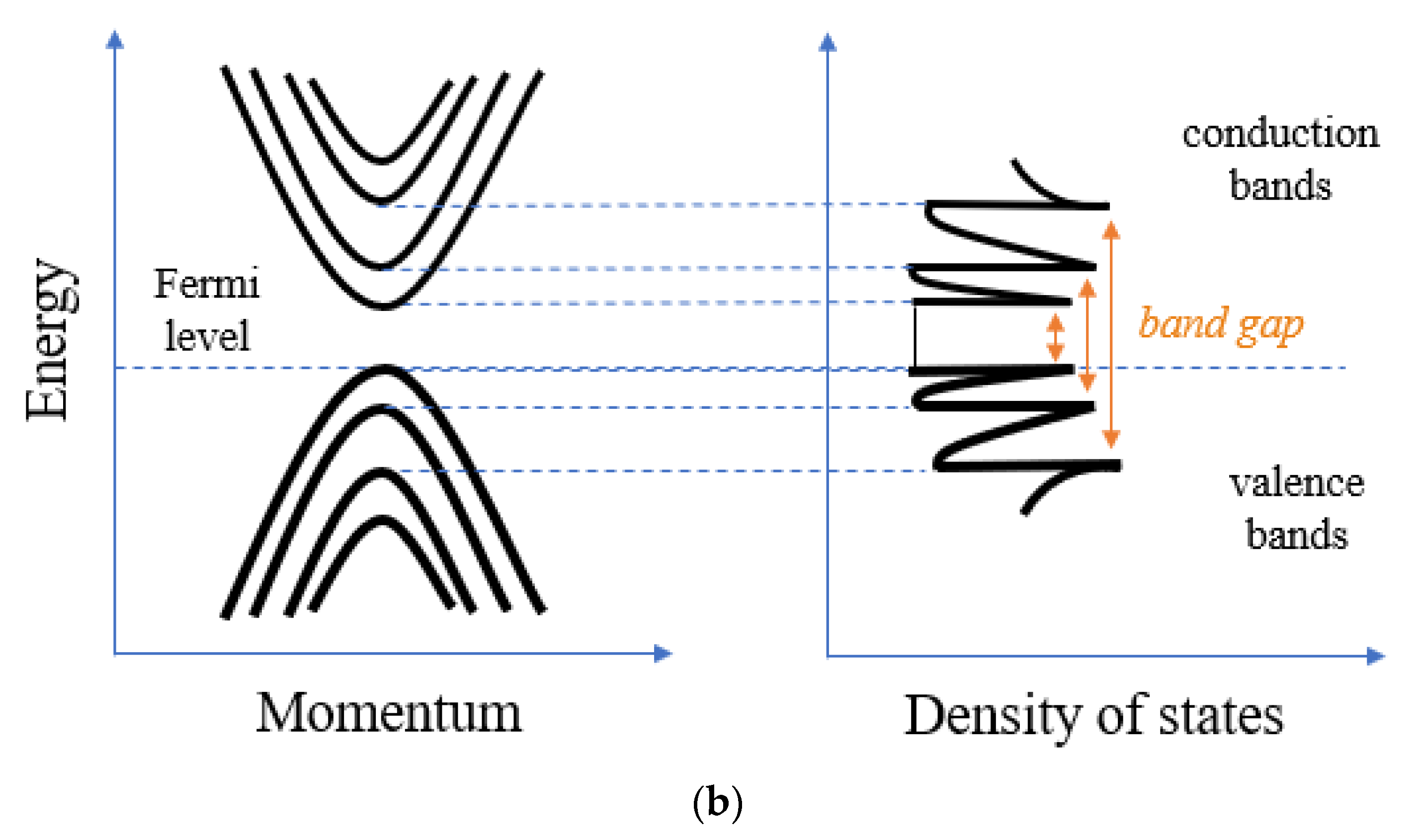
3. MWCNT Electrochemical Properties
4. Overview of MWCNT Applications in Electrochemical Sensors
5. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sudha, P.N.; Sangeetha, K.; Vijayalakshmi, K.; Barhoum, A. Nanomaterials history, classification, unique properties, production and market. In Emerging Applications of Nanoparticles and Architecture Nanostructures—Current Prospects and Future Trends; A Volume in Micro and Nano Technologies; Makhlouf, A.S.H., Barhoum, A., Eds.; Elsevier: Cambridge, MA, USA, 2018; pp. 341–384. ISBN 978-0-323-51254-1. [Google Scholar]
- Soriano, M.S.; Zougagh, M.; Valcárcel, M.; Ríos, Á. Analytical Nanoscience and Nanotechnology: Where we are and where we are heading. Talanta 2018, 177, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Adams, F.C.; Barbante, C. Nanoscience, nanotechnology and spectrometry. Spectrochim. Acta Part B 2013, 86, 3–13. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Lu, J.; Zhu, H. The formation mechanism of chiral carbon nanotubes. Physica B 2018, 530, 277–282. [Google Scholar] [CrossRef]
- Kurkowska, M.; Awietjan, S.; Kozera, R.; Jezierska, E.; Boczkowska, A. Application of electroless deposition for surface modification of the multiwall carbon nanotubes. Chem. Phys. Lett. 2018, 702, 38–43. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Dumitrescu, I.; Unwin, P.R.; Macpherson, J.V. Electrochemistry at carbon nanotubes: Perspective and issues. Chem. Commun. 2009, 6886–6901. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Sawada, S.-I.; Oshiyama, A. New one-dimensional conductors: Graphitic microtubules. Phys. Rev. Lett. 1992, 68, 1579–1581. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Thiol functionalized carbon nanotubes: Synthesis by sulfur chemistry and their multi-purpose applications. Appl. Surf. Sci. 2018, 447, 235–243. [Google Scholar] [CrossRef]
- Xiao, Z.; Elike, J.; Reynolds, A.; Moten, R.; Zhao, X. The fabrication of carbon nanotube electronic circuits with dielectrophoresis. Microelectron. Eng. 2016, 164, 123–127. [Google Scholar] [CrossRef]
- Su, L.; Wang, X.; Wang, Y.; Zhang, Q. Roles of carbon nanotubes in novel energy storage devices. Carbon 2017, 122, 462–474. [Google Scholar] [CrossRef]
- Guo, Y.; Shen, G.; Sun, X.; Wang, X. Electrochemical aptasensor based on multiwalled carbon nanotubes and graphene for tetracycline detection. IEES Sens. J. 2015, 15, 1951–1958. [Google Scholar] [CrossRef]
- Liu, L.; Niu, Z.; Chen, J. Flexible supercapacitors based on carbon nanotubes. Chin. Chem. Lett. 2018, 29, 571–581. [Google Scholar] [CrossRef]
- Parveen, S.; Kumar, A.; Husain, S.; Husain, M. Fowler Nordheim theory of carbon nanotube based field emitters. Phys. B Condens. Matter. 2017, 505, 1–8. [Google Scholar] [CrossRef]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Kim, S.N.; Rusling, J.F.; Papadimitrakopoulos, F. Carbon nanotubes for electronic and electrochemical detection of biomolecules. Adv. Mater. 2007, 19, 3214–3228. [Google Scholar] [CrossRef] [PubMed]
- López-Lorente, Á.; Valcárcel, M. The third way in analytical nanoscience and nanotechnology: Involvement of nanotools and nanoanalytes in the same analytical process. Trends Analyt. Chem. 2016, 75, 1–9. [Google Scholar] [CrossRef]
- Harris, P.J. Engineering carbon materials with electricity. Carbon 2017, 122, 504–513. [Google Scholar] [CrossRef]
- Abdalla, S.; Al-Marzouki, F.; Al-Ghamdi, A.A.; Abdel-Daiem, A. Different technical applications of carbon nanotubes. Nanoscale Res. Lett. 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Vosguerichian, M.; Zhenan Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, S.; Ghaderi, A.; Boochani, A.; Solaymani, S. Synthesis of multiwalled carbon nanotubes on Cu-Fe nano-catalyst substrate. Res. Phys. 2017, 7, 3640–3644. [Google Scholar] [CrossRef]
- Dhore, V.G.; Rathod, W.S.; Patil, K.N. Synthesis and characterization of high yield multiwalled carbon nanotubes by ternary catalyst. Mater. Today Proc. 2018, 5, 3432–3437. [Google Scholar] [CrossRef]
- Monthioux, M.; Serp, P.; Flahaut, E.; Razafinimanana, M.; Laurent, C.; Peigney, A.; Bacsa, W.; Broto, J.-M. Introduction to carbon nanotubes. In Springer Handbook of Nanotechnology, 2nd ed.; Bhushan, B., Ed.; Springer: Berlin, Germany, 2007; pp. 47–118. ISBN 3-540-29855-X. [Google Scholar]
- Araga, R.; Sharma, C.S. One step direct synthesis of multiwalled carbon nanotubes from coconut shell derived charcoal. Mater. Lett. 2017, 188, 205–207. [Google Scholar] [CrossRef]
- Rius, G.; Baldi, A.; Ziaie, B.; Atashbar, M.Z. Introduction to micro-/nanofabrication. In Springer Handbook of Nanotechnology, 4th ed.; Bhushan, B., Ed.; Springer: Berlin, Germany, 2017; pp. 51–86. ISBN 978-3-662-54355-9. [Google Scholar]
- Yáñez-Sedeño, P.; Pingarrón, J.M.; Riu, J.; Rius, F.X. Electrochemical sensing based on carbon nanotubes. Trends Anal. Chem. 2010, 29, 939–953. [Google Scholar] [CrossRef]
- Bandaru, P.R. Electrical properties and applications of carbon nanotube structures. J. Nanosci. Nanotechnol. 2007, 7, 1–29. [Google Scholar] [CrossRef]
- Ahammad, A.J.S.; Lee, J.-J.; Rahman, M.A. Electrochemical sensors based on carbon nanotubes. Sensors 2009, 9, 2289–2319. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.; Li, H.; Yu, L.; Hu, X. Electrochemical sensor based on multi-walled carbon nanotubes and chitosan-nickel complex for sensitive determination of metronidazole. J. Electroanal. Chem. 2017, 799, 257–262. [Google Scholar] [CrossRef]
- Holanda, L.F.; Ribeiro, F.W.P.; Sousa, C.P.; Casciano, P.N.S.; De Lima-Neto, P.; Correia, A.N. Multi-walled carbon nanotubes-cobalt phthalocyanine modified electrode for electroanalytical determination of acetaminophen. J. Electroanal. Chem. 2016, 772, 9–16. [Google Scholar] [CrossRef]
- Montes, R.H.O.; Lima, A.P.; Cunha, R.R.; Guedes, T.J.; Dos Santos, W.T.P.; Nosso, E.; Richter, E.M.; Munoz, R.A.A. Size effects of multi-walled carbon nanotubes on the electrochemical oxidation of propionic acid derivative drugs: Ibuprofen and naproxen. J. Electroanal. Chem. 2016, 775, 9–16. [Google Scholar] [CrossRef]
- Pavinatto, A.; Mercante, L.A.; Leandro, C.S.; Mattoso, L.H.C.; Correa, D.S. Layer-by-Layer assembled films of chitosan and multi-walled carbon nanotubes for the electrochemical detection of 17α-ethinylestradiol. J. Electroanal. Chem. 2015, 755, 215–220. [Google Scholar] [CrossRef]
- Chen, L.; Li, K.; Zhu, H.; Meng, L.; Chen, J.; Li, M.; Zhu, Z. A chiral electrochemical sensor for propranolol based on multi-walled carbon nanotubes/ionic liquids nanocomposite. Talanta 2013, 105, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Hundari, F.F.; Souza, J.C.; Zanoni, M.V.B. Adsorptive stripping voltammetry for simultaneous determination of hydrochlorothiazide and triamterene in hemodialysis samples using a multi-walled carbon nanotube-modified glassy carbon electrode. Talanta 2018, 179, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Wang, H.; Wanh, S.; Chen, Z.; Wang, S.; Zhou, Q.; Pan, Y. Electrochemical determination of mangiferin and icariin based on Au-AgNPs/MWNTs-SGSs modified glassy carbon electrode. Sens. Actuators B Chem. 2018, 255, 1771–1780. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Heng, Y.; Wang, F. Facile fabrication of 3D graphene-multi walled carbon nanotubes network and its use as a platform for natamycin detection. J. Electroanal. Chem. 2018, 816, 54–61. [Google Scholar] [CrossRef]
- Deng, K.; Liu, X.; Li, C.; Hou, Z.; Huang, H. An electrochemical omeprazole sensor based on shortened multi-walled carbon nanotubes-Fe3O4nanoparticles and poly(2,6-pyridinedicarboxylic acid). Sens. Actuators B Chem. 2017, 253, 1–9. [Google Scholar] [CrossRef]
- Khaled, E.; Khalil, M.M.; El Aziz, G.M.A. Calixarene/carbon nanotubes based screen printed sensors for potentiometric determination of gentamicin sulphate in pharmaceutical preparations and spiked surface water samples. Sens. Actuators B Chem. 2017, 244, 876–884. [Google Scholar] [CrossRef]
- Başkaya, G.; Yıldız, Y.; Savk, A.; Okyay, T.O.; Eriş, S.; Sert, H.; Şen, F. Rapid, sensitive, and reusable detection of glucose by highly monodisperse nickel nanoparticles decorated functionalized multi-walled carbon nanotubes. Biosens. Bioelectron. 2017, 91, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, B.; Liu, J.; Guo, X.; Abudukeyoumu, G.; Zhang, Y.; Ye, B.-C.; Li, Y. A novel electrochemical sensor based on Cu@Ni/MWCNTs nanocomposite for simultaneous determination of guanine and adenine. Biosens. Bioelectron. 2018, 102, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lee, E.-C. Functionalized multi-wall carbon nanotubes as an efficient additive for electrochemical DNA sensor. Sens. Actuators B Chem. 2017, 239, 652–659. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, X.-L.; Zeng, Q.; Wang, H.-S.; Wang, L.-S. A multi-walled carbon nanotubes based molecularly imprinted polymers electrochemical sensor for the sensitive determination of HIV-p24. Talanta 2017, 164, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Alexander, S. A potentiometric sensor for the trace level determination of hemoglobin in real samples using multiwalled carbon nanotube based molecular imprinted polymer. Eur. Polym. J. 2017, 97, 84–93. [Google Scholar] [CrossRef]
- Gutierrez, F.A.; Rubianes, M.D.; Rivas, G.A. Electrochemical sensor for amino acids and glucose based on glassy carbon electrodes modified with multi-walled carbon nanotubes and copper microparticles dispersed in polyethylenimine. J. Electroanal. Chem. 2016, 765, 16–21. [Google Scholar] [CrossRef]
- Ji, J.; Zhou, Z.; Zhao, X.; Sun, J.; Sun, X. Electrochemical sensor based on molecularly imprinted film at Au nanoparticles-carbon nanotubes modified electrode for determination of cholesterol. Biosens. Bioelectron. 2015, 66, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Taurino, I.; Van Hoof, V.; De Micheli, G.; Carrara, S. Superior sensing performance of multi-walled carbon nanotube-based electrodes to detect unconjugated bilirubin. Thin Solid Films 2013, 548, 546–550. [Google Scholar] [CrossRef]
- Sharma, V.V.; Gualandi, I.; Vlamidis, Y.; Tonelli, D. Electrochemical behavior of reduced graphene oxide and multi-walled carbon nanotubes composites for catechol and dopamine oxidation. Electrochim. Acta 2017, 246, 415–423. [Google Scholar] [CrossRef]
- Li, J.; Sun, Q.; Mao, Y.; Bai, Z.; Ning, X.; Zheng, J. Sensitive and low-potential detection of NADH based on boronic acid functionalized multi-walled carbon nanotubes coupling with an electrocatalysis. J. Electroanal. Chem. 2017, 794, 1–7. [Google Scholar] [CrossRef]
- Wayu, M.B.; DiPasquale, L.T.; Schwarzmann, M.A.; Gillespie, S.D.; Leopold, M.C. Electropolymerization of β-cyclodextrin onto multi-walled carbon nanotube composite films for enhanced selective detection of uric acid. J. Electroanal. Chem. 2016, 783, 192–200. [Google Scholar] [CrossRef]
- Tarditto, L.V.; Arévalo, F.J.; Zon, M.A.; Ovando, H.G.; Vettorazzi, N.R.; Fernández, H. Electrochemical sensor for the determination of enterotoxigenic Escherichia coli in swine feces using glassy carbon electrodes modified with multi-walled carbon nanotubes. Microchem. J. 2016, 127, 220–225. [Google Scholar] [CrossRef]
- Sipa, K.; Brycht, M.; Leniart, A.; Urbaniak, P.; Nosal-Wiercińska, A.; Pałecz, B.; Skrzypek, S. β-Cyclodextrins incorporated multi-walled carbon nanotubes modified electrode for the voltammetric determination of the pesticide dichlorophen. Talanta 2018, 176, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Özcan, A.; Gürbüz, M. Development of a modified electrode by using a nanocomposite containing acid-activated multi-walled carbon nanotube and fumed silica for the voltammetric determination of clopyralid. Sens. Actuators B Chem. 2018, 255, 262–267. [Google Scholar] [CrossRef]
- Ghodsi, J.; Rafati, A.A. A voltammetric sensor for diazinon pesticide based on electrode modified with TiO2 nanoparticles covered multi walled carbon nanotube nanocomposite. J. Electroanal. Chem. 2017, 807, 1–9. [Google Scholar] [CrossRef]
- Wei, X.-P.; Luo, Y.-L.; Xu, F.; Chen, Y.-S.; Yang, L.H. In-situ non-covalent dressing of multi-walled carbon nanotubes@titanium dioxides with carboxymethyl chitosan nanocomposite electrochemical sensors for detection of pesticide residues. Mater. Des. 2016, 111, 445–452. [Google Scholar] [CrossRef]
- Ertan, B.; Eren, T.; Ermiş, İ.; Saral, H.; Atar, N.; Yola, M.L. Sensitive analysis of simazine based on platinum nanoparticles on polyoxometalate/multi-walled carbon nanotubes. J. Colloid. Interface Sci. 2016, 470, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Park, J.Y. A miniaturized and flexible cadmium and lead ion detection sensorbased on micro-patterned reduced graphene oxide/carbonnanotube/bismuth composite electrodes. Sens. Actuators B Chem. 2018, 255, 1220–1227. [Google Scholar] [CrossRef]
- Roushani, M.; Saedi, Z.; Hamdi, F.; Dizajdizi, B.Z. Preparation an electrochemical sensor for detection of manganese (II) ions using glassy carbon electrode modified with multi walled carbon nanotube-chitosan-ionic liquid nanocomposite decorated with ion imprinted polymer. J. Electroanl. Chem. 2017, 804, 1–6. [Google Scholar] [CrossRef]
- Firmino, M.L.M.; Morais, S.; Correia, A.N.; De Lima-Neto, P.; Carvalho, F.A.O.; Castro, S.S.L.; Oliveira, T.M.B.F. Sensor based on β-NiOx hybrid film/multi-walled carbon nanotubes composite electrode for groundwater salinization inspection. Chem. Eng. J. 2017, 323, 47–55. [Google Scholar] [CrossRef]
- Sudha, V.; Kumar, S.M.S.; Thangamuthu, R. Simultaneous electrochemical sensing of sulphite and nitrite on acid-functionalized multi-walled carbon nanotubes modified electrodes. J. Alloys Compd. 2018, 749, 990–999. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Ding, L.; Zhou, D.; Cui, H.; Wei, Z.; Zhai, J. Synthesis of silver/multi-walled carbon nanotubes composite and its application for electrocatalytic reduction of bromate. Chem. Eng. J. 2013, 217, 28–33. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Leng, J.; Yu, Y.; Wang, W.; Jiang, M.; Bai, L. An enhanced electrochemical platform based on graphene oxide and multi-walled carbon nanotubes nanocomposite for sensitive determination of sunset yellow and tartrazine. Food Chem. 2019, 190, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Jin, B. Poly (crystal violet)-Multi-walled carbon nanotubes modified electrode for electroanalytical determination of luteolin. J. Electroanal. Chem. 2016, 780, 46–52. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Bedi, R.K.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Non-covalently anchored multi-walled carbon nanotubes with hexa-decafluorinated zinc phthalocyanine as ppb level chemiresistive chlorine sensor. Appl. Surf. Sci. 2018, 427, 202–209. [Google Scholar] [CrossRef]
- Jesionek, M.; Nowak, M.; Mistewicz, K.; Kępińska, M.; Stróż, D.; Bednarczyk, I.; Paszkiewicz, R. Sonochemical growth of nanomaterials in carbon nanotube. Ultrasonics 2018, 83, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Bora, A.; Mohan, K.; Pegu, D.; Gohain, C.B.; Dolui, S.K. A room temperature methanol vapor sensor based on highlyconducting carboxylated multi-walled carbon nanotube/polyanilinenanotube composite. Sens. Actuators B Chem. 2017, 253, 977–986. [Google Scholar] [CrossRef]
- Arévalo, F.J.; Osuna-Sánchez, Y.; Sandoval-Cortés, J.; Tocco, A.D.; Granero, A.M.; Robledo, S.N.; Zon, M.A.; Vettorazzi, N.R.; Martínez, J.L.; Segura, E.P.; et al. Development of an electrochemical sensor for the determination of glycerol based on glassy carbon electrodes modified with a copper oxide nanoparticles/multiwalled carbon nanotubes/pectin composite. Sens. Actuators B Chem. 2017, 244, 949–957. [Google Scholar] [CrossRef]
- Yu, H.; Feng, X.; Chen, X.-X.; Qiao, J.-L.; Gao, X.-L.; Xu, B.; Gao, L.-J. Electrochemical determination of bisphenol A on a glassy carbon electrode modified with gold nanoparticles loaded on reduced graphene oxide-multi walled carbon nanotubes composite. Chin. J. Anal. Chem. 2017, 45, 713–720. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, Z.; Zhang, J.; Li, G.; Li, P.; Zhang, W.; Lian, K. Synthesis of palladium nanoparticle modified reduced graphene oxide and multi-walled carbon nanotube hybrid structures for electrochemical applications. Appl. Surf. Sci. 2017, 396, 523–529. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, K.; Zhang, N.; Zhang, L.; Wang, H.; Xu, J.; Shi, H.; Zhuo, X.; Qin, M.; Wu, X. A simple strategy for fabricating a prussian blue/chitosan/carbon nanotube composite and its application for the sensitive determination of hydrogen peroxide. Micro Nano Lett. 2016, 12, 23–26. [Google Scholar] [CrossRef]



| Sensor | Modification Procedure | Analyte(s) | Technique(s)/Detection Limit | Application | Stability | Reference |
|---|---|---|---|---|---|---|
| Pharmaceuticals | ||||||
| Ni-CTS/MWCNT/GCE | GCE modified with MWCNT and Ni-CTS complex through drop coating and self-assembly, respectively | metronidazole | DPV/0.025 μmol L−1 | tablet and biological samples | 81% after one month | [29] |
| Co-Pht@f-MWCNT/Au-NP/GCE | suspension of Co-Pht and f-MWCNT immobilized on Au-NP modified GCE by drop coating | acetaminophen | SWV/0.135 μmol L−1 | commercial formulations | n.r. | [30] |
| MWCNT(shorter diameter)/GCE | MWCNT (diameter × length: 6–9 nm × 5 μm) dropped on GCE | ibuprofen | CV/1.90 μmol L−1 | tablet and liquid commercial formulations | n.r. | [31] |
| Three bilayer MWCNT@CTS/FTO | FTO coated with nanostructured Layer-by-Layer films of MWCNT@CTS | 17-α-ethinylestradiol | SWV/0.09 μmol L−1 | synthetic urine samples | n.r. | [32] |
| MWCNT@IL/GCE | immobilization of MWCNT and IL (1-octyl-3-methyl-imidazolium hexa-fluorophosphate) nanocomposite on GCE | propranolol | LSV/n.r. | commercial reagent and wastewater | n.r. | [33] |
| MWCNT/GCE | suspension of MWCNT dropped on GCE | hydrochlorothiazide and triamterene | ASV/2.8 × 10−8 and 2.9 × 10−8 mol L−1 for hydrochlorothiazide and triamterene, respectively | hemodialysis samples | n.r. | [34] |
| Au@Ag-NP/MWCNT-SGSs/GCE | layer-by-layer assembly of Au@Ag-NP and MWCNT-SGSs on GCE | mangiferin and icariin | DPV/0.017 μmol L−1 for both compounds | Rhizoma anemarrhenae, Artemisia capillaris Herba and Epimedium macranthum samples | ≤95.1% after one month | [35] |
| 3D-Grf@MWCNT/GCE | electrodeposition of 3D-Grf@MWCNT suspension on GCE | natamycin | LSV/1.0 × 10−8 mol L−1 | red wine and beverage samples | 94.6% after two weeks | [36] |
| Fe3O4-NP@MWCNT/PDDA/GCE | casting of PDDA modified GCE with Fe3O4-NP@MWCNT hybrid film | omeprazole | LSV/15 nmol L−1 | tablet, capsules, wastewater, serum, and urine | 92.1% after three weeks | [37] |
| Calixarene/MWCNT/SPE | dip coating of graphite-based SPE in composite matrix of calixarene and MWCNT | gentamicin sulphate | potentiometry/7.5 × 10−8 mol L−1 | dosage forms and spiked surface water samples | n.r. | [38] |
| Biologically active molecules | ||||||
| Ni-NP@f-MWCNTs/GCE | drop coating of hybrid film (Ni-NP and f-MWCNT) on GCE | glucose | CV and amperometry/0.021 μmol L−1 | human blood serum samples | practically constant signal after 1000th cycle | [39] |
| Cu@Ni-NP/MWCNT/GCE | immobilization of hybrid film of Cu@Ni-NP and MWCNT on GCE | guanine and adenine | DPV/0.17 μmol L−1 and 0.33 μmol L−1 for guanine and adenine, respectively | ds-DNA from mice brain tissues | 96.7% for 30 days | [40] |
| Gold electrode | measurements with unmodified gold electrode, keeping f-MWCNT additive and DNA sequence in electrolyte solution | breast cancer marker (5′-GTG TTG TCT CCT AGG TTG GCT CTG-3′; 24-base fraction of the p53 gene) | DPV/141.2 pmol L−1 | solution containing the complementary sequence (5′-CAG AGC CAA CCT AGG AGA CAA CAC-3′) | n.r. | [41] |
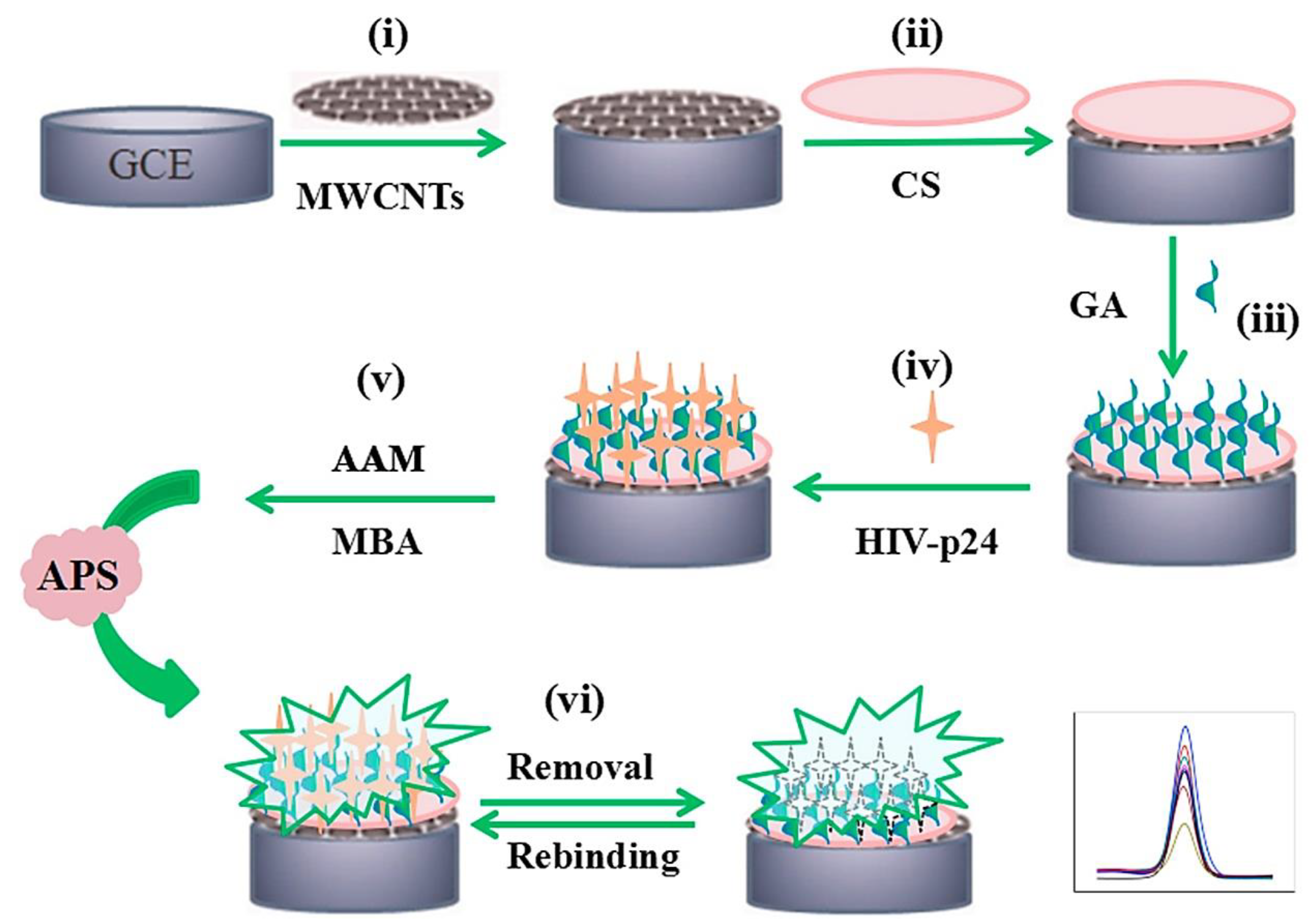
| HIV-p24/MIP /MWCNT/GCE | HIV-p24 crosslinking MIP (acrylamide functional monomer, N,N′-methylenebisacrylamide as crosslinking agent and ammonium persulphate as initiator) immobilized on MWCNT/GCE | HIV-p24 protein | DPV/0.083 pg cm−3 | real human serum samples | 98.6% after 10 days | [42] |
| MIP/MWCNT/Cu | MIP (itaconic acid monomer, ethylene glycol dimethacrylate cross-linker and α,α′-azobisisobutyronitrile as initiator) on MWCNT modified Cu electrode | hemoglobin | potentiometry/1.0 µg mlL−1 | human bile juice and urine samples | 6 months without significant change in the electrode performance | [43] |
| Cu-MP@polyethylenimine /MWCNT/GCE | Cu-MP dispersed in polyethylenimine and dropped on MWCNT/GCE | amino acids, albumin and glucose | SWV and amperometry/0.10–0.37 μmol L−1 for the amino acids (L-cystine, L-histidine and L-serine); 1.2 mg mL−1 for albumin; and 182 nmol L−1 for glucose | pharmaceutical products and beverages | 7.7% RSD after 10 successive calibration plots using the same surface | [44] |
| MIP/Au-NP/MWCNT/GCE | Au-NP electrodeposited on MWCNT/GCE, and assembled with MIP (tetrabutylammonium perchlorate) | cholesterol | DPV/3.3 × 10−14 mol L−1 | n.r. | 91.7% after one month | [45] |
| MWCNT/SPE | MWCNT films casted onto SPE | bilirubin | CV/9.4 µmol L−1 | n.r. | n.r. | [46] |
| Grf-Ox@MWCNT/GCE | drop coating of Grf-Ox@MWCNT suspension on GCE | catechol and dopamine | CV/n.r. | n.r. | n.r. | [47] |
| Phenazine methosulfate/3-aminophenylboronic acid/f-MWCNT/GCE | drop coating of phenazine methosulfate and 3-aminophenyl boronic acid on f-MWCNT/GCE | NADH | amperometry/0.16 μmol L−1 | human serum | 96.7% after five consecutive measurements | [48] |
| HPU/β-CD/MWCNT@Nafion®/GCE | layer-by-layer of HPU, β-CD and composite film (MWCNT@Nafion®) on GCE | uric acid | amperometry/n.r. | n.r. | n.r. | [49] |
| Microorganisms | ||||||
| MWCNT@Nafion®/GCE | dip coating of composite suspension (MWCNT@Nafion®) on GCE | Enterotoxigenic Escherichia coli F4 (K88) | SWV/6 × 104 CFU mL−1 | swine stool samples | n.r. | [50] |
| Pesticides | ||||||
| β-CD/MWCNT/GCE | β-CD and MWCNT composite suspension dropped on GCE | dichlorophen | SWAdSV/ 4.4 × 10−8 mol L−1 | river water | 93.9% after one week | [51] |
| Fumed silica/acid-activated MWCNT/GCE | drop coating of a nanocomposite suspension (Fumed silica and acid-activated MWCNT) on GCE | clopyralid | DPV/0.8 nmol L−1 | urine, river water, sugar beet, wheat, and herbicide formulations (Phaeton and Lontrel) | 91% after three weeks | [52] |
| TiO2-NP@MWCNT/GCE | TiO2-NP@MWCNT nanocomposite dropped on GCE | diazinon | CV an SWV/3.0 nmol L−1 | well and tap water | 89% after 28 days | [53] |
| Nafion®/TiO2-NP@MWCNT @carboxymethyl chitosan/GCE | Nafion® assembled on composite film (TiO2-NP@MWCNT @carboxymethyl chitosan) previously immobilized on GCE | trichlorfon | DPV/4.0 × 10−7 mol L−1 | apple, mushroom, and cucumber | 98% after one week | [54] |
| MIP/Pt-NP@polyoxometalate@f-MWCNT/GCE | MIP (pyrrole in the presence of the analyte) assembled on hybrid film (Pt-NP@polyoxometalate@f-MWCNT) immobilized on GCE | simazine | DPV/2.0 × 10−11 mol L−1 | wastewater samples | 96.9% after 45 days | [55] |
| Metallic cations | ||||||
| BiF/Grf-Red/MWCNT/SPE | layer-by-layer of BiF, Grf-Red and MWCNT on SPE (gold support) | Cd2+ and Pb2+ | SWV/0.6 ppb for Cd and 0.2 ppb for Pb | drinking water | n.r. | [56] |
| Mn2+-imprinted polymer /IL@CTS@MWCNT/GCE | thermal immobilization of Mn2+-imprinted polymer on composite layer (IL@CTS@MWCNT) dropped on GCE | Mn2+ | SWAdSV/0.15 μmol L−1 | wastewater | 94.8% after two weeks | [57] |
| β-NiOx/MWCNT-modified CPE | electrodeposition of hybrid film of β-NiOx on MWCNT-modified CPE | Na+ | SWV/9.86 × 10−8 mol L−1 | groundwater | practically constant signal for more than five hundred consecutive cycles | [58] |
| Anions | ||||||
| f-MWCNT/GCE | drop coating of f-MWCNT (COOH-functionalized structures) as suspension on GCE | SO32− and NO2− | DPV/215 nmol L−1 for SO32− and 565 nmol L−1 for NO2− | groundwater | ≥96.4% after one week | [59] |
| Ag-NP@MWCNT/GCE | drop coating of nanocomposite (Ag-NP@MWCNT) on GCE | BrO3− | amperometry/n.r. | n.r. | n.r. | [60] |
| Dyes | ||||||
| Grf-Ox@MWCNT/GCE | suspension of Grf-Ox@MWCNT immobilized on GCE by drop coating | sunset yellow and tartrazine | LSV/0.025 µmol L−1 for sunset yellow and 0.010 µmol L−1 for tartrazine | orange juice | 89–93% after one month | [61] |
| Poly(crystal violet)/MWCNT/GCE | electropolymerization of crystal violet on MWCNT/GCE | luteolin | DPV/5.0 × 10−9 mol L−1 | Chrysanthemum samples | 93% after one month | [62] |
| Gas/Vapor | ||||||
| Hexa-decafluorinated zinc phthalocyanine @f-MWCNT/SPE (gold support) | drop coating of the composite (Hexa-decafluorinated zinc phthalocyanine@f-MWCNT) on SPE | Cl2 | resistance/0.06 ppb | n.r. | n.r. | [63] |
| SbSI@CNTs/Au-microelectrode | ultrasonic bonding of SbSI@CNTs composite on Au-microelectrode | CO2 | amperometry/n.r. | n.r. | n.r. | [64] |
| MWCNT@polyaniline/FTO | drop coating of MWCNT@polyaniline nanocomposite on FTO | methanol vapor | resistance/≈50 ppm | n.r. | the signal remained almost constant for up to 20 days | [65] |
| Industrial by-products | ||||||
| CuO-NP/MWCNT/GCE | electrodeposition of CuO-NP on MWCNT/GCE | glycerol | amperometry/5.8 × 10−6 g dm−3 | biodiesel samples | n.r. | [66] |
| Au-NP/Grf-Red@MWCNT/GCE | electrodeposition of Au-NP on Grf-Red@MWCNT/GCE | bisphenol A | DPV/1.0 × 10−9 mol L−1 | river water and shopping receipt samples | 98% after 30 days | [67] |
| Pd-NP/Grf-Red@MWCNT/GCE | electrodeposition of Pd-NP on Grf-Red@MWCNT/GCE | hydrazine | amperometry/0.15 µmol L−1 | tap water spiked with hydrazine | n.r. | [68] |
| Prussian blue/CTS@MWCNT /GCE | electrodeposition of Prussian blue complex on GCE Modified with CTS@MWCNT nanocomposite | hydrogen peroxide | amperometry/0.10 µmol L−1 | routine analysis in pure electrolyte | 90.5–92.6% after two weeks | [69] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, T.M.B.F.; Morais, S. New Generation of Electrochemical Sensors Based on Multi-Walled Carbon Nanotubes. Appl. Sci. 2018, 8, 1925. https://doi.org/10.3390/app8101925
Oliveira TMBF, Morais S. New Generation of Electrochemical Sensors Based on Multi-Walled Carbon Nanotubes. Applied Sciences. 2018; 8(10):1925. https://doi.org/10.3390/app8101925
Chicago/Turabian StyleOliveira, Thiago M. B. F., and Simone Morais. 2018. "New Generation of Electrochemical Sensors Based on Multi-Walled Carbon Nanotubes" Applied Sciences 8, no. 10: 1925. https://doi.org/10.3390/app8101925
APA StyleOliveira, T. M. B. F., & Morais, S. (2018). New Generation of Electrochemical Sensors Based on Multi-Walled Carbon Nanotubes. Applied Sciences, 8(10), 1925. https://doi.org/10.3390/app8101925






