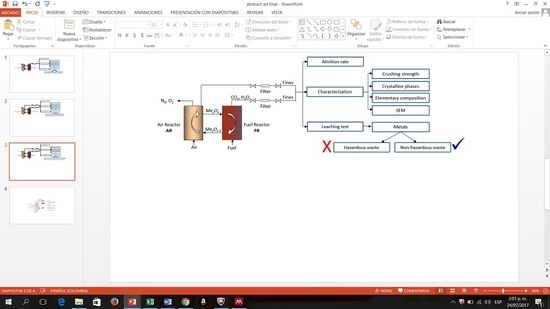
Characterization for Disposal of the Residues Produced by Materials Used as Solid Oxygen Carriers in an Advanced Chemical Looping Combustion Process
Abstract
Featured Application
- The residues of six low-cost materials found in Colombia were characterized with CH4 and H2 used as fuels in a chemical looping combustion (CLC) system.
- The residues of the low-cost materials evaluated would not have a negative impact on the environment when disposed of in a landfill.
- The residues are not considered toxic waste or dangerous according to current Colombian legislation or European regulations, respectively.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
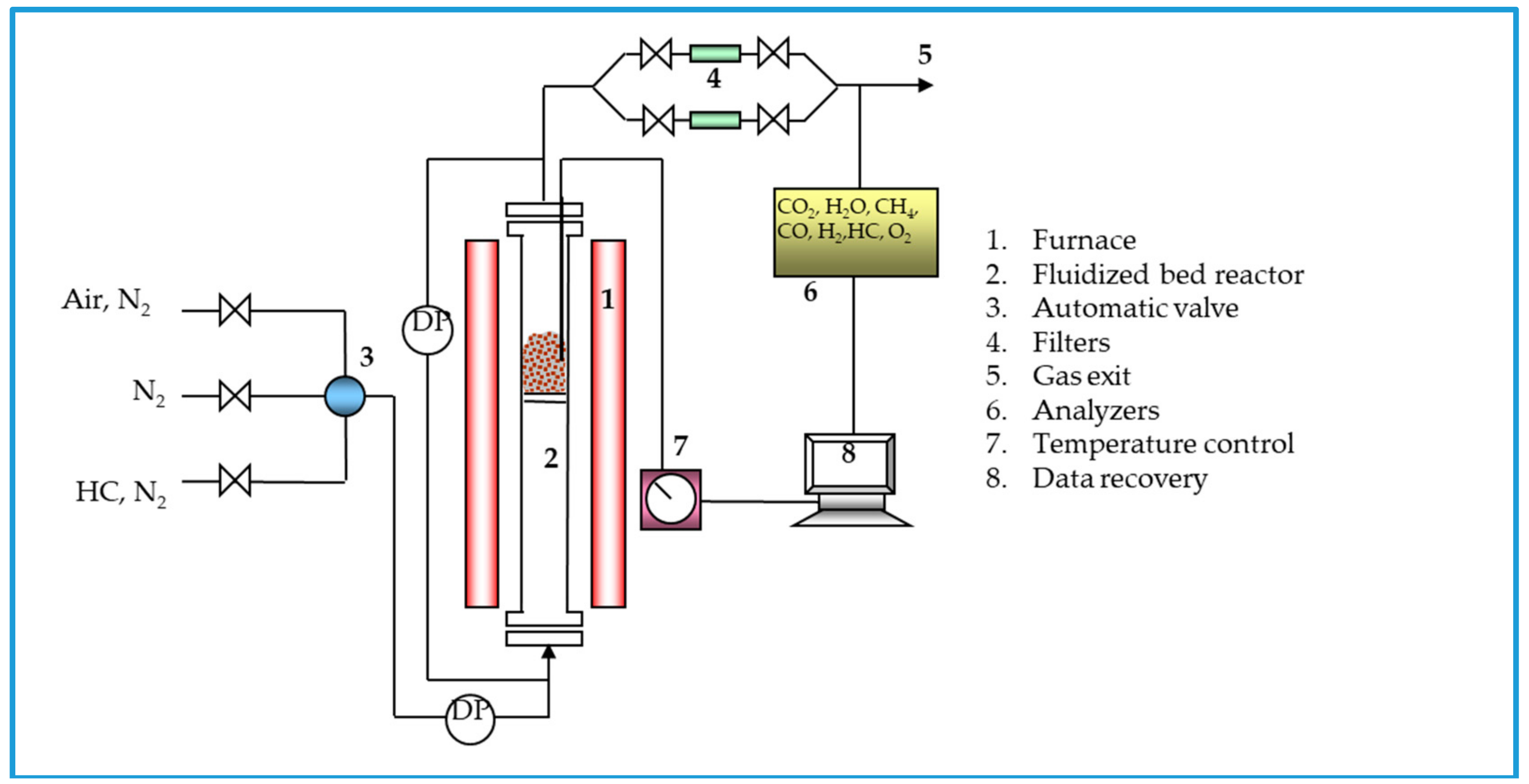
2.2. Experimental Procedure
2.2.1. Characterization of OC Residues
2.2.2. Leaching Behavior Tests
3. Results and Discussion
3.1. Attrition
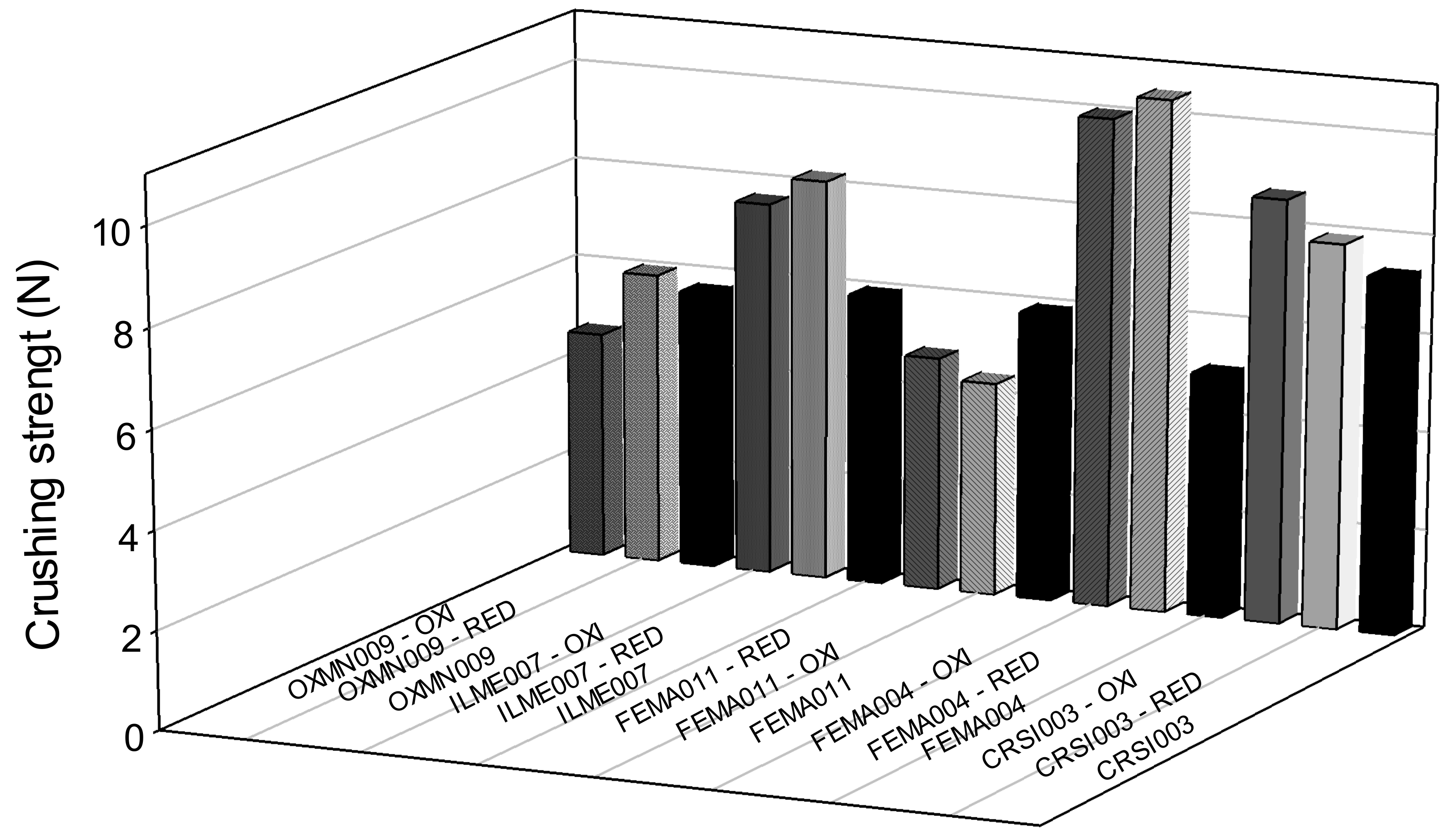
3.2. Crushing Strength
3.3. Crystalline Phases
3.4. Morphological Behaviour
3.5. Elementary Composition Using XRF
3.6. Hazardous Metal Leaching and Identification Test
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- International Energy Agency (IEA). International Energy Outlook 2016; IEA: New York, NY, USA, 2016. [Google Scholar]
- UNFCCC. Conferencia de las Partes 21 Periodo de Sesiones Cop 21, París del 30 de Noviembre al 11 de Diciembre del 2015; Convención Marco de las Naciones Unidas Sobre Cambio Climático: Paris, France, 2015. [Google Scholar]
- IPCC. Special Report on Carbon Dioxide Capture and Storage; Prepared by Working Group III of the Intergovernmental Panel on Climate Change; Metz, B., Davidson, O., de Coninck, H.C., Loos, M., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005; p. 442. [Google Scholar]
- Institute, G.C. The Global Status of CCS: 2016 Summary Report; Global Carbon Capture and Storage Institute Ltd: Melbourne, Australia, 2016. [Google Scholar]
- International Energy Agency (IEA). Energy Technology Perspectives 2016; IEA: Paris, France, 2016. [Google Scholar]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Ishida, M.; Jin, H. A new advanced power-generation system using chemical-looping combustion. Energy 1994, 19, 415–422. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Leckner, B.; Mattisson, T. A fluidized-bed combustion process with inherent CO2 separation; application of chemical-looping combustion. Chem. Eng. Sci. 2001, 56, 3101–3113. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. The use of petroleum coke as fuel in chemical-looping combustion. Fuel 2007, 86, 1947–1958. [Google Scholar] [CrossRef]
- Mattisson, T.; Lyngfelt, A. Applications of Chemical Looping Combustion with Capture of CO2. In Second Nordic Minisymposium on Carbon Dioxide Capture and Storage, Göteborg, 26 October 2001; Center for Environment and Sustainability, Chalmers: Göteborg, Sweden, 2001. [Google Scholar]
- Shafiefarhood, A.; Stewart, A.; Li, F. Iron-containing mixed-oxide composites as oxygen carriers for chemical looping with oxygen uncoupling (clou). Fuel 2015, 139, 1–10. [Google Scholar] [CrossRef]
- Abad, A.; Cuadrat, A.; Mendiara, T.; García-Labiano, F.; Gayán, P.; de Diego, L.F.; Adánez, J. Low-cost Fe-based oxygen carrier materials for the IG-CLC process with coal. 2. Ind. Eng. Chem. Res. 2012, 51, 16230–16241. [Google Scholar] [CrossRef]
- Mendiara, T.; Pérez, R.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Low-cost Fe-based oxygen carrier materials for the IG-CLC process with coal. 1. IInd. Eng. Chem. Res. 2012, 51, 16216–16229. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. Use of ores and industrial products as oxygen carriers in chemical-looping combustion. Energy Fuels 2009, 23, 2307–2315. [Google Scholar] [CrossRef]
- Fossdal, A.; Bakken, E.; Øye, B.A.; Schøning, C.; Kaus, I.; Mokkelbost, T.; Larring, Y. Study of inexpensive oxygen carriers for chemical looping combustion. Int. J. Greenh. Gas Control 2011, 5, 483–488. [Google Scholar] [CrossRef]
- Mendiara, T.; García-Labiano, F.; Gayán, P.; Abad, A.; de Diego, L.F.; Adánez, J. Evaluation of the use of different coals in chemical looping combustion using a bauxite waste as oxygen carrier. Fuel 2013, 106, 814–826. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Basu, P. Chemical-looping gasification of biomass for hydrogen-enriched gas production with in-process carbon dioxide capture. Energy Fuels 2009, 23, 5077–5083. [Google Scholar] [CrossRef]
- Arjmand, M.; Leion, H.; Mattisson, T.; Lyngfelt, A. Investigation of different manganese ores as oxygen carriers in chemical-looping combustion (CLC) for solid fuels. Appl. Energy 2014, 113, 1883–1894. [Google Scholar] [CrossRef]
- Wen, Y.-Y.; Li, Z.-S.; Xu, L.; Cai, N.-S. Experimental study of natural Cu ore particles as oxygen carriers in chemical looping with oxygen uncoupling (clou). Energy Fuels 2012, 26, 3919–3927. [Google Scholar] [CrossRef]
- Moldenhauer, P.; Rydén, M.; Lyngfelt, A. Testing of minerals and industrial by-products as oxygen carriers for chemical-looping combustion in a circulating fluidized-bed 300 W laboratory reactor. Fuel 2012, 93, 351–363. [Google Scholar] [CrossRef]
- Frohn, P.; Arjmand, M.; Azimi, G.; Leion, H.; Mattisson, T.; Lyngfelt, A. On the high-gasification rate of brazilian manganese ore in chemical-looping combustion (CLC) for solid fuels. AIChE J. 2013, 59, 4346–4354. [Google Scholar] [CrossRef]
- Velasco-Sarria, F.J.; Forero, C.R.; Arango, E.; Adánez, J. Reduction and oxidation kinetics of fe–mn-based minerals from southwestern colombia for chemical looping combustion. Energy Fuels 2018, 32, 1923–1933. [Google Scholar] [CrossRef]
- Velasco-Sarria, F.J.; Forero, C.R.; Adánez-Rubio, I.; Abad, A.; Adánez, J. Assessment of low-cost oxygen carrier in south-western colombia, and its use in the in-situ gasification chemical looping combustion technology. Fuel 2018, 218, 417–424. [Google Scholar] [CrossRef]
- Ortega Montero, C.R.; Rojas, E.E. Yacimientos minerales en colombia. In XII Congreso Colombiano de Geología; Congreso Las Geociencias: Paipa, Colombia, 2009. [Google Scholar]
- Leion, H.; Lyngfelt, A.; Johansson, M.; Jerndal, E.; Mattisson, T. The use of ilmenite as an oxygen carrier in chemical-looping combustion. Chem. Eng. Res. Des. 2008, 86, 1017–1026. [Google Scholar] [CrossRef]
- Adánez, J.; Cuadrat, A.; Abad, A.; Gayán, P.; de Diego, L.F.; García-Labiano, F. Ilmenite activation during consecutive redox cycles in chemical-looping combustion. Energy Fuels 2010, 24, 1402–1413. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; Adánez, J.; de Diego, L.F.; García-Labiano, F.; Gayán, P. Behavior of ilmenite as oxygen carrier in chemical-looping combustion. Fuel Process. Technol. 2012, 94, 101–112. [Google Scholar] [CrossRef]
- Abad, A.; Adánez, J.; Cuadrat, A.; García-Labiano, F.; Gayán, P.; de Diego, L.F. Kinetics of redox reactions of ilmenite for chemical-looping combustion. Chem. Eng. Sci. 2011, 66, 689–702. [Google Scholar] [CrossRef]
- Leion, H.; Jerndal, E.; Steenari, B.M.; Hermansson, S.; Israelsson, M.; Jansson, E.; Johnsson, M.; Thunberg, R.; Vadenbo, A.; Mattisson, T.; et al. Solid fuels in chemical-looping combustion using oxide scale and unprocessed iron ore as oxygen carriers. Fuel 2009, 88, 1945–1954. [Google Scholar] [CrossRef]
- Abad, A.; Adánez, J.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Celaya, J. Mapping of the range of operational conditions for Cu-, Fe-, and Ni-based oxygen carriers in chemical-looping combustion. Chem. Eng. Sci. 2007, 62, 533–549. [Google Scholar] [CrossRef]
- Mattisson, T.; Lyngfelt, A.; Cho, P. The use of iron oxide as an oxygen carrier in chemical-looping combustion of methane with inherent separation of CO2. Fuel 2001, 80, 1953–1962. [Google Scholar] [CrossRef]
- Jerndal, E.; Mattisson, T.; Lyngfelt, A. Thermal analysis of chemical-looping combustion. Chem. Chem. Eng. Res. Des. 2006, 84, 795–806. [Google Scholar] [CrossRef]
- Cho, P.; Mattisson, T.; Lyngfelt, A. Defluidization conditions for a fluidized bed of iron oxide- nickel oxide- and manganese oxide-containing oxygen carriers for chemical-looping combustion. Ind. Eng. Chem. Res. 2006, 45, 968–977. [Google Scholar] [CrossRef]
- Mattisson, T.; Johansson, M.; Lyngfelt, A. Multicycle reduction and oxidation of different types of iron oxide particles-application to chemical-looping combustion. Energy Fuels 2004, 18, 628–637. [Google Scholar] [CrossRef]
- Rydén, M.; Cleverstam, E.; Johansson, M.; Lyngfelt, A.; Mattisson, T. Fe2O3 on Ce-, Ca-, or Mg-stabilized ZrO2 as oxygen carrier for chemical-looping combustion using NiO as additive. AIChE J. 2010, 56, 2211–2220. [Google Scholar]
- Cho, P.; Mattisson, T.; Lyngfelt, A. Carbon formation on nickel and iron oxide-containing oxygen carriers for chemical-looping combustion. Ind. Eng.Chem. Res. 2005, 44, 668–676. [Google Scholar] [CrossRef]
- Mei, D.; Mendiara, T.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J.; Zhao, H. Evaluation of manganese minerals for chemical looping combustion. Energy Fuels 2015, 29, 6605–6615. [Google Scholar] [CrossRef]
- Larring, Y.; Pishahang, M.; Sunding, M.F.; Tsakalakis, K. Fe–Mn based minerals with remarkable redox characteristics for chemical looping combustion. Fuel 2015, 159, 169–178. [Google Scholar] [CrossRef]
- Stobbe, E.R.; de Boer, B.A.; Geus, J.W. The reduction and oxidation behaviour of manganese oxides. Catal. Today 1999, 47, 161–167. [Google Scholar] [CrossRef]
- Zafar, Q.; Abad, A.; Mattisson, T.; Gevert, B.; Strand, M. Reduction and oxidation kinetics of Mn3O4/Mg-ZrO2 oxygen carrier particles for chemical-looping combustion. Chem. Eng. Sci. 2007, 62, 6556–6567. [Google Scholar] [CrossRef]
- Rydén, M.; Leion, H.; Mattisson, T.; Lyngfelt, A. Combined oxides as oxygen-carrier material for chemical-looping with oxygen uncoupling. Appl. Energy 2014, 113, 1924–1932. [Google Scholar] [CrossRef]
- Källén, M.; Hallberg, P.; Rydén, M.; Mattisson, T.; Lyngfelt, A. Combined oxides of iron, manganese and silica as oxygen carriers for chemical-looping combustion. Fuel Process.Technol. 2014, 124, 87–96. [Google Scholar] [CrossRef]
- Jing, D.; Arjmand, M.; Mattisson, T.; Rydén, M.; Snijkers, F.; Leion, H.; Lyngfelt, A. Examination of oxygen uncoupling behaviour and reactivity towards methane for manganese silicate oxygen carriers in chemical-looping combustion. Int. J. Greenh. Gas Control 2014, 29, 70–81. [Google Scholar] [CrossRef]
- Mendiara, T.; Gayán, P.; Abad, A.; García-Labiano, F.; de Diego, L.F.; Adánez, J. Characterization for disposal of fe-based oxygen carriers from a clc unit burning coal. Fuel Process. Technol. 2015, 138, 750–757. [Google Scholar] [CrossRef]
- García-Labiano, F.; Gayán, P.; Adánez, J.; de Diego, L.F.; Forero, C.R. Solid waste management of a chemical-looping combustion plant using cu-based oxygen carriers. Environ. Sci. Technol. 2007, 41, 5882–5887. [Google Scholar] [CrossRef] [PubMed]
- UNE-EN-12457-3. Caracterización de Residuos–Lixiviación–Ensayo de Conformidad Para la Lixiviación de Residuos Granulares y Lodos; AENOR: Madrid, Spain, 2003; p. 35. [Google Scholar]
- European Union. Council decision 2003/33/ec establishing criteria and procedures for the acceptance of waste at landfills pursuant to article 16 of and annex ii to directive 1999/31/ec. Off. J. Eur. Union 2003, L 11, 27–49. [Google Scholar]
- Arango, E.; Vasquez, F.G. Determinación de los Parámetros Cinéticos Para la Combustión Usando Minerales del Suroccidente Colombiano Como Transportadores Sólidos de Oxígeno; Universidad del Valle: Cali-Colombia, Colombia, 2016. [Google Scholar]
- Gonzáles, D.A.; Parra, T.X. Análisis de Reactividad de Materiales Basados en Cromita, Óxidos de Hierro y Óxidos de Manganeso Como Posibles Transportadores Sólidos de Oxígeno; Universidad del Valle: Cali-Colombia, Colombia, 2015. [Google Scholar]
- Mejía, A.F.; Jaramillo, C.C. Análisis de Reactividad de Ilmenita y Olivino Como Posibles Transportadores Sólidos de Oxígeno en el Proceso Chemical Looping Combustion (CLC); Universidad del Valle: Cali-Colombia, Colombia, 2015. [Google Scholar]
- Forero, C.R. Combustión de Gas Con Captura de CO2 Mediante Transportadores Sólidos de Oxígeno a Base de Cuo; Universidad de Zaragoza-Consejo Superior de Investigaciones Científicas (CSIC): Zaragoza, España, 2011. [Google Scholar]
- IDEAM. Protocolos de muestreo y análisis de laboratorio para la caracterización fisicoquímica de los residuos o desechos peligrosos en el país. In Resolución 0062; Instituto de Hidrología: Bogota, Colombia, 2007; Volume Resolución 0062. [Google Scholar]
- Ministerio de Ambiente, Vivienda, y Desarrollo Territorial. Decreto 4741 de Diciembre 30 de 2005; Decreto 4741 de diciembre 30 de 2005; Ministerio de Ambiente: Bogotá, Colombia, 2005; p. 27. [Google Scholar]
- Ministerio de Ambiente, Vivienda, y Desarrollo Territorial. Gestión Integral de Residuos o Desechos Peligrosos. Bases Conceptuales; Ministerio de Ambiente, Vivienda y Desarrollo Territorial, Dirección de Desarrollo Sectorial Sostenible/Organización de Control Ambiental y Desarrollo Empresarial OCADE: Bogotá, Colombia, 2007; p. 186. [Google Scholar]
- Adánez Elorza, J.; de Diego Poza, L.F.; García Labiano, F.; Gayán Sanz, P.; Abad Secades, A. Material Transportador de O2 Obtenible a Partir de CuO y MgAl2O4 y USO de Dicho Material en la Combustión de Sólidos con Captura Inherente de CO2. Patente ES2390334 A1, 12 November 2012. [Google Scholar]
- Mao, L.; Deng, N.; Liu, L.; Cui, H.; Zhang, W. Inhibition of Cr(III) oxidation during thermal treatment of simulated tannery sludge: The role of phosphate. Chem. Eng. J. 2016, 294, 1–8. [Google Scholar] [CrossRef]
| OC | CRSI003 | FEMA004 | FEMA011 | ILME007 | OXMN009 | OXMN010A |
|---|---|---|---|---|---|---|
| Origin | Chromite Ore, Antioquia | Iron Ore, Antioquia | Iron Ore, Cauca | Ilmenite Ore, Antioquia | Manganese Ore, Valle | Manganese Waste, Nariño |
| Element | Composition (%) a | |||||
| Si | 0.8 | 0.0 | 1.6 | 2.4 | 1.8 | 13.0 |
| Al | 3.5 | 0.9 | 0.4 | 0.5 | 0.2 | 2.5 |
| Fe | 27.2 | 41.5 | 65.9 | 30.7 | 1.0 | 3.5 |
| Ca | 0.2 | 0.3 | 0.8 | 0.9 | 0.5 | 1.9 |
| Mg | 2.8 | 1.3 | 0.1 | 0.3 | 0.1 | 0.8 |
| Na | - | - | - | 0.1 | - | 0.4 |
| K | - | - | - | - | - | 0.2 |
| Cu | - | - | - | - | - | 0.1 |
| Zn | 0.2 | 0.1 | - | - | - | - |
| Ti | 2.6 | 12.9 | - | 24.5 | - | 0.2 |
| V | 0.2 | 0.2 | - | 0.2 | 0.1 | - |
| Cr | 28.7 | 7.0 | - | - | - | - |
| Ni | - | - | - | - | - | - |
| P | - | - | - | - | 0.1 | 0.1 |
| Mn | 0.5 | - | - | 2.5 | 53.4 | 35.7 |
| Ba | - | - | - | - | - | 0.4 |
| O | 33.1 | 31.8 | 31.0 | 34.2 | 18.7 | 30.7 |
| LOI b | 0.0 | 0.6 | 0.0 | 3.5 | 23.1 | 10.3 |
| Total | 100.0 | 96.6 | 100.0 | 99.9 | 99.1 | 99.7 |
| Crushing strength (N) | 7.3 | 5.0 | 5.8 | 5.9 | 5.6 | 2.3 |
| Crystalline phases | FeCr2O4 | Fe2O3, Fe3O4, FeTiO3, FeCr2O4 | Fe3O4, Fe2SiO4 | Fe2O3, FeTiO3, CaO | SiO2, Mn3O4 | MnSiO3, FeO |
| Density (kg/m3) | 4964 | 4943 | 3116 | 4639 | 3286 | 3009 |
| Variable | Description |
|---|---|
| Temperature | 950 °C |
| Number of cycles | 30 |
| Particle size | 100–300 microns |
| Reducing gas | H2 (25%)–N2 (75%), CH4 (20%)–N2 (80%) |
| Oxidizing gas | 100% air |
| Flow (l/h) | >2 × minimum fluidization velocity (>2 × Umf) |
| Weight OC (g) | 300 |
| Waste from OC | Reducing Gas | Operation Time (h) | Attrition Rate (%/h) |
|---|---|---|---|
| CRSI003 | H2 | 8.3 | 0.0027 |
| FEMA004 | H2 | 7.8 | 0.0074 |
| FEMA011 | H2 | 7.3 | 0.2007 |
| FEMA011 | CH4 | 3.7 | 0.4156 |
| ILME007 | H2 | 6.5 | 0.0008 |
| OXMN009 | H2 | 4.1 | 0.0653 |
| OXMN010A | H2 | 9.1 | 0.0059 |
| OXMN010A | CH4 | 4.5 | 0.1506 |
| Residue/Gas | Oxidation State | Crystalline Phases | Possible Reactions | ||
|---|---|---|---|---|---|
| CRSI003 H2 | Reduced | - | (2) | [16] | |
| Oxidized | Fe2O3, TiO2, SiO2, FeCr2O4 | (3) | [16] | ||
| FEMA004 H2 | Reduced | Fe2O3, Fe3O4, FeTiO3 | (4) | [16] | |
| (5) | [16] | ||||
| Oxidized | Fe2O3 | (6) | [14] | ||
| (7) | [14] | ||||
| FEMA011 CH4 | Reduced | Fe2O3, Fe3O4 | (8) | [14] | |
| Oxidized | Fe2O3, Fe3O4 | (7) | [14] | ||
| FEMA011 H2 | Reduced | Fe2O3, Fe3O4 | (6) | [14] | |
| Oxidized | Fe2O3, Fe3O4 | (7) | [14] | ||
| ILME007 H2 | Reduced | FeTiO3, TiO2 | (4) | [16] | |
| Oxidized | Fe2O3, TiO2, SiO2, Fe2TiO5 | (5) | [16] | ||
| OXMN009 H2 | Reduced | - | (9) | [15] | |
| Oxidized | Mn3O4,Mn2O3 | (10) | [15] | ||
| OXMN010 CH4 | Reduced | SiO2, MnSiO3, SiO2, FeO, Mn2SiO4 | (11) | [27] | |
| Oxidized | SiO2, MnSiO3, SiO2 | (12) | [27] | ||
| OXMN010 H2 | Reduced | SiO2, Mn2SiO4, Mn2O3, MnO | (9) | [15] | |
| (10) | [15] | ||||
| Oxidized | SiO2, Mn3O4, Mn2O3, MnFe2O4 | (11) | [27] | ||
| (12) | [27] | ||||
| OC | Fresh | Reduced | Oxidized | Reduced | Oxidized |
|---|---|---|---|---|---|
| Gas | H2 | H2 | CH4 | CH4 | |
| CRSI003 |  |  |  | ||
| FEMA004 |  |  |  | ||
| FEMA011 |  |  |  |  |  |
| ILME007 |  |  |  | ||
| OXMN009 |  |  |  | ||
| OXMN010A |  |  |  |  |  |
| Max. Allowed | Ba | Cu | Cr | Ni | Zn |
|---|---|---|---|---|---|
| Decree 4741 (mg/L) | 100 | - | 5 | - | - |
| CRSI003 RED H2 | 0.421 | - | <0.01 | 0.049 | 3.625 |
| CRSI003 OXI H2 | 0.182 | - | 0.137 | 0.011 | 0.281 |
| FEMA004 RED H2 | - | 0.139 | <0.009 | 0.027 | 0.674 |
| FEMA004 OXI H2 | - | 0.136 | <0.007 | 0.006 | 0.426 |
| FEMA011 RED H2 | - | <0.004 | <0.005 | ≈0 | - |
| FEMA011 OXI H2 | - | <0.007 | <0.011 | 0.339 | - |
| FEMA011 RED CH4 | - | 0.158 | <0.009 | 0.098 | - |
| FEMA011 OXI CH4 | - | 0.197 | <0.009 | 0.040 | - |
| ILME007 RED H2 | - | - | <0.008 | 0.019 | 0.596 |
| ILME007 OXI H2 | - | - | <0.008 | 0.009 | 0.738 |
| OXMN009 RED H2 | 3.447 | 0.010 | 0.086 | 0.026 | 0.127 |
| OXMN009 OXI H2 | 2.107 | <0.008 | 0.119 | 0.026 | 0.350 |
| OXMN010A RED H2 | 0.816 | 0.054 | <0.006 | 0.291 | 0.230 |
| OXMN010A OXI H2 | 1.829 | 0.225 | 0.558 | 0.030 | 0.559 |
| OXMN010A RED CH4 | 0.429 | <0.006 | 0.351 | ≈0 | <0.001 |
| OXMN010A OXI CH4 | 1.591 | <0.005 | 0.274 | ≈0 | <0.001 |
| Max. Allowed (mg/kg Dry Matter) | Ba | Cu | Cr | Ni | Zn |
|---|---|---|---|---|---|
| Inert Waste | 36.25 | 3.375 | 0.875 | 0.65 | 6.5 |
| Non-Hazardous Waste | 187.5 | 81.25 | 17.5 | 16.25 | 81.25 |
| CRSI003 RED H2 | 8.421 | - | <0.2 | 0.981 | 72.500 |
| CRSI003 OXI H2 | 3.646 | - | 2.748 | 0.212 | 5.624 |
| FEMA004 RED H2 | - | 2.790 | <0.18 | 0.535 | 13.477 |
| FEMA004 OXI H2 | - | 2.719 | <0.14 | 0.115 | 8.517 |
| FEMA011 RED H2 | - | <0.08 | <0.1 | ≈0 | - |
| FEMA011 OXI H2 | - | <0.14 | <0.22 | 6.785 | - |
| FEMA011 RED CH4 | - | 3.160 | <0.18 | 1.965 | - |
| FEMA011 OXI CH4 | - | 3.933 | <0.18 | 0.807 | - |
| ILME007 RED H2 | - | - | <0.16 | 0.382 | 11.917 |
| ILME007 OXI H2 | - | - | <0.16 | 0.172 | 14.754 |
| OXMN009 RED H2 | 68.947 | 0.197 | 1.727 | 0.525 | 2.536 |
| OXMN009 OXI H2 | 42.135 | <0.16 | 2.388 | 0.510 | 6.994 |
| OXMN010A RED H2 | 16.319 | 1.075 | <0.12 | 5.813 | 4.598 |
| OXMN010A OXI H2 | 36.587 | 4.500 | 11.160 | 0.605 | 11.183 |
| OXMN010A RED CH4 | 8.589 | <0.12 | 7.029 | ≈0 | <0.02 |
| OXMN010A OXI CH4 | 31.820 | <0.1 | 5.471 | ≈0 | <0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo, A.L.; Forero, C.R. Characterization for Disposal of the Residues Produced by Materials Used as Solid Oxygen Carriers in an Advanced Chemical Looping Combustion Process. Appl. Sci. 2018, 8, 1787. https://doi.org/10.3390/app8101787
Carrillo AL, Forero CR. Characterization for Disposal of the Residues Produced by Materials Used as Solid Oxygen Carriers in an Advanced Chemical Looping Combustion Process. Applied Sciences. 2018; 8(10):1787. https://doi.org/10.3390/app8101787
Chicago/Turabian StyleCarrillo, Adriana L., and Carmen R. Forero. 2018. "Characterization for Disposal of the Residues Produced by Materials Used as Solid Oxygen Carriers in an Advanced Chemical Looping Combustion Process" Applied Sciences 8, no. 10: 1787. https://doi.org/10.3390/app8101787
APA StyleCarrillo, A. L., & Forero, C. R. (2018). Characterization for Disposal of the Residues Produced by Materials Used as Solid Oxygen Carriers in an Advanced Chemical Looping Combustion Process. Applied Sciences, 8(10), 1787. https://doi.org/10.3390/app8101787