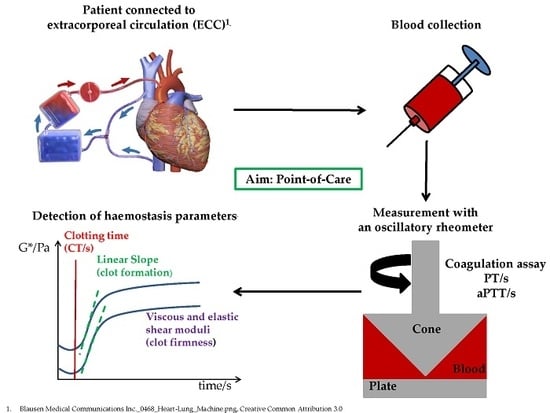
Dynamic and Quantitative Assessment of Blood Coagulation Status with an Oscillatory Rheometer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oscillatory Rheometer
2.2. Ball Coagulometer (KC 1A)
2.3. Blood
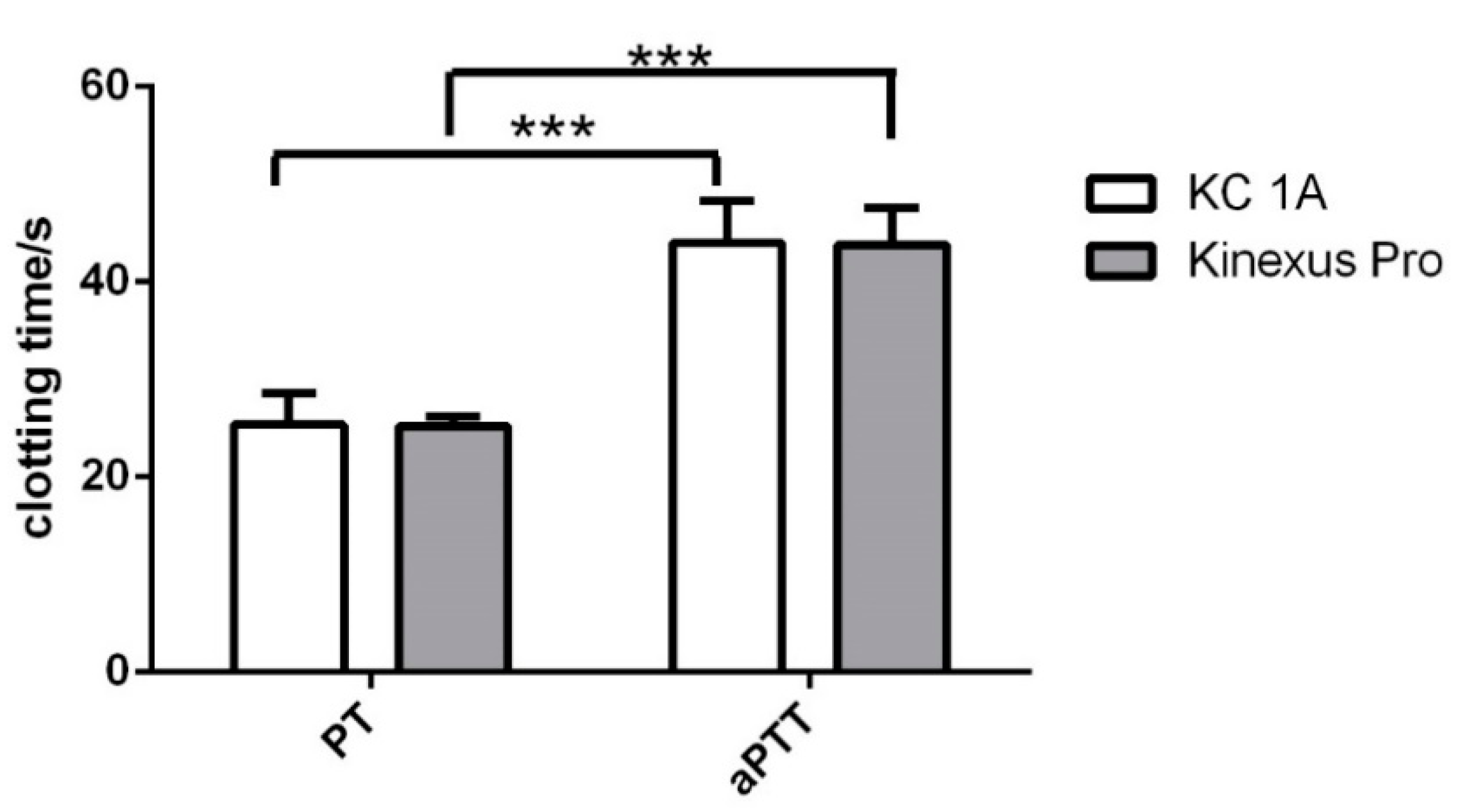
2.4. Prothrombin Time (PT)
2.5. Activated Partial Thromboplastin Time (aPTT)
2.6. Heparin and Protamine
2.7. Statistical Analyses
3. Results
3.1. Extrinsic Coagulation
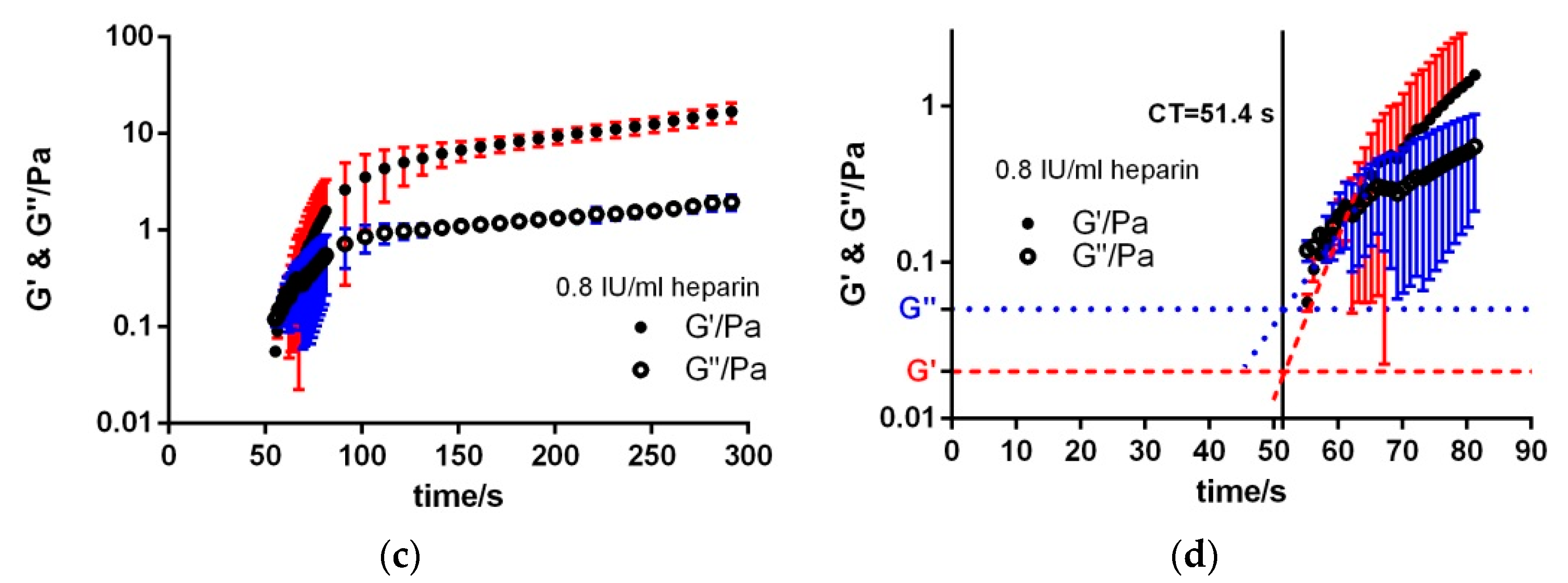
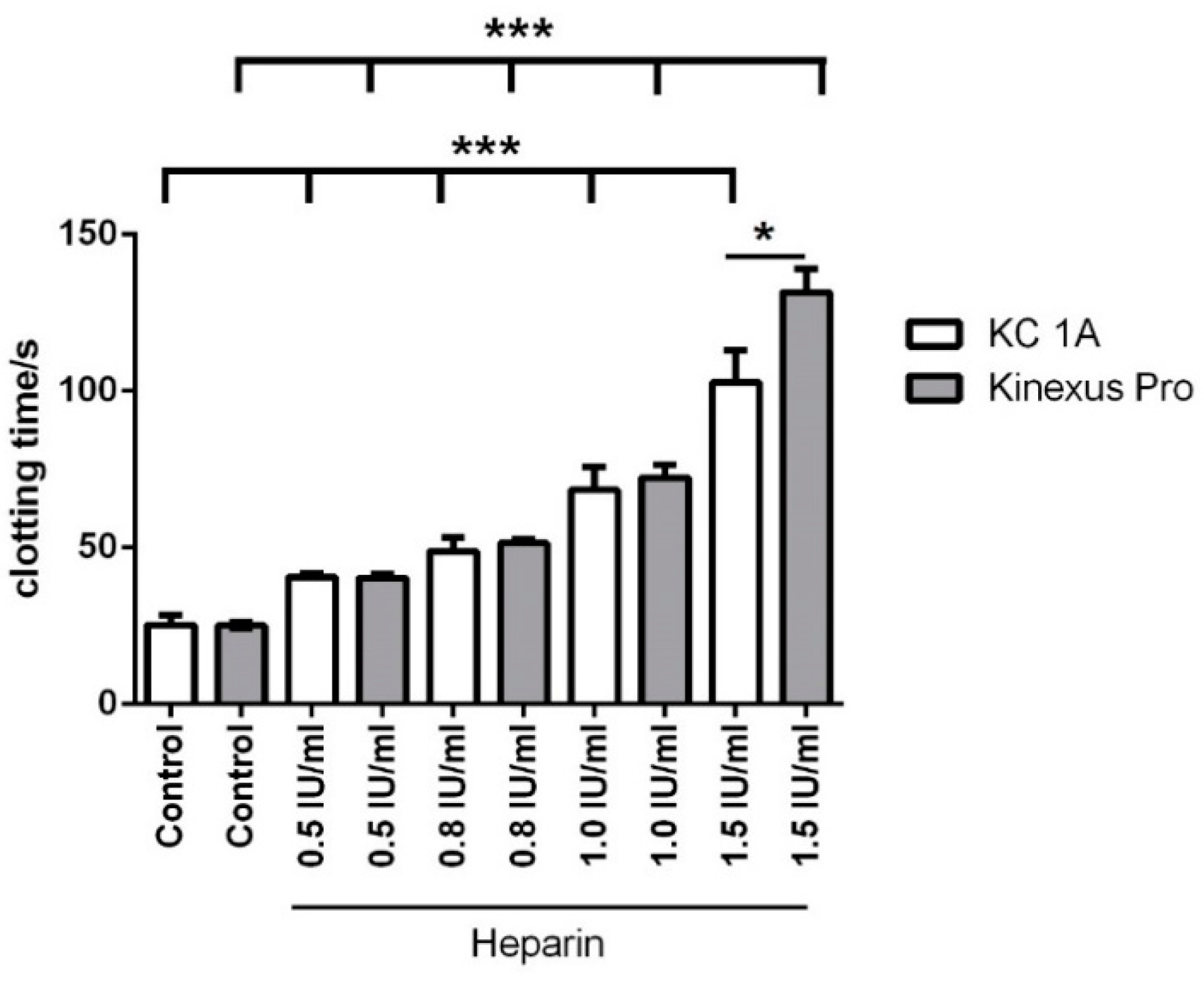
3.2. Heparin Titration
3.3. Intrinsic Coagulation
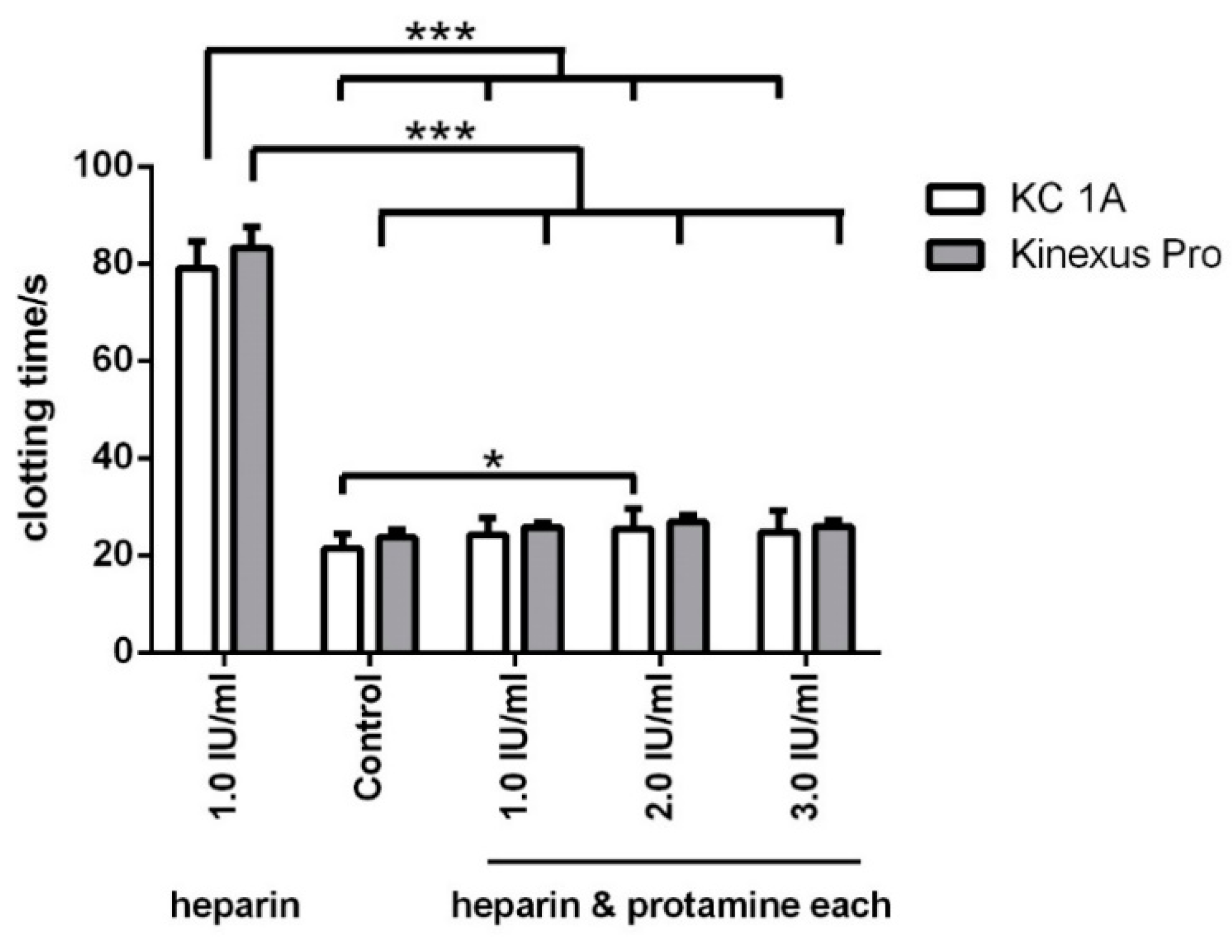
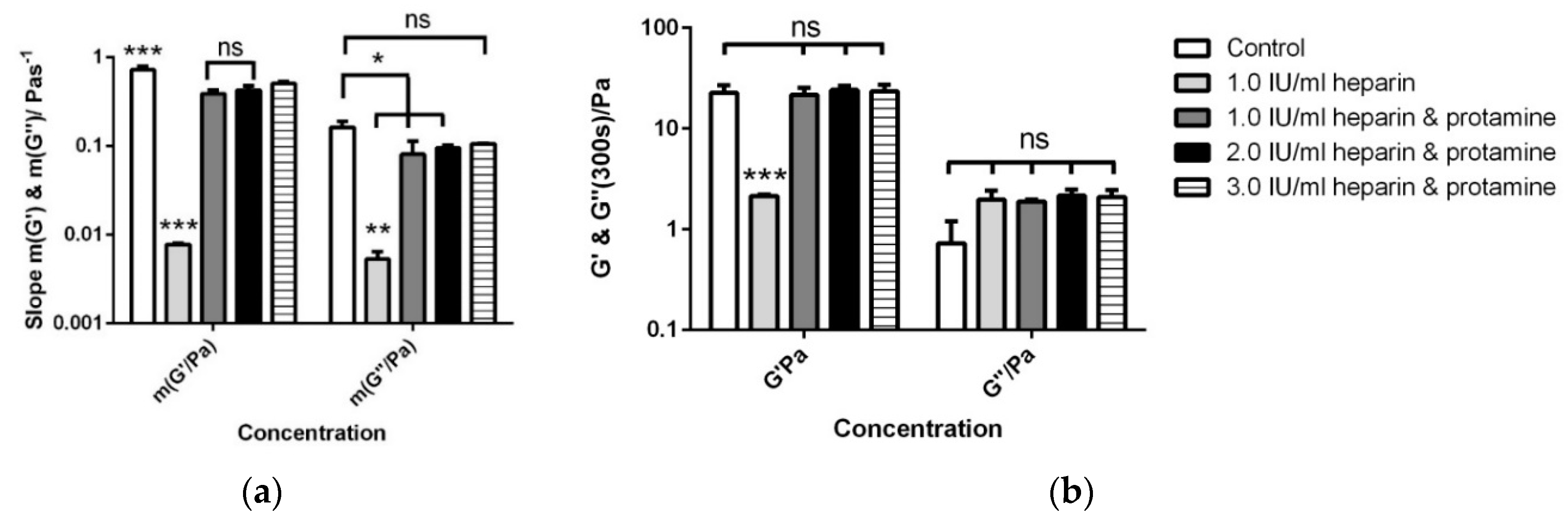
3.4. Antagonising Heparin with Equimolar Concentrations of Protamine
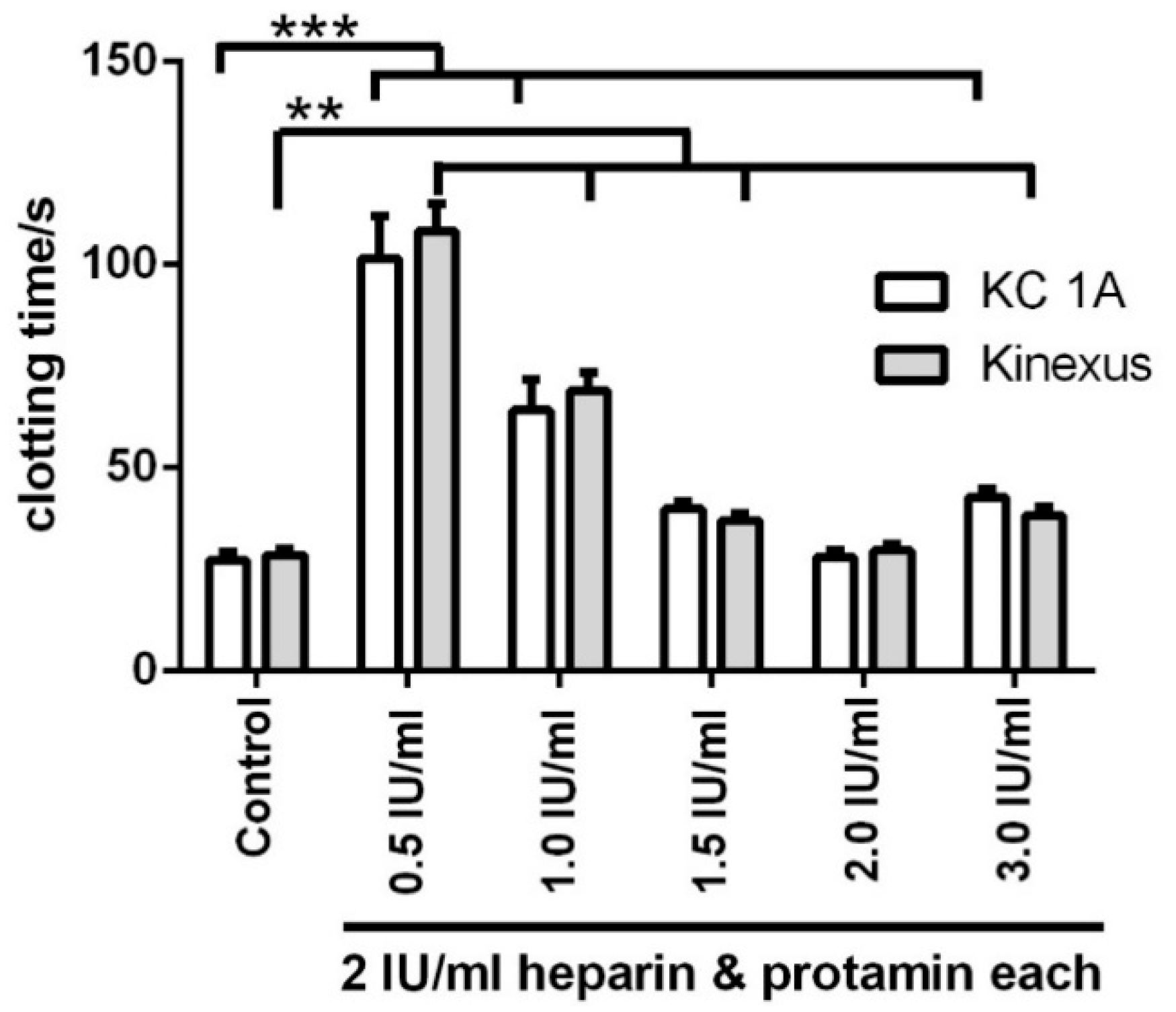
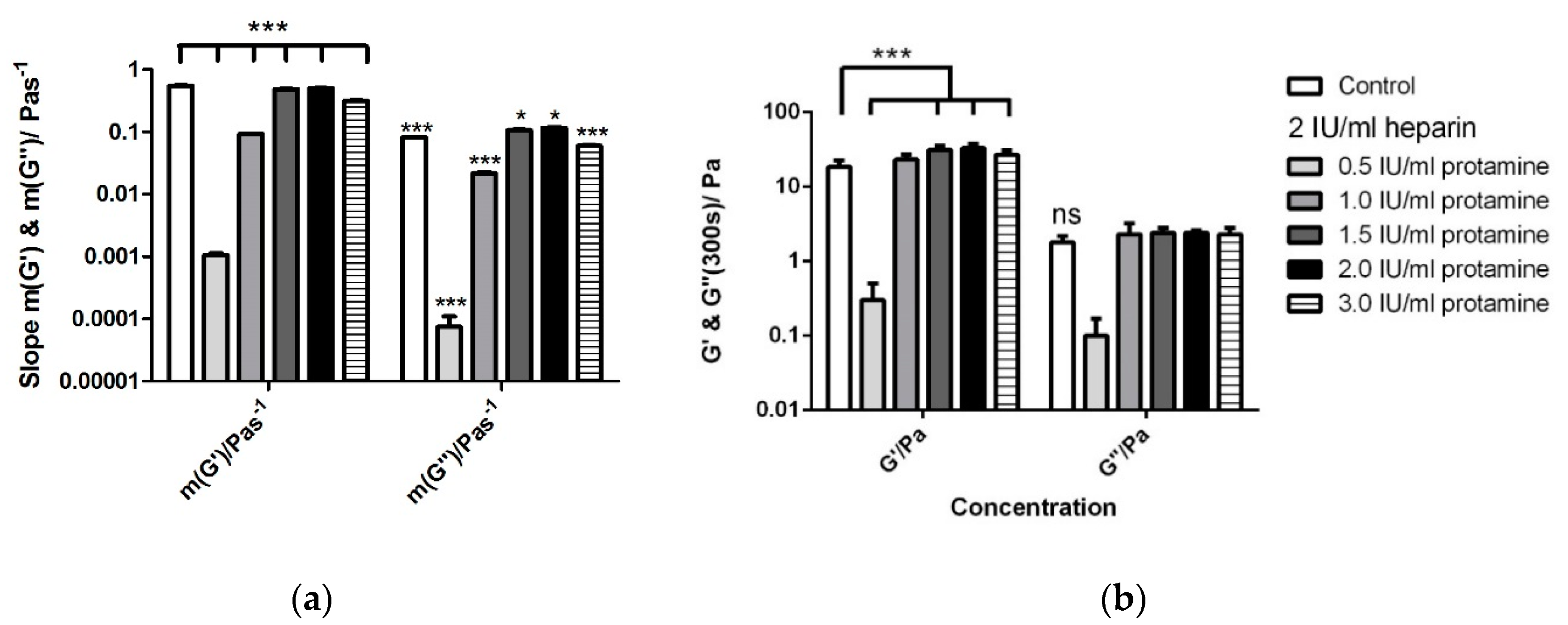
3.5. Titration of Various Protamine Concentrations to a Fixed Heparin Concentration
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A




References
- Punjabi, P.P.; Taylor, K.M. The science and practice of cardiopulmonary bypass: From cross circulation to ecmo and sirs. Glob. Cardiol. Sci. Pract. 2013, 2013, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, S.; Krauss, S.; Kurz, J.; Neumann, B.; Schlensak, C.; Wendel, H.P. Real-time measurement of free thrombin: Evaluation of the usability of a new thrombin assay for coagulation monitoring during extracorporeal circulation. Thromb. Res. 2014, 133, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.A.; Gelijns, A.C.; Moskowitz, A.J.; Heitjan, D.F.; Stevenson, L.W.; Dembitsky, W.; Long, J.W.; Ascheim, D.D.; Tierney, A.R.; Levitan, R.G.; et al. Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 2001, 345, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Niimi, Y.; Ishiguro, Y.; Nakata, Y.; Goto, T.; Morita, S.; Yamane, S. Platelet adhesion to heparin coated oxygenator fibers under in vitro static conditions: Impact of temperature. ASAIO J. 2001, 47, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Metzelder, S.M. Einfluss Dreier Verschiedener Techniken der Extrakorporalen Zirkulation auf Parameter der Hämodynamik, Inflammation, Blutgerinnung und Hämolyse bei Aortokoronaren Bypass-Operationen; RWTH Aachen University: Aachen, Germany, 2009. [Google Scholar]
- Myers, D.R.; Qiu, Y.; Fay, M.E.; Tennenbaum, M.; Chester, D.; Cuadrado, J.; Sakurai, Y.; Baek, J.; Tran, R.; Ciciliano, J.C.; et al. Single-platelet nanomechanics measured by high-throughput cytometry. Nat. Mater. 2017, 16, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, J.; Chen, Z. Dynamic and quantitative assessment of blood coagulation using optical coherence elastography. Sci. Rep. 2016, 6, 24294. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.M.; Hajjarian, Z.; Van Cott, E.M.; Nadkarni, S.K. Assessing blood coagulation status with laser speckle rheology. Biomed. Opt. Express 2014, 5, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.M.; Egawa, S.; Wirth, A.G.; Tshikudi, D.M.; Van Cott, E.M.; Nadkarni, S.K. Clinical evaluation of whole blood prothrombin time (PT) and international normalised ratio (INR) using a laser speckle rheology sensor. Sci. Rep. 2017, 7, 9169. [Google Scholar] [CrossRef] [PubMed]
- Marn, P.T.J. Numerical study of blood flow in stenotic artery. Appl. Rheol. 2009, 19, 13060. [Google Scholar]
- Collins, W.E.; Shibeshi, S.S. Correleation of hemorheology parameter hematocrit with hemodynamic factors and arterial diseases. Appl. Rheol. 2010, 20, 64092. [Google Scholar]
- Versteeg, H.H.; Heemskerk, J.W.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Dempfle, C.E.; Kalsch, T.; Elmas, E.; Suvajac, N.; Lucke, T.; Munch, E.; Borggrefe, M. Impact of fibrinogen concentration in severely ill patients on mechanical properties of whole blood clots. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2008, 19, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Mezger, T. Das Rheologie Handbuch: Für Anwender von Rotations-und Oszillations-Rheometern; Vincentz: Hannover, Germany, 2010. [Google Scholar]
- Frank, A.J. Understanding Instrument Inertia Corrections in Oscillation; TA Instruments: New Castle, DE, USA, 2005. [Google Scholar]
- Kinexus Pro+®—Forschungs Rheometer zur Charakterisierung Komplexer Fluide. Malvern Instruments Ltd.: Herrenberg, Germany. Available online: http://www.Malvern.Com/de/products/product-range/kinexus-range/kinexus-pro-plus/default.Aspx (accessed on 28 September 2016).
- ABW Medizin und Technik GmbH. Mc 1—Micro Macro Vet. Available online: http://www.Merlinmedical.Net/index.Php?Id=15&l=1 (accessed on 2 July 2014).
- ABW Medizin und Technik GmbH/Heinrich Amelung GmbH. Operation Manual mc1. Instructions. Merlin Medical Experience the Power of Innovation. Available online: http://www.merlinmedical.net/en/info-box/operation-manuals/ (accessed on 5 January 2017).
- ABW Medizin und Technik GmbH. Mechanical Ball Method-Measuring Principle. Available online: http://www.Merlinmedical.Net/index.Php?Id=6&l=1 (accessed on 5 July 2014).
- Siemens Healthcare Diagnostics Inc. Thromborel S (Package Insert). Available online: https://de.scribd.com/document/256489960/Insert-de-TP-Thromborel-S (accessed on 5 January 2017).
- Hartmann, M.; Sucker, C.; Boehm, O.; Koch, A.; Loer, S.; Zacharowski, K. Effects of cardiac surgery on hemostasis. Transfus. Med. Rev. 2006, 20, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuolo, G.; Carciati, A.; Caserta, S.; Guido, S. Blood linear viscoelasticity by small amplitude oscillatory flow. Rheol. Acta 2016, 55, 485–495. [Google Scholar] [CrossRef]
- Kappes, U. Zur Überwachung der antikoagulanzien-therapie. In HEPTEST-STAT [Arbeitsanleitung]; HEPTEST Laboratories Inc.: Augsburg, Germany, 2010. [Google Scholar]
- Evans, P.A.; Hawkins, K.; Lawrence, M.; Barrow, M.S.; Williams, P.R.; Williams, R.L. Studies of whole blood coagulation by oscillatory shear, thromboelastography and free oscillation rheometry. Clin. Hemorheol. Microcirc. 2008, 38, 267–277. [Google Scholar] [PubMed]
- Matthias, N. Einfluss Verschiedener Heparinisierungsprotokolle auf den Postoperativen Blutverlust und das Gerinnungssystem bei Kardiochirurgischen Operationen unter Einsatz der Extrakorporalen Zirkulation; Universität Hamburg: Hamburg, Germany, 2002. [Google Scholar]
- Ainle, F.N.; Preston, R.J.S.; Jenkins, P.V.; Nel, H.J.; Johnson, J.A.; Smith, O.P.; White, B.; Fallon, P.G.; O’Donnell, J.S. Protamine sulfate down-regulates thrombin generation by inhibiting factor v activation. Blood 2009, 114, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Krebs, C.R.; Lin, F.C.; Wolberg, A.S.; Oldenburg, A.L. High sensitivity micro-elastometry: Applications in blood coagulopathy. Ann. Biomed. Eng. 2013, 41, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Winstedt, D.; Tynngard, N.; Olanders, K.; Schott, U. Free oscillation rheometry monitoring of haemodilution and hypothermia and correction with fibrinogen and factor xiii concentrates. Scand. J. Trauma Resusc. Emerg. Med. 2013, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Hans, G.A.; Besser, M.W. The place of viscoelastic testing in clinical practice. Br. J. Haematol. 2016, 173, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.; DiNardo, J.A. Teg and rotem: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, D.; Seeberger, M.D.; Tanaka, K.A. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus. Med. Rev. 2012, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Spannagl, M. Blutgerinnungsanalytik. In Poct—Patientennahe Labordiagnostik; Luppa, B.P., Schlebusch, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 91–99. [Google Scholar]
- Schöchl, H.; Solomon, C.; Schulz, A.; Voeckel, W.; Hanke, A.; Griensven, M.V.; Redl, H.; Bahrami, S. Thromboelastometry (tem®) findings in disseminated intravascular coagulation in a pig model of endotoxinemia. Feinstein Inst. Med. Res. 2011, 17, 266–272. [Google Scholar]
- Calatzis, A.; Spannagl, M.; Vorweg, M. Zielgerichtete Behandlung akuter Hämostasestörungen mit Hilfe der Rotem Analyse; Tem Innovations GmbH: München, Germany, 2011. [Google Scholar]
- Daraktchiev, A.T. Evaluation Eines Neuen Thromboelastographie-Verfahrens-Rotem (Rotationselastometrie); Philipps-University Marburg: Marburg, Germany, 2009. [Google Scholar]
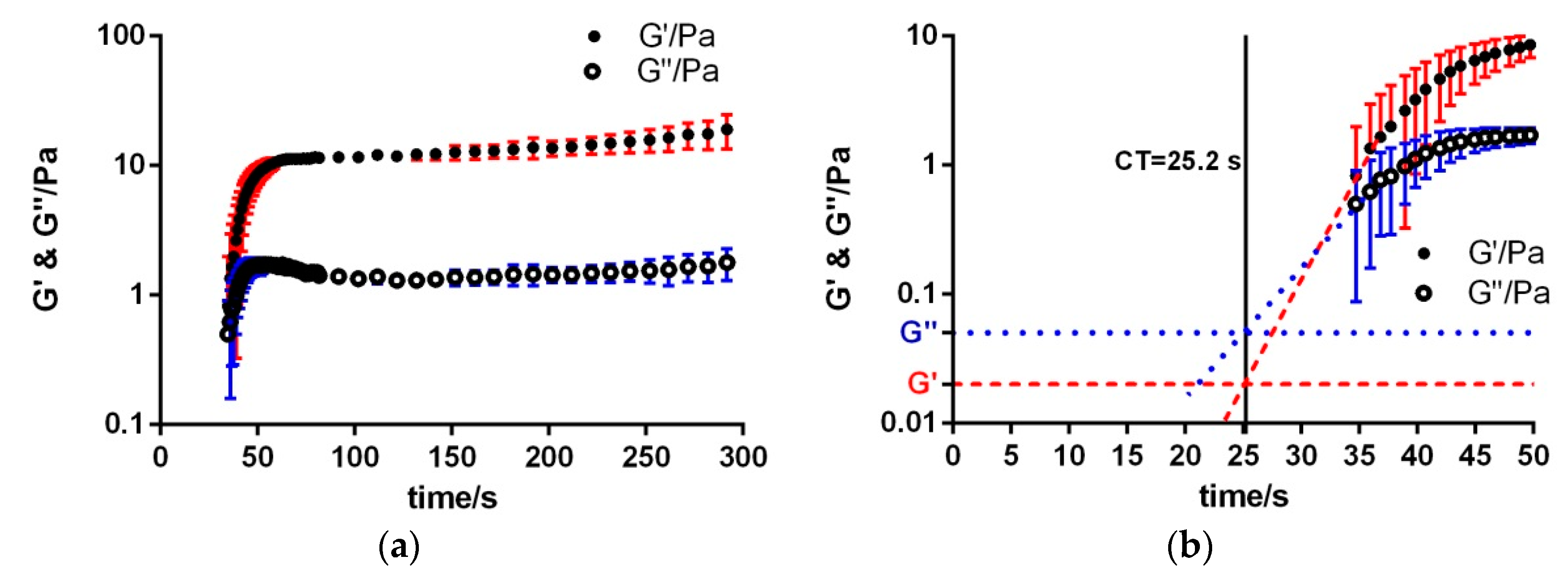
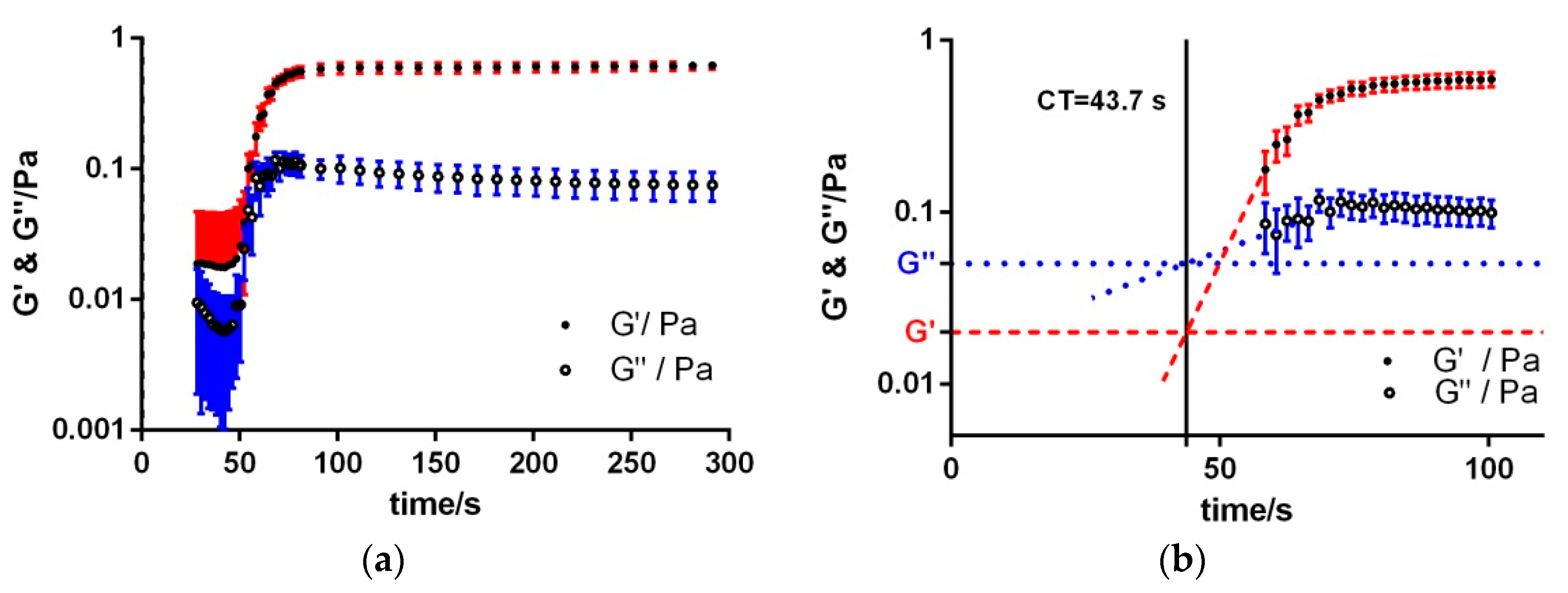
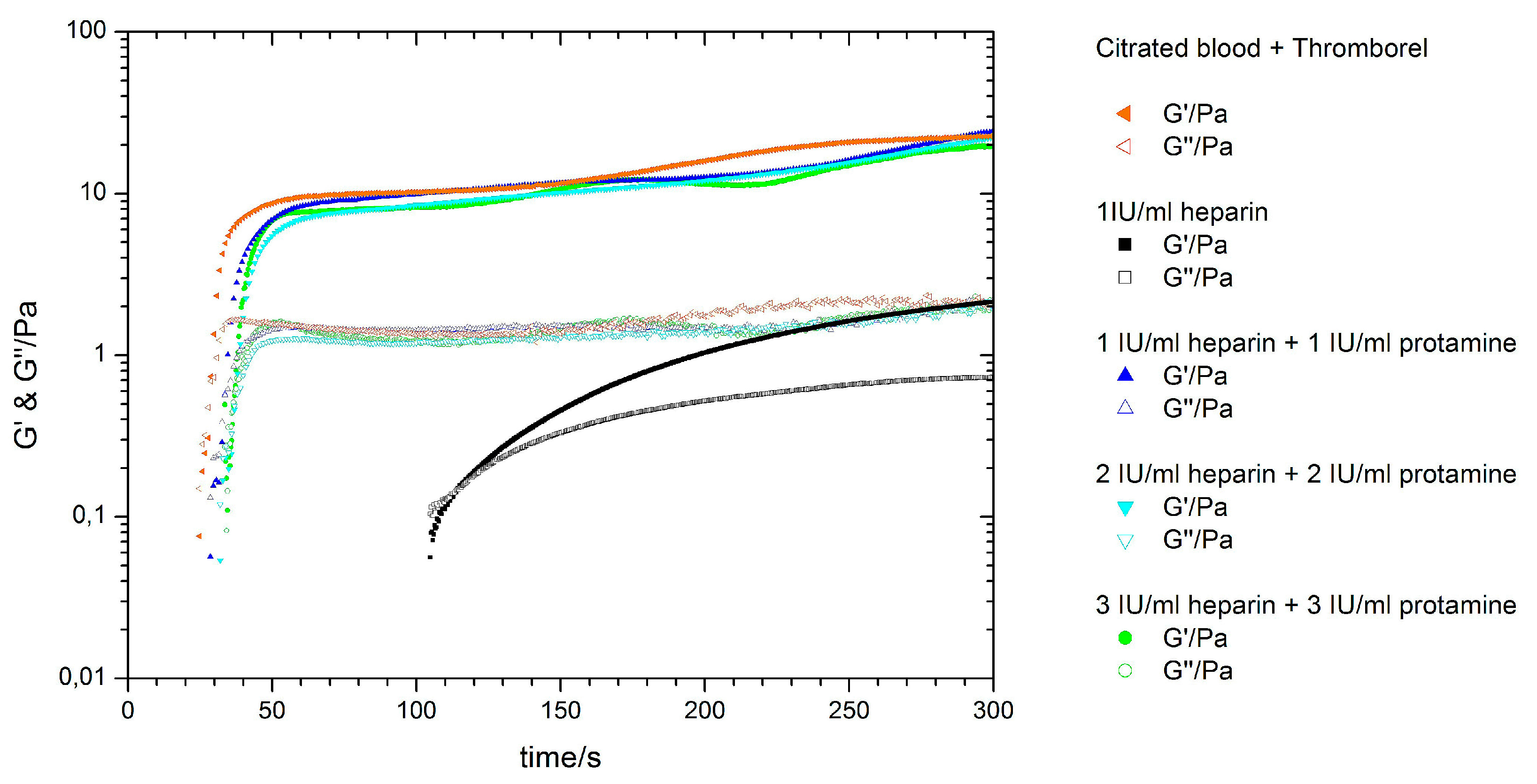
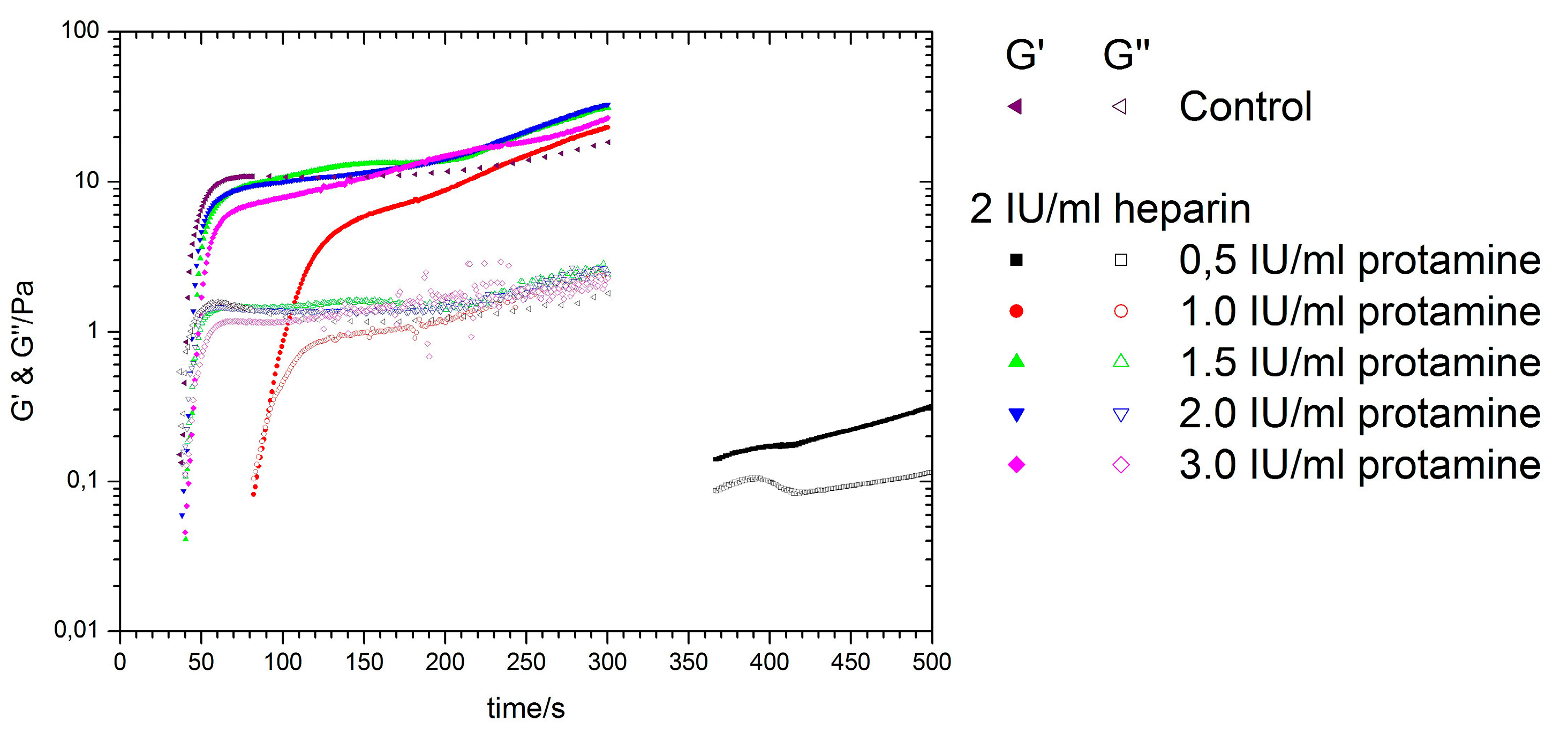
| Sample | Kinexus Pro | KC 1A | Kinexus Pro | |||
|---|---|---|---|---|---|---|
| CT/s | CT/s | G′ (Pa) at 300 s | G″ (Pa) at 300 s | m (G′/Pa s−1) | m (G″/Pa s−1) | |
| Control | 25.2 ± 1.0 | 25.4 ± 3.3 | 18.5 ± 4.5 | 1.7 ± 0.4 | 6.0 × 10−1 ± 0.8 × 10−1 | 1.2 × 10−1 ± 0.3 × 10−1 |
| 0.5 IU/mL | 40.3 ± 1.2 | 40.5 ± 1.3 | 18.6 ± 3.9 | 1.8 ± 0.2 | 4.4 × 10−1 ± 0.7 × 10−1 | 9.0 × 10−2 ± 1.8 × 10−2 |
| 0.8 IU/mL | 51.4 ± 1.3 | 48.8 ± 4.5 | 17.5 ± 3.9 | 2.0 ± 0.4 | 1.4 × 10−1 ± 0.8 × 10−1 | 2.3 × 10−2 ± 1.3 × 10−2 |
| 1.0 IU/mL | 72.3 ± 4.2 | 68.4 ± 7.3 | 1.8 ± 0.5 | 6.5 × 10−1 ± 0.7 × 10−1 | 8.6 × 10−3 ± 2.3 × 10−3 | 4.2 × 10−3 ± 1.2 × 10−3 |
| 1.5 IU/mL (n = 4) | 131.5 ± 7.4 | 102.7 ± 10.3 | 0.5 ± 0.3 | 3.0 × 10−1 ± 0.5 × 10−1 | 1.2 × 10−3 ± 0.8 × 10−3 | 9.0 × 10−4 ± 6.3 × 10−4 |
| Test | Kinexus Pro | KC 1A | Kinexus Pro | |||
|---|---|---|---|---|---|---|
| CT/s | CT/s | G′ (Pa) at 300 s | G″ (Pa) at 300 s | m (G′/Pa s−1) | m (G″/Pa s−1) | |
| aPTT | 43.7 ± 3.9 | 44.1 ± 4.3 | 6.2 × 10−1 ± 0.4 × 10−1 | 7.4 × 10−2 ± 1.9 × 10−2 | 2.6 × 10−2 ± 0.4 × 10−2 | 6.4 × 10−3 ± 1.4 × 10−3 |
| Sample | Kinexus Pro | KC 1A | Kinexus Pro | |||
|---|---|---|---|---|---|---|
| CT/s | CT/s | G′ (Pa)at 300 s | G″ (Pa)at 300 s | m (G′/Pa s−1) | m (G″/Pa s−1) | |
| Control | 23.7 ± 1.6 | 21.4 ± 2.9 | 22.7 ± 4.3 | 2.0 ± 0.5 | 72.3 × 10−2 ± 7.5 × 10−2 | 16.1 × 10−2 ± 3.0 × 10−2 |
| 1.0 IU/mL heparin | 83.3 ± 4.2 | 79.0 ± 5.6 | 2.1 ± 0.05 | 0.7 ± 0.07 | 7.8 × 10−3 ± 2.2 × 10−3 | 5.3 × 10−3 ± 1.2 × 10−3 |
| 1.0 IU/mL heparin and protamine | 25.8 ± 0.9 | 24.3 ± 3.5 | 21.5 ± 3.8 | 1.9 ± 0.3 | 39.0 × 10−2 ± 4.0 × 10−2 | 8.1 × 10−2 ± 3.3 × 10−2 |
| 2.0 IU/mL heparin and protamine | 26.9 ± 1.3 | 25.4 ± 4.1 | 24.1 ± 2.4 | 2.2 ± 0.4 | 42.7 × 10−2 ± 5.0 × 10−2 | 9.5 × 10−2 ± 0.7 × 10−2 |
| 3.0 IU/mL heparin and protamine | 25.9 ± 1.3 | 24.7 ± 4.5 | 23.5 ± 3.6 | 2.1 ± 0.1 | 51.0 × 10−2 ± 3.2 × 10−2 | 11.0 × 10−2 ± 0.1 × 10−2 |
| Sample | Kinexus Pro | KC 1A | Kinexus Pro | |||
|---|---|---|---|---|---|---|
| CT/s | CT/s (KC 1A) | G′ (Pa) | G″ (Pa) | m (G′/Pa s−1) | m (G″/Pa s−1) | |
| Control | 28.5 ± 1.4 | 27.2 ± 1.9 | 18.4 ± 4.1 | 1.8 ± 0.4 | 554.5 × 10−3 ± 6.5 × 10−3 | 82.0 × 10−3 ± 1.6 × 10−3 |
| 2 IU/mL heparin with: | ||||||
| 0.5 IU/mL protamine | 108.3 ± 6.8 | 101.4 ± 10.5 | 0.3 ± 0.2 | 1.1 × 10−1 ± 6.9 × 10−1 | 110.0 × 10−5 ± 2.1 × 10−5 | 7.5 × 10−5 ± 3.5 × 10−5 |
| 1.0 IU/mL protamine | 69.1 ± 4.4 | 64.2 ± 7.5 | 23.1 ± 3.9 | 2.3 ± 0.9 | 93.5 × 10−3 ± 0.6 × 10−3 | 21.6 × 10−3 ± 1.0 × 10−3 |
| 1.5 IU/mL protamine | 37.1 ± 1.8 | 39.9 ± 1.9 | 31.3 ± 3.7 | 2.4 ± 0.4 | 490.0 × 10−3 ± 0.6 × 10−3 | 106.0 × 10−3 ± 3.1 × 10−3 |
| 2.0 IU/mL protamine | 29.7 ± 1.6 | 28.1 ± 1.7 | 32.9 ± 4.6 | 2.4 ± 0.2 | 505.0 × 10−3 ± 4.2 × 10−3 | 116.0 × 10−3 ± 2.4 × 10−3 |
| 3.0 IU/mL protamine | 38.3 ± 2.2 | 42.7 ± 2.1 | 26.8 ± 4.1 | 2.3 ± 0.5 | 317.2 × 10−3 ± 4.8 × 10−3 | 60.0 × 10−3 ± 1.4 × 10−3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunnakattu, S.-J.; Groß, T.; Knieps, J.; Kemper, T.; Fennrich, S.; Rauch, N.; Wendel, H.P.; Stoppelkamp, S. Dynamic and Quantitative Assessment of Blood Coagulation Status with an Oscillatory Rheometer. Appl. Sci. 2018, 8, 84. https://doi.org/10.3390/app8010084
Kunnakattu S-J, Groß T, Knieps J, Kemper T, Fennrich S, Rauch N, Wendel HP, Stoppelkamp S. Dynamic and Quantitative Assessment of Blood Coagulation Status with an Oscillatory Rheometer. Applied Sciences. 2018; 8(1):84. https://doi.org/10.3390/app8010084
Chicago/Turabian StyleKunnakattu, Silju-John, Theresia Groß, Juvano Knieps, Tim Kemper, Stefan Fennrich, Nicole Rauch, Hans P. Wendel, and Sandra Stoppelkamp. 2018. "Dynamic and Quantitative Assessment of Blood Coagulation Status with an Oscillatory Rheometer" Applied Sciences 8, no. 1: 84. https://doi.org/10.3390/app8010084
APA StyleKunnakattu, S.-J., Groß, T., Knieps, J., Kemper, T., Fennrich, S., Rauch, N., Wendel, H. P., & Stoppelkamp, S. (2018). Dynamic and Quantitative Assessment of Blood Coagulation Status with an Oscillatory Rheometer. Applied Sciences, 8(1), 84. https://doi.org/10.3390/app8010084