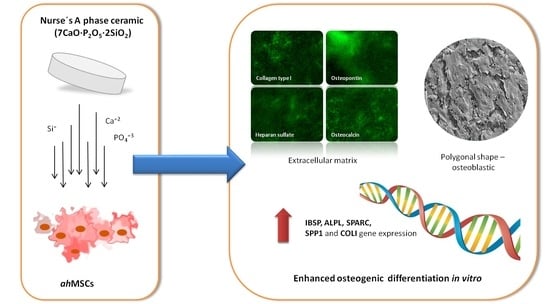
Impact of a Porous Si-Ca-P Monophasic Ceramic on Variation of Osteogenesis-Related Gene Expression of Adult Human Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Porous Si-Ca-P Monophasic Ceramic (SCP-c)
2.2. Isolation, Characterization and Primary Culture of Adult Human Bone Marrow-Derived Mesenchymal Stem Cells (ahMSCs)
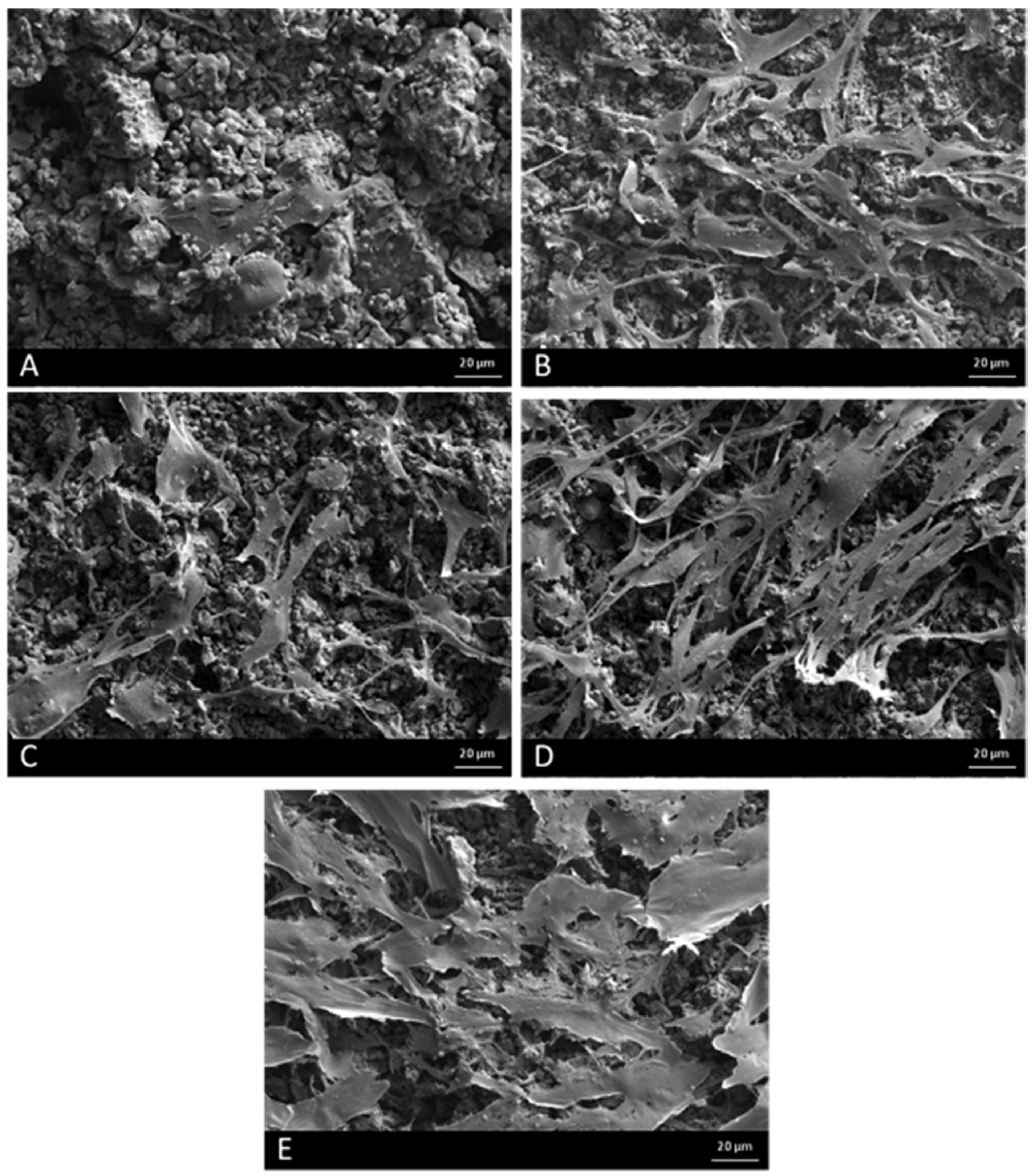
2.3. Field Emission Scanning Electron Microscopy (FESEM)
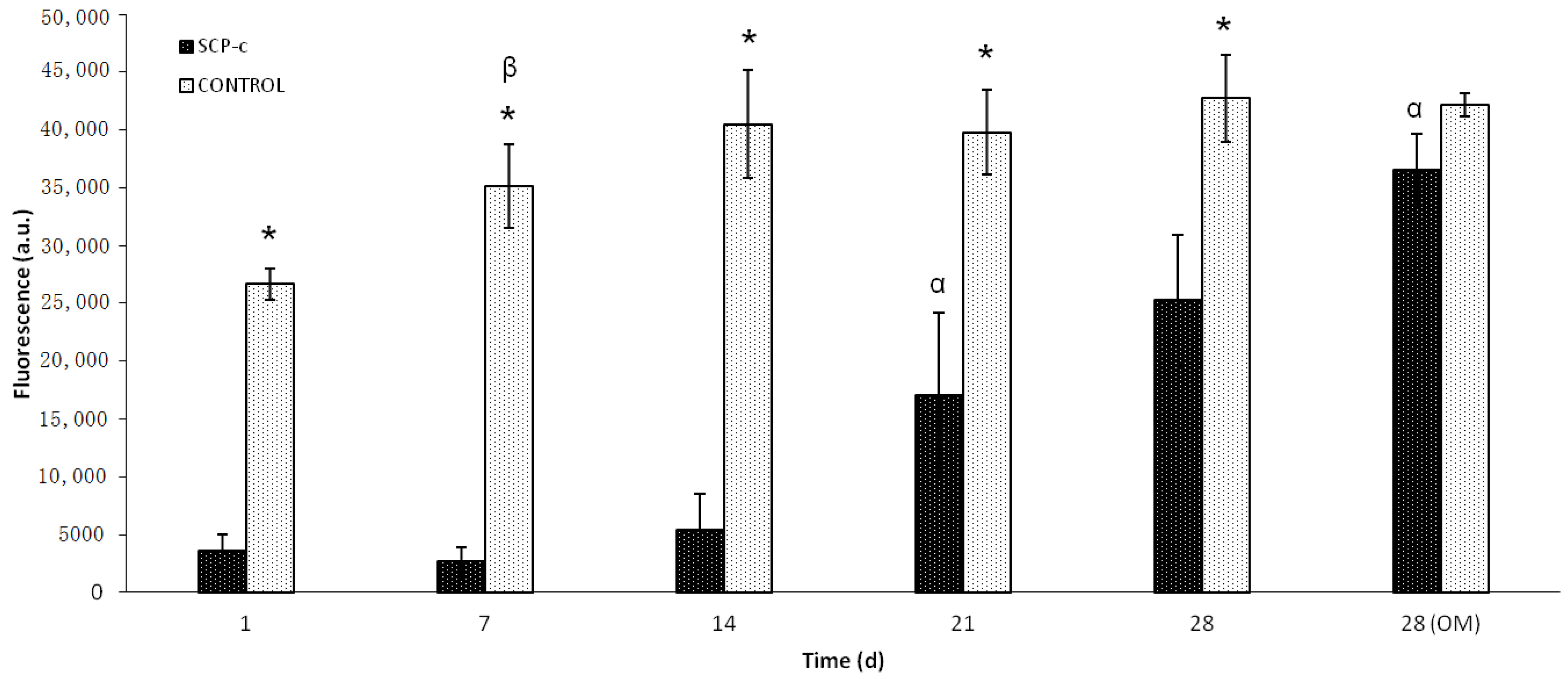
2.4. Cellular Metabolic Activity Assay
2.5. Osteogenic Differentiation Assays
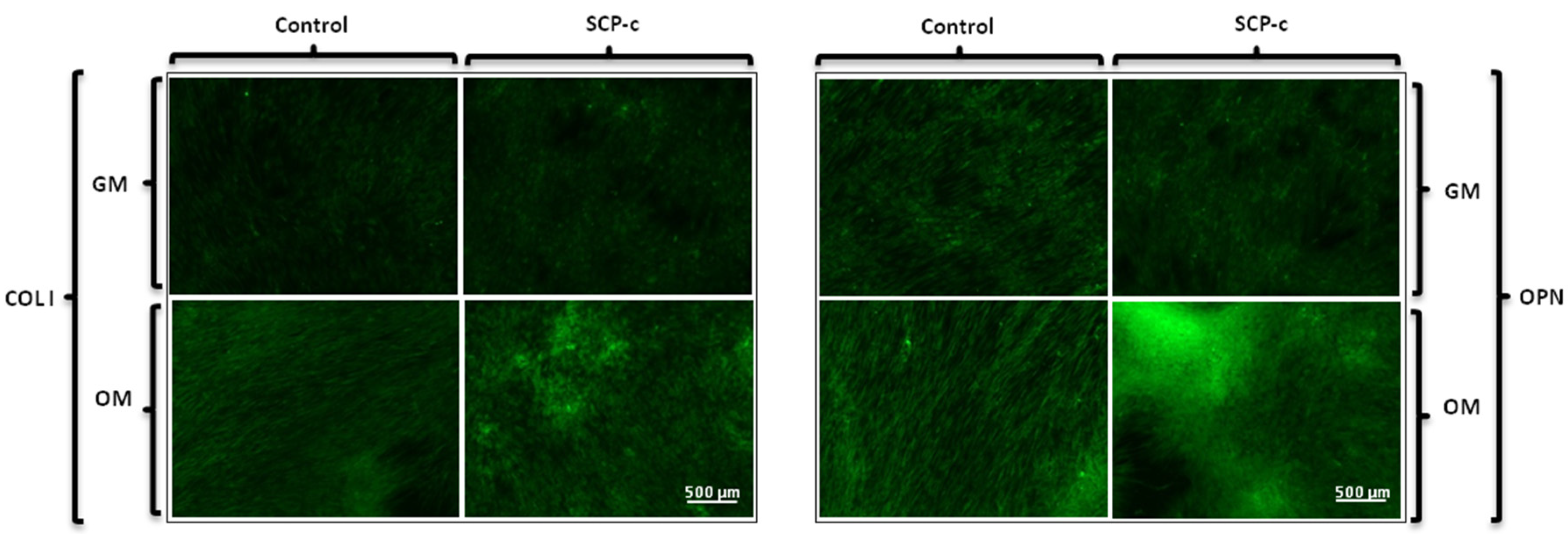
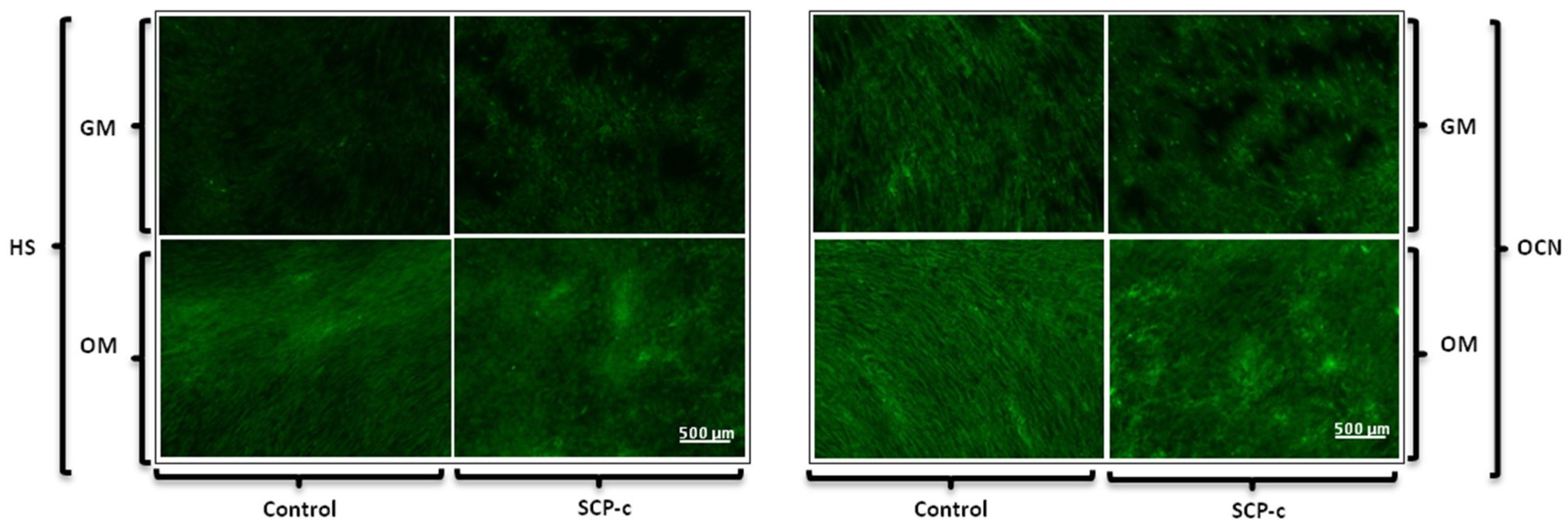
2.5.1. Immunofluorescence Staining Assay: Heparan Sulphate (HS), Osteocalcin (OCN), Osteopontin (OPN) and Collagen Type I (Col I) Expression
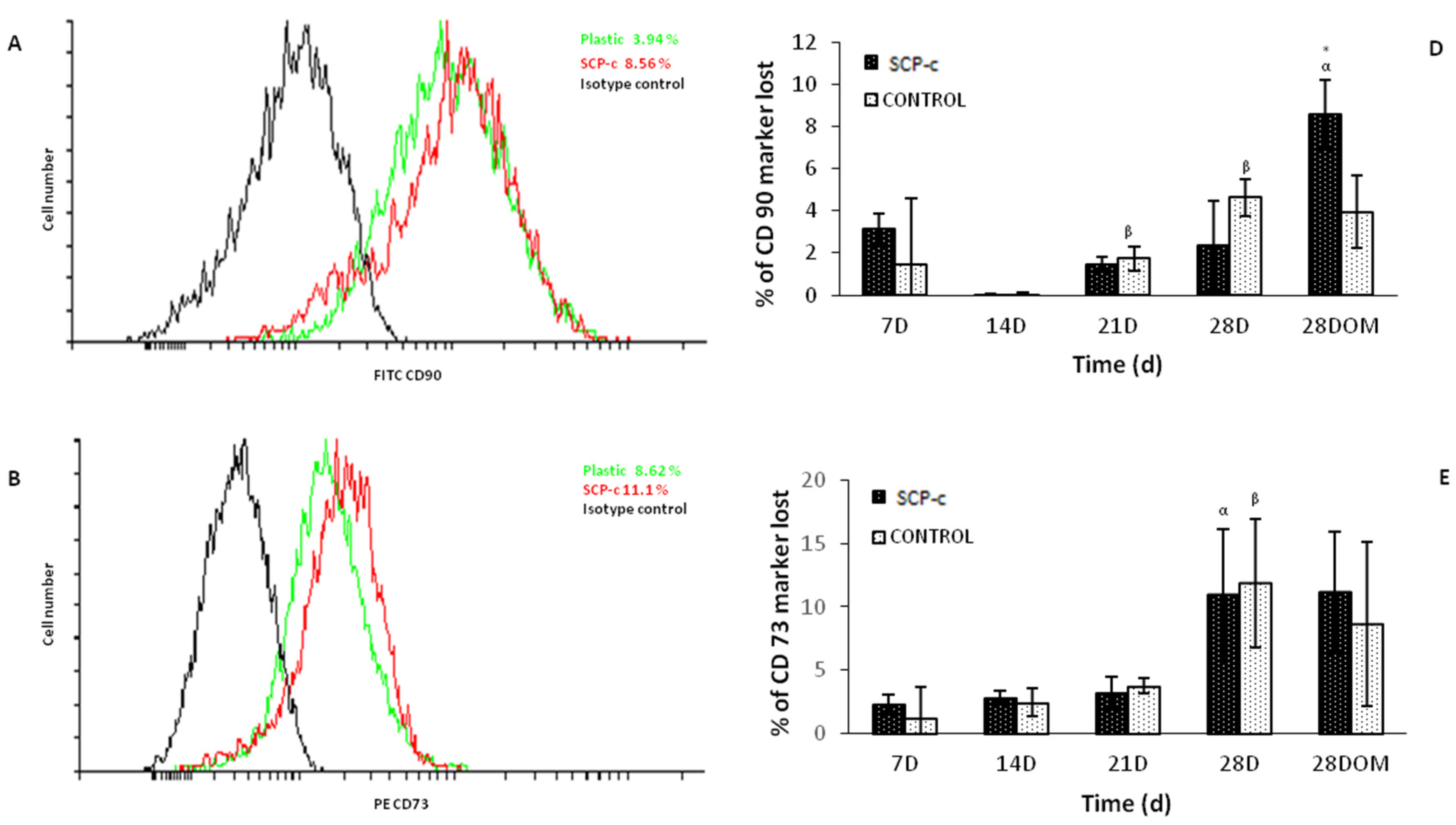
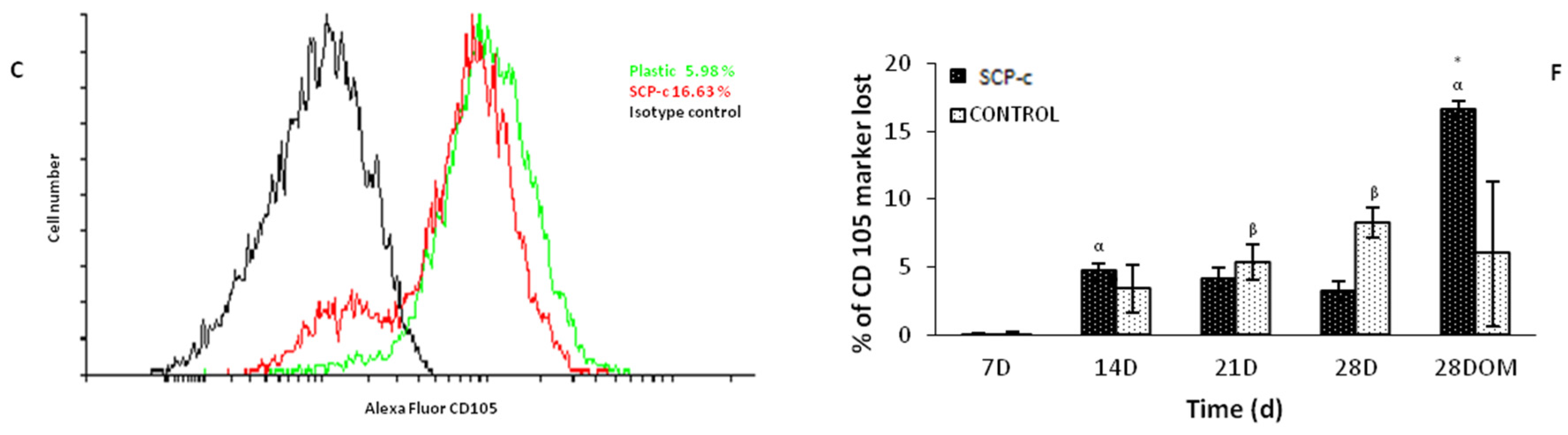
2.5.2. Surface Markers Cluster Differentiation (CD)
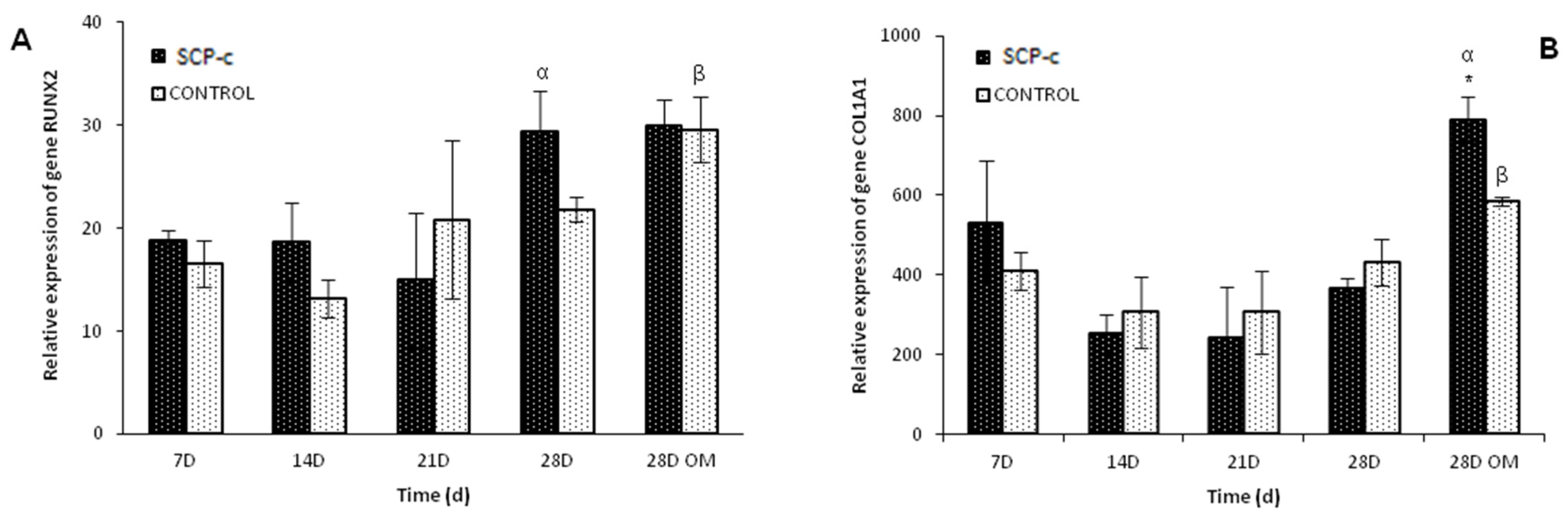
2.5.3. Osteogenic Gene Expression: Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assay
3. Calculation
4. Results
4.1. Field Emission Scanning Electron Microscopy (FESEM)
4.2. Cellular Metabolic Activity Assay
4.3. Differentiation Assays
4.3.1. Immunofluorescence Staining Assay: Heparan Sulphate (HS), Osteocalcin (OCN), Osteopontin (OPN) and Collagen Type I (Col I) Expression
4.3.2. Surface Markers CD
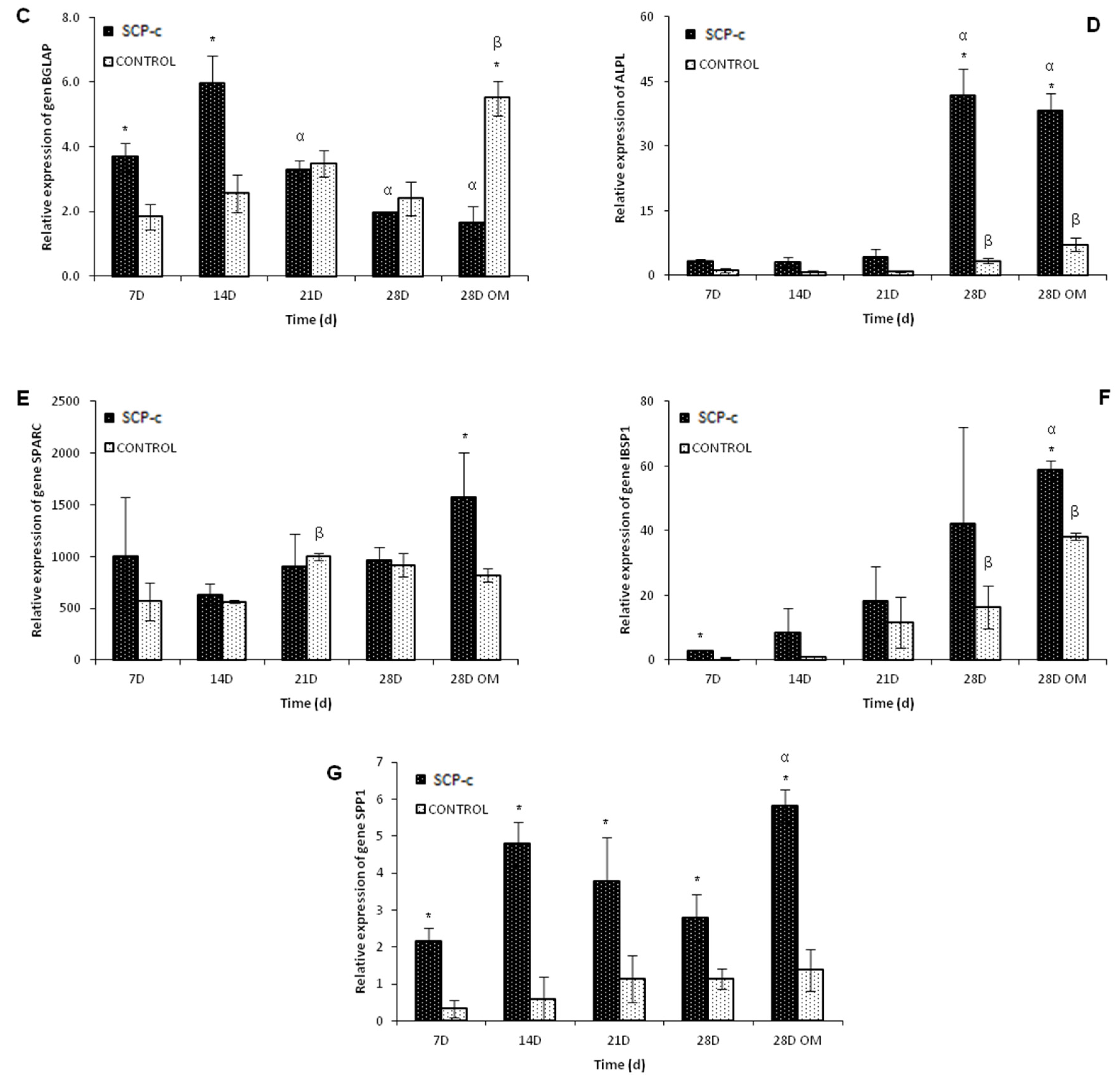
4.3.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assay
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef]
- Bosetti, M.; Cannas, M. The effect of bioactive glasses on bone marrow stromal cells differentiation. Biomaterials 2005, 26, 3873–3879. [Google Scholar] [CrossRef] [PubMed]
- Lugo, G.; Mazón, P.; De Aza, P. Phase transitions in single phase Si–Ca–P-based ceramic under thermal treatment. J. Eur. Ceram. Soc. 2015, 35, 3693–3700. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F.; Piras, A.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Hild, N.; Schneider, O.D.; Mohn, D.; Luechinger, N.A.; Koehler, F.M.; Hofmann, S.; Vetsch, J.R.; Thimm, B.W.; Müller, R.; Stark, W.J. Two-layer membranes of calcium phosphate/collagen/plga nanofibres: In vitro biomineralisation and osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2011, 3, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, Z.; Wang, C.; Chen, J.; Zhang, X. Dynamic study of calcium phosphate formation on porous HA/TCP ceramics. J. Mater. Sci. Mater. Med. 2004, 15, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhao, X.; Darvell, B.W.; Lu, W.W. Apatite-formation ability–predictor of “bioactivity”? Acta Biomater. 2010, 6, 4181–4188. [Google Scholar] [CrossRef] [PubMed]
- Jonasova, L.; Müller, F.A.; Helebrant, A.; Strnad, J.; Greil, P. Hydroxyapatite formation on alkali-treated titanium with different content of Na+ in the surface layer. Biomaterials 2002, 23, 3095–3101. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Valerio, P.; Pereira, M.M.; Goes, A.M.; Leite, M.F. The effect of ionic products from bioactive glass dissolution on osteoblast proliferation and collagen production. Biomaterials 2004, 25, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.-Y.; Ding, S.-J.; Chang, H.-C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef] [PubMed]
- Rabadan-Ros, R.; Mazón, P.; Serena, S.; Sainz, M.; Meseguer-Olmo, L.; De Aza, P. In vitro behaviour of nurse's a ss-phase: A new calcium silicophosphate ceramic. J. Eur. Ceram. Soc. 2017, 37, 2943–2952. [Google Scholar] [CrossRef]
- Rabadan-Ros, R.; Velásquez, P.A.; Meseguer-Olmo, L.; De Aza, P.N. Morphological and structural study of a novel porous nurse’s a ceramic with osteoconductive properties for tissue engineering. Materials 2016, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Rabadan-Ros, R.; Aznar-Cervantes, S.; Mazón, P.; Ros-Tarraga, P.; De Aza, P.N.; Meseguer-Olmo, L. Nurse’s a-phase material enhance adhesion, growth and differentiation of human bone marrow-derived stromal mesenchymal stem cells. Materials 2017, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Lugo, G.J.; Mazón, P.; Piedad, N. Material processing of a new calcium silicophosphate ceramic. Ceram. Int. 2016, 42, 673–680. [Google Scholar] [CrossRef]
- Lugo, G.J.; Mazón, P.; Baudin, C.; De Aza, P.N. Nurse′ s a-phase: Synthesis and characterization in the binary system ca2sio4–ca3 (po4) 2. J. Am. Ceram. Soc. 2015, 98, 3042–3046. [Google Scholar] [CrossRef]
- De Aza, P.N.; García-Bernal, D.; Cragnolini, F.; Velasquez, P.; Meseguer-Olmo, L. The effects of ca2sio4-ca3 (po4) 2 ceramics on adult human mesenchymal stem cell viability, adhesion, proliferation, differentiation and function. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4009–4020. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- García-Páez, I.H.; Carrodeguas, R.G.; Antonio, H.; Baudín, C.; Pena, P. Effect of mg and si co-substitution on microstructure and strength of tricalcium phosphate ceramics. J. Mech. Behav. Biomed. Mater. 2014, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, S.; Moztarzadeh, F. Influence of strontium on the structure and biological properties of sol–gel-derived mesoporous bioactive glass (MBG) powder. J. Sol-Gel Sci. Technol. 2016, 78, 539–549. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.B.; Varma, H.; John, A. Triphasic ceramic coated hydroxyapatite as a niche for goat stem cell-derived osteoblasts for bone regeneration and repair. J. Mater. Sci. Mater. Med. 2009, 20, 251–258. [Google Scholar] [CrossRef] [PubMed]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and rhoa regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Sordella, R.; Jiang, W.; Chen, G.-C.; Curto, M.; Settleman, J. Modulation of rho gtpase signaling regulates a switch between adipogenesis and myogenesis. Cell 2003, 113, 147–158. [Google Scholar] [CrossRef]
- Thomas, C.H.; Collier, J.H.; Sfeir, C.S.; Healy, K.E. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc. Natl. Acad. Sci. USA 2002, 99, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Shih, Y.R.V.; Kuo, T.K.; Lee, O.K.; Wei, Y.H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2008, 26, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Conget, P.A.; Minguell, J.J. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J. Cell. Physiol. 1999, 181, 67–73. [Google Scholar] [CrossRef]
- Mafi, P.; Hindocha, S.; Mafi, R.; Griffin, M.; Khan, W. Adult mesenchymal stem cells and cell surface characterization—A systematic review of the literature. Open Orthop. J. 2011, 5, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Setzer, B.; Bächle, M.; Metzger, M.C.; Kohal, R.J. The gene-expression and phenotypic response of hfob 1.19 osteoblasts to surface-modified titanium and zirconia. Biomaterials 2009, 30, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.-I.; Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nature 2003, 423, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Lee, K.-S.; Kim, H.-J.; Li, Q.-L.; Chi, X.-Z.; Ueta, C.; Komori, T.; Wozney, J.M.; Kim, E.-G.; Choi, J.-Y.; Ryoo, H.-M. Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between RUNX2 and SMAD5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line c2c12. Mol. Cell. Biol. 2000, 20, 8783–8792. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R.T.; Xiao, G. Regulation of the osteoblast-specific transcription factor, runx2: Responsiveness to multiple signal transduction pathways. J. Cell. Biochem. 2003, 88, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R. The developmental control of osteoblast-specific gene expression: Role of specific transcription factors and the extracellular matrix environment. Crit. Rev. Oral Biol. Med. 1999, 10, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Jiao, G.; Liu, H.; Wu, W.; Li, S.; Wang, Q.; Xu, D.; Li, X.; Liu, H.; Chen, Y. Biological silicon stimulates collagen type 1 and osteocalcin synthesis in human osteoblast-like cells through the BMP-2/SMAD/RUNX2 signaling pathway. Biol. Trace Elem. Res. 2016, 173, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-L.; Yang, J.W.; Rifas, L.; Zhang, S.-F.; Avioli, L.V. Differentiation of human bone marrow osteogenic stromal cells in vitro: Induction of the osteoblast phenotype by dexamethasone. Endocrinology 1994, 134, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Jikko, A.; Harris, S.E.; Chen, D.; Mendrick, D.L.; Damsky, C.H. Collagen integrin receptors regulate early osteoblast differentiation induced by BMP-2. J. Bone Miner. Res. 1999, 14, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Schipani, E.; Kronenberg, H.M. Adult Mesenchymal Stem Cells; Harvard Stem Cell Institute: Cambridge, MA, USA, 2009. [Google Scholar]
- Kulterer, B.; Friedl, G.; Jandrositz, A.; Sanchez-Cabo, F.; Prokesch, A.; Paar, C.; Scheideler, M.; Windhager, R.; Preisegger, K.-H.; Trajanoski, Z. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genom. 2007, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Allabouch, A.; Colat-Parros, J.; Salmon, R.; Naim, S.; Meunier, J. Biocompatibility of some materials used in dental implantology: Histological study. Coll. Surf. B Biointerfaces 1993, 1, 323–329. [Google Scholar] [CrossRef]
- Aybar, B.; Bilir, A.; Akçakaya, H.; Ceyhan, T. Effects of tricalcium phosphate bone graft materials on primary cultures of osteoblast cells in vitro. Clin. Oral Implant. Res. 2004, 15, 119–125. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, L.; Cui, L.; Liu, W.; Cao, Y. Tissue-engineered bone formation using human bone marrow stromal cells and novel β-tricalcium phosphate. Biomed. Mater. 2007, 2, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Payer, M.; Lohberger, B.; Stadelmeyer, E.; Bartmann, C.; Windhager, R.; Jakse, N. Behaviour of multipotent maxillary bone derived cells on β-tricalcium phosphate and highly porous bovine bone mineral. Clin. Oral Implant. Res. 2010, 21, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Yefang, Z.; Hutmacher, D.; Varawan, S.-L.; Meng, L.T. Comparison of human alveolar osteoblasts cultured on polymer-ceramic composite scaffolds and tissue culture plates. Int. J. Oral Maxillofac. Surg. 2007, 36, 137–145. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruben, R.-R.; Beatriz, R.-N.; Patricia, M.; Salvador, A.-C.; Patricia, R.-T.; Piedad N., D.A.; Luis, M.-O. Impact of a Porous Si-Ca-P Monophasic Ceramic on Variation of Osteogenesis-Related Gene Expression of Adult Human Mesenchymal Stem Cells. Appl. Sci. 2018, 8, 46. https://doi.org/10.3390/app8010046
Ruben R-R, Beatriz R-N, Patricia M, Salvador A-C, Patricia R-T, Piedad N. DA, Luis M-O. Impact of a Porous Si-Ca-P Monophasic Ceramic on Variation of Osteogenesis-Related Gene Expression of Adult Human Mesenchymal Stem Cells. Applied Sciences. 2018; 8(1):46. https://doi.org/10.3390/app8010046
Chicago/Turabian StyleRuben, Rabadan-Ros, Revilla-Nuin Beatriz, Mazón Patricia, Aznar-Cervantes Salvador, Ros-Tarraga Patricia, De Aza Piedad N., and Meseguer-Olmo Luis. 2018. "Impact of a Porous Si-Ca-P Monophasic Ceramic on Variation of Osteogenesis-Related Gene Expression of Adult Human Mesenchymal Stem Cells" Applied Sciences 8, no. 1: 46. https://doi.org/10.3390/app8010046
APA StyleRuben, R.-R., Beatriz, R.-N., Patricia, M., Salvador, A.-C., Patricia, R.-T., Piedad N., D. A., & Luis, M.-O. (2018). Impact of a Porous Si-Ca-P Monophasic Ceramic on Variation of Osteogenesis-Related Gene Expression of Adult Human Mesenchymal Stem Cells. Applied Sciences, 8(1), 46. https://doi.org/10.3390/app8010046