Structure and Capacitance of Electrical Double Layers at the Graphene–Ionic Liquid Interface
Abstract
1. Introduction
2. Simulation System and Methods
3. Results and Discussion
3.1. Ion Distributions in EDL
3.2. Differential Capacitance Calculation and Analysis
4. Conclusions
- (1)
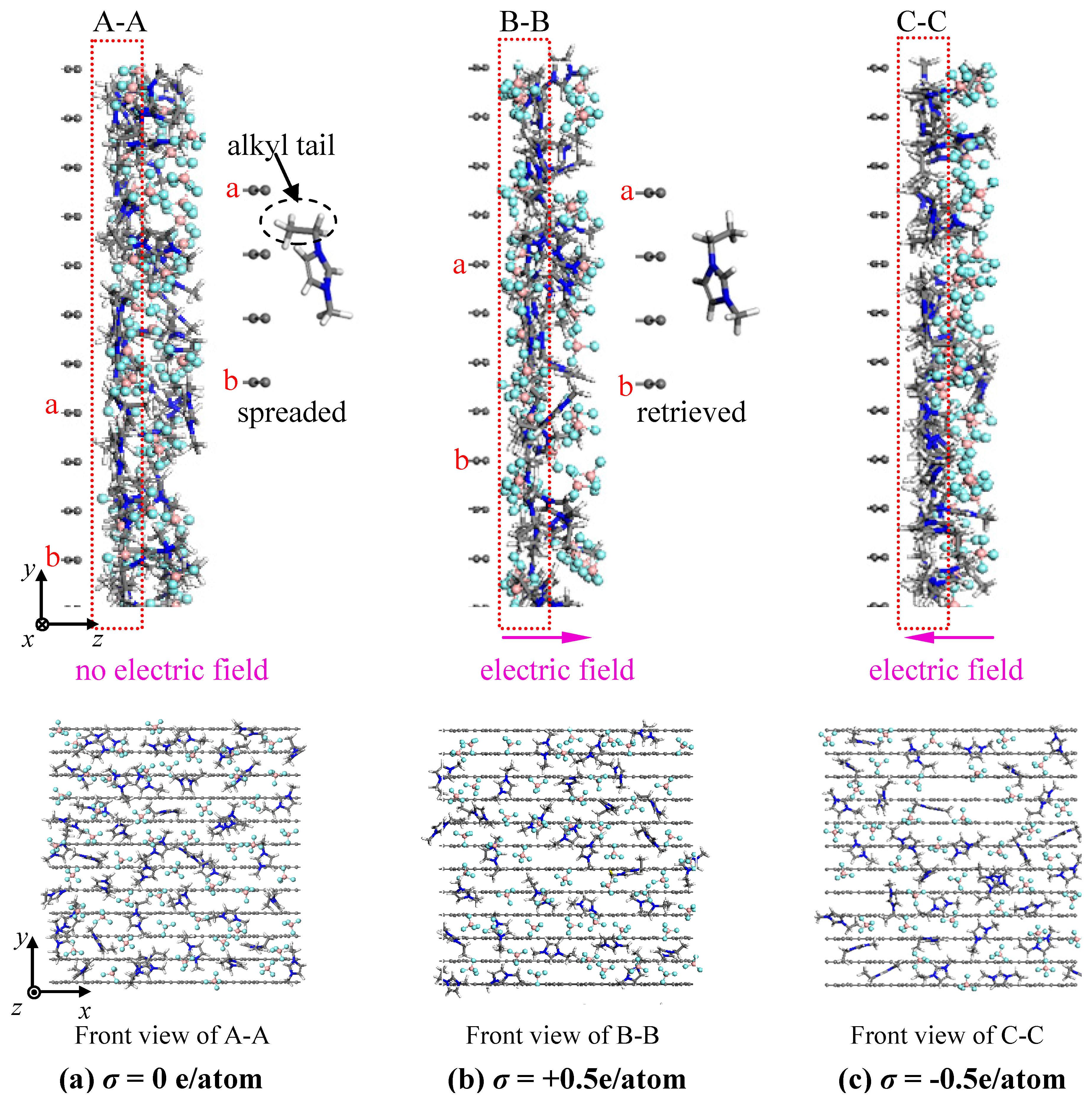
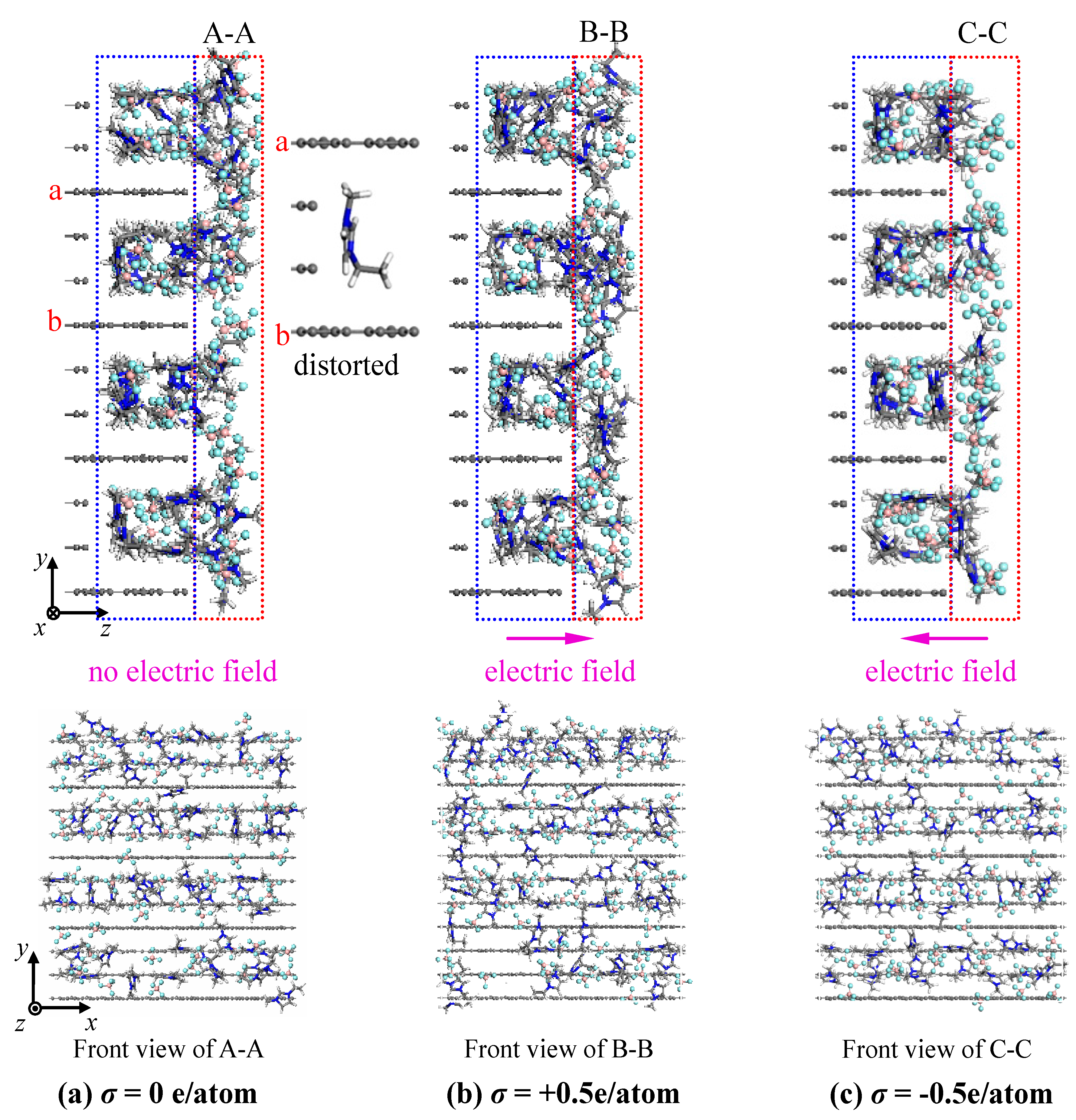
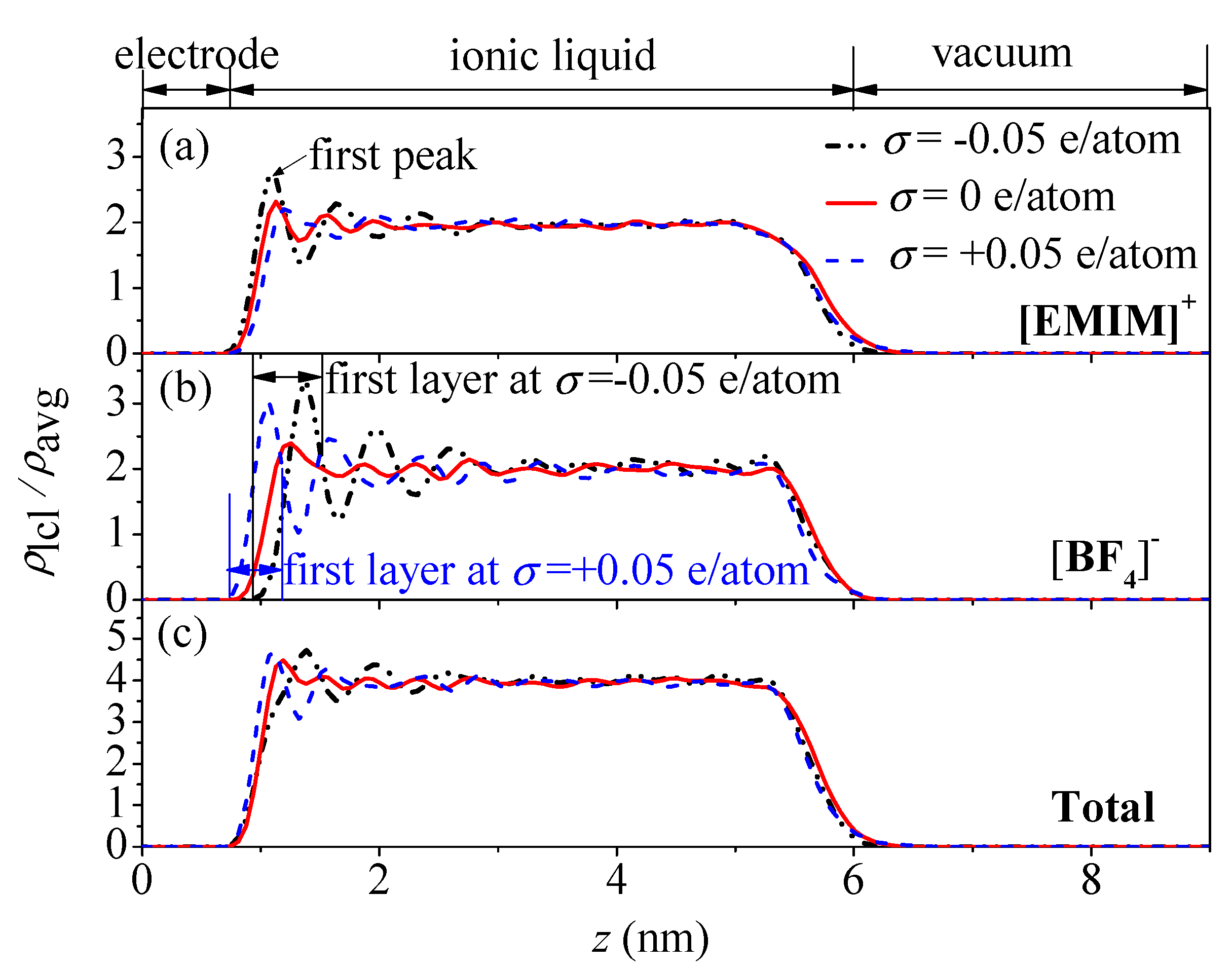
- The concentration profiles of ions exhibit periodical oscillations with decaying amplitude along the direction perpendicular to the charged/uncharged electrode surface, which suggests an alternate distribution of anions and cations in several consecutive layers in the EDL on the electrode surface. When the electrode is charged, the alternate layers of [BF4]− anions experience more-significant migration than the layers of [EMIM]+ cations, owing to the good mobility of the [BF4]− anion due to its small size and steric effect. Additionally, these ion layers can be extended deeper into the bulk electrolyte solution by the stronger interaction of the rough electrode compared to the situation for the non-rough electrode.
- (2)
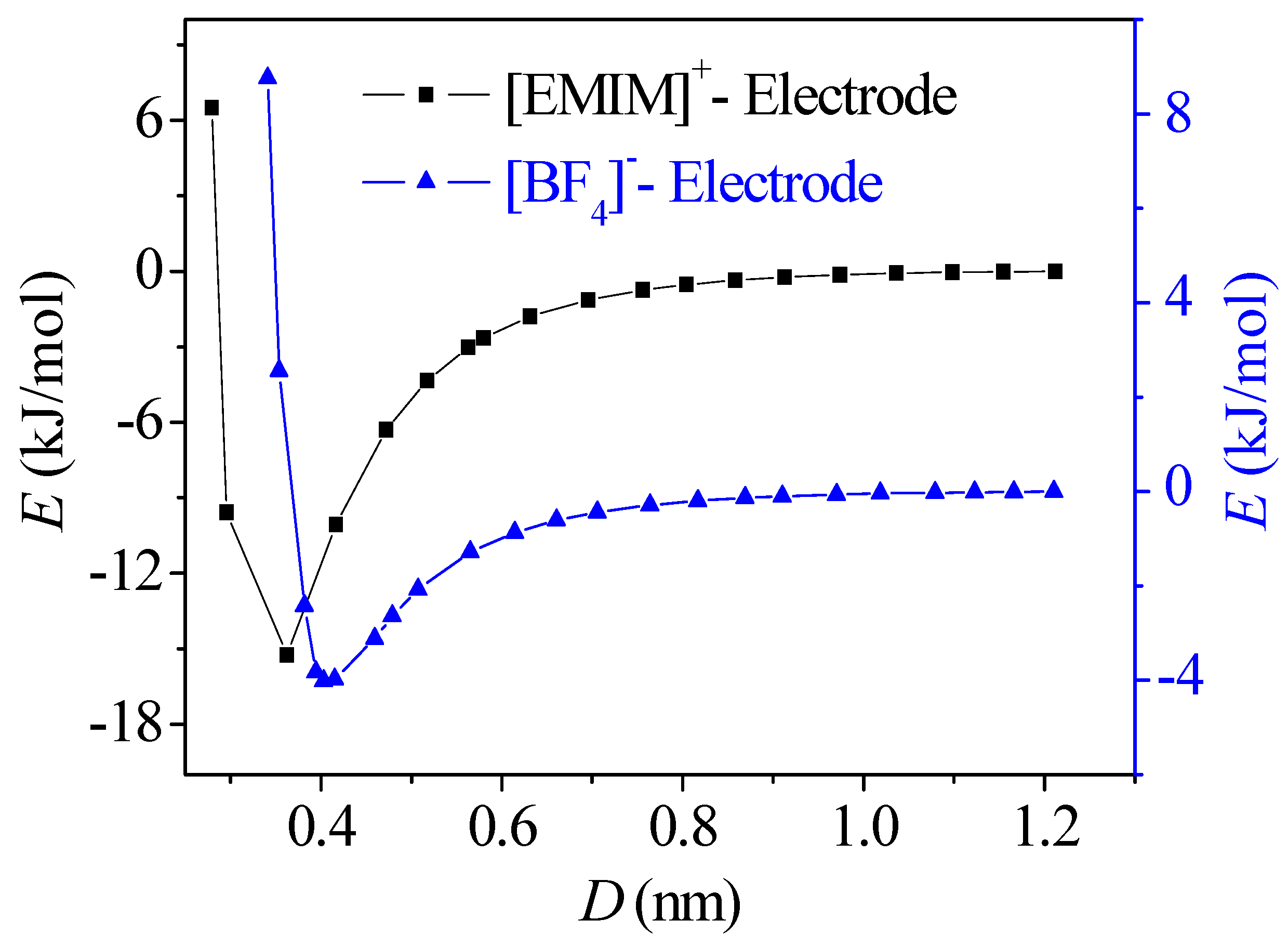
- The potential energy valley of ions near the neutral electrode surface establishes a potential energy difference to compensate the energy cost of the ion accumulation. As a result, ions are able to accumulate in the location of the valley to form the first layers near the electrode, allowing the potential drop across the EDL on the uncharged electrode surface to be produced.
- (3)
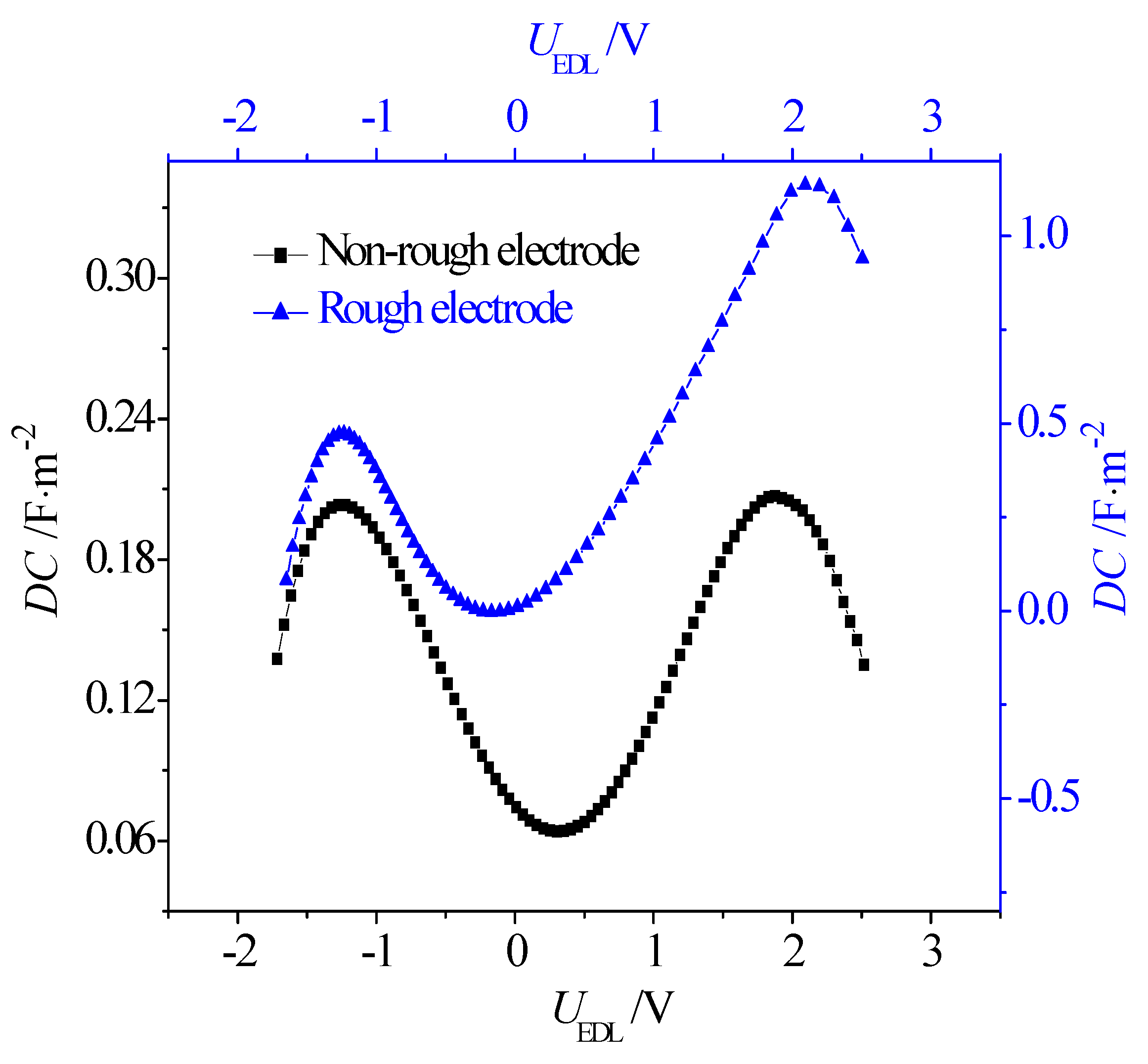
- Due to the greater effective contact area between the ions and electrode, the rough electrode possesses a larger capacitance than the non-rough one when it is negatively and positively charged. In addition, when the electrode is charged, it is harder for the larger-sized [EMIM]+ cations to accumulate in the narrow grooves on the rough electrode when compared with the smaller [BF4]−. Consequently, when compared with the symmetric double-hump-shaped C–V curve for the non-rough electrode surface, the double-hump-shaped C–V curve for the rough electrode is asymmetric, where the capacitance is increased more significantly when the electrode is positively charged.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef] [PubMed]
- Fronk, B.M.; Neal, R.; Garimella, S. Evolution of the transition to a world driven by renewable energy. J. Energy Resour. Technol. 2010, 132, 021009. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Ye, D.D.; Zhang, L.; Zhu, X.; Liao, Q. A hybrid microbial fuel cell stack based on single and double chamber microbial fuel cells for self-sustaining pH control. J. Power Sources 2016, 306, 685–691. [Google Scholar] [CrossRef]
- Zhu, X.B.; Zhao, T.S.; Wei, Z.H.; Tian, P.; An, L. A high-rate and long cycle life solid-state lithium-air battery. Energy Environ. Sci. 2015, 8, 3745–3754. [Google Scholar] [CrossRef]
- Demirocak, D.E.; Srimivasan, S.S.; Stefanakos, E.K. A Review on Nanocomposite Materials for Rechargeable Li-ion Batteries. Appl. Sci. 2017, 7, 731. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef] [PubMed]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A brief review on electrode materials for supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Rajput, N.N.; Monk, J.; Hung, F.R. Ionic liquids confined in a realistic activated carbon model: A molecular simulation study. J. Phys. Chem. C 2014, 118, 1540–1553. [Google Scholar] [CrossRef]
- Yang, L.; Fishbine, B.H.; Migliori, A.; Pratt, L.R. Molecular simulation of electric double-layer capacitors based on carbon nanotube forests. J. Am. Chem. Soc. 2008, 131, 12373–12376. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Jiang, D.E.; Cummings, P.T. Curvature effect on the capacitance of electric double layers at ionic liquid/onion-like carbon interfaces. J. Chem. Theory Comput. 2012, 8, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.Q.; Li, M.; Zhu, S.J.; Wang, T. Three-dimensional (3D) nanocomposites of MnO2-modified mesoporous carbon filled with carbon spheres/carbon blacks for supercapacitors. Int. J. Electrochem. Sci. 2016, 11, 23–33. [Google Scholar]
- Shim, Y.; Kim, H.J. Nanoporous carbon supercapacitors in an ionic liquid: A computer simulation study. ACS Nano 2010, 4, 2345–2355. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, S.; Bao, J.; Wu, W.; Pei, S.S. Sum frequency generation study on the orientation of room-temperaturre ionic liquid at the graphehe-ionic liquid interface. Chem. Phys. Lett. 2011, 516, 171–173. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Schwenzer, B.; Shutthanandan, V.; Hu, J.Z.; Liu, J.; Aksay, I.A. Elucidating graphehe-ionic liquid interfacial region: A combined expermental and computational study. Nano Energy 2014, 3, 152–158. [Google Scholar] [CrossRef]
- Mo, Y.F.; Wan, Y.F.; Chou, A.; Huang, F.C. Graphene/ionic liquid composite films and ion exchange. Sci. Rep. 2014, 4, 5466. [Google Scholar] [CrossRef] [PubMed]
- Vatamanu, J.; Bedrov, D. Capacitive energy storage: Current and future challenges. J. Phys. Chem. Lett. 2015, 6, 3594–3609. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Kandlikar, S.G. A theoretical model for axial heat conduction effects during single-phase flow in microchannels. J. Heat Transf. 2012, 134, 020902. [Google Scholar] [CrossRef]
- Li, W.M.; Qu, X.P.; Alam, T.; Yang, F.H.; Chang, W.; Khan, J.; Li, C. Enhanced flow boiling in microchannels through integrating multiple micro-nozzles and reentry microcavities. Appl. Phys. Lett. 2017, 110, 014104. [Google Scholar] [CrossRef]
- Helmholtz, H. Studies of electrical interfaces. Ann. Phys. 1879, 243, 337–382. [Google Scholar] [CrossRef]
- Bolt, G.H. Analysis of the validity of the Gouy-Chapman theory of the electric double layer. J. Colloid Sci. 1955, 10, 206–218. [Google Scholar] [CrossRef]
- Oldham, K.B. A Gouy-Chapman-Stern model of the double layer at a (metal)/(ionic liquid) interface. J. Electroanal. Chem. 2008, 613, 131–138. [Google Scholar] [CrossRef]
- Kornyshev, A.A. Double-layer in ionic liquids: paradigm change? J. Phys. Chem. B 2007, 111, 5545–5557. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Qiao, R.; Huang, J.S.; Dai, S.; Sumpter, B.J.; Meunier, V. The importance of ion size and electrode curvature on electrical double layers in ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Feng, G.; Bauelos, J.L.; Rother, G.; Fulvio, P.F.; Dai, S.; Cummings, P.T. Distinctive nanoscale organization of dicationic versus monocationic ionic liquids. J. Phys. Chem. C 2013, 117, 18251–18257. [Google Scholar] [CrossRef]
- Vatamanu, J.; Hu, Z.Z.; Bedrov, D. Increasing energy storage in electrochemical capacitors with ionic liquid electrolytes and nanostructured carbon electrodes. J. Phys. Chem. Lett. 2013, 4, 2829–2837. [Google Scholar] [CrossRef]
- Fedorov, M.V.; Kornyshev, A.A. Ionic liquid near a charged wall: Structure and capacitance of electrical double layer. J. Phys. Chem. B 2008, 112, 11868–11872. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, M.V.; Georgi, N.; Kornyshev, A.A. Double layer in ionic liquids: The nature of the camel shape of capacitance. Electrochem. Commun. 2010, 12, 296–299. [Google Scholar] [CrossRef]
- Vatamanu, J.; Cao, L.L.; Borodin, O.; Bedrov, D.; Smith, G.D. On the influence of surface topographyc double layer structure and differential capacitance of graphite/ionic liquid interfaces. J. Phys. Chem. Lett. 2011, 2, 2267–2272. [Google Scholar] [CrossRef]
- Bo, Z.; Zhu, W.G.; Ma, W.; Wen, Z.H.; Shuai, X.R.; Chen, J.H. Vertically oriented graphene bridging active-layer/current-collector interface for ultrahigh rate supercapacitors. Adv. Mater. 2013, 25, 5799–5806. [Google Scholar] [CrossRef] [PubMed]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A., III; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar]
- De Andrade, J.; Böes, E.S.; Stassen, H. Computational study of room temperature molten salts composed by 1-alkyl-3-methylimidazolium cations—Force-field proposal and validation. J. Phys. Chem. B 2002, 106, 13344–13351. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Zhang, C.B.; Lu, P.F.; Chen, Y.P. Molecular dynamics simulation of electroosmotic flow in rough nanochannels. Int. J. Heat Mass Transf. 2014, 59, 101–105. [Google Scholar] [CrossRef]
- Nymand, T.M.; Linse, P. Ewald summation and reaction field methods for potentials with atomic charges, dipoles, and polarizabilities. J. Chem. Phys. 2000, 112, 6152–6160. [Google Scholar] [CrossRef]
- Nyden, M.R.; Stoliarov, S.I.; Westmoreland, P.R.; Guo, Z.X.; Jee, C. Applications of reactive molecular dynamics ot the study of the thermal decomposition of polymers and nanoscale structures. Mater. Sci. Eng. A 2004, 365, 114–121. [Google Scholar] [CrossRef]
- Dell, A.E.; Tsang, B.; Jiang, L.X.; Granick, S.; Schweizer, K.S. Correlated two-particle diffusion in dense colloidal suspensions at early times: Theory and comparison to experiment. Phys. Rev. E 2015, 92, 052304. [Google Scholar] [CrossRef] [PubMed]
- Outhwaite, C.W. Modified Poisson-Boltzmann equation in electric double layer theory based on the Bogoliubov-Born-Green-Yvon integral equations. J. Chem. Soc. 1978, 74, 1214–1221. [Google Scholar] [CrossRef]
- Singh, T.; Kumar, A. Static dielectric constant of room temperature ionic liquids: internal pressure and cohesive energy density approach. J. Phys. Chem. B 2008, 112, 12968–12972. [Google Scholar] [CrossRef] [PubMed]
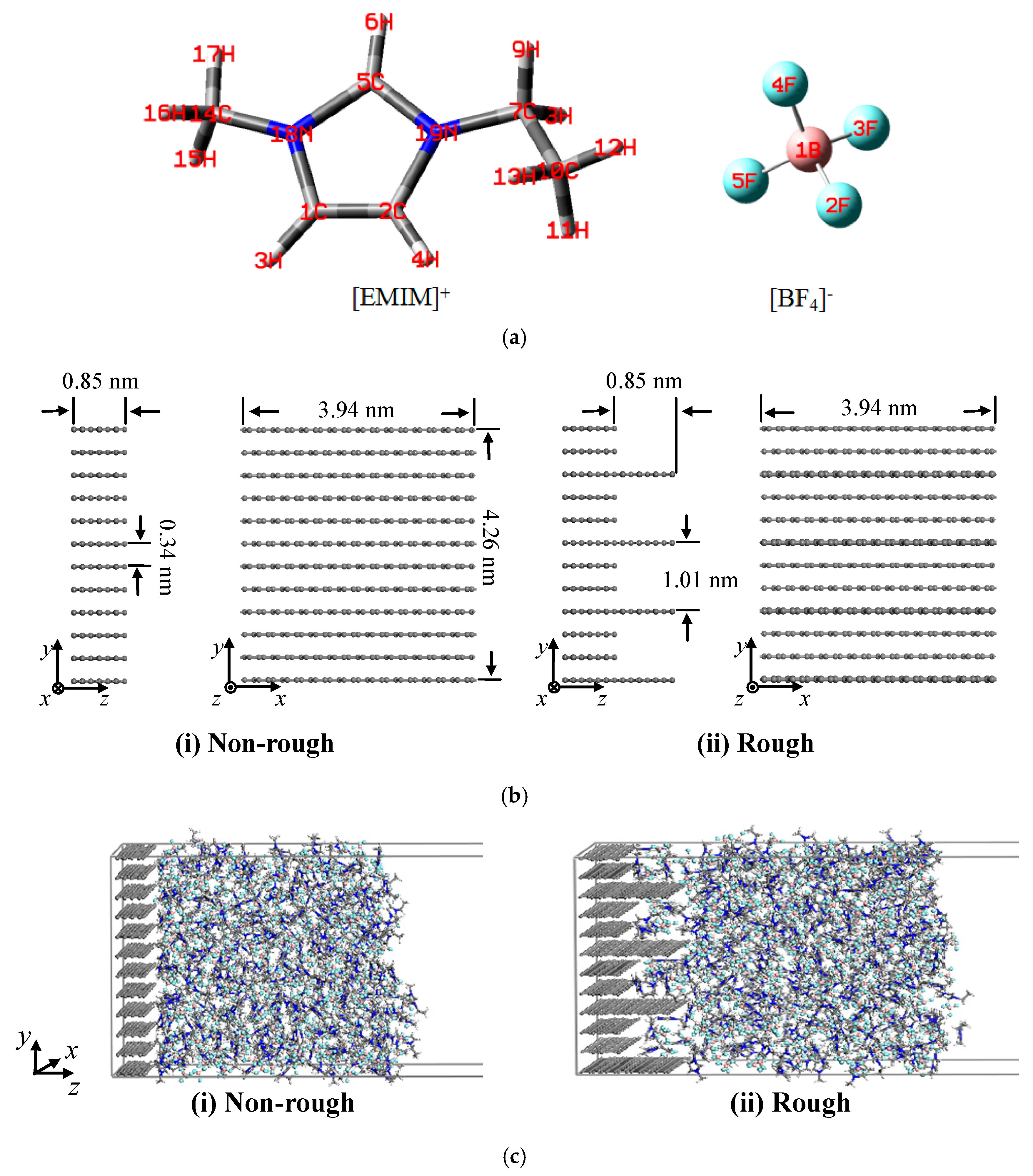



| Atoms | Atom Type | q(e) | Atoms | Atom Type | q(e) |
|---|---|---|---|---|---|
| 1C | C_R | −0.167 | 14C | C_3 | −0.166 |
| 2C | C_R | −0.192 | 15H | H_b | 0.129 |
| 3H | C_R | 0.248 | 16H | H_b | 0.129 |
| 4H | H_b | 0.259 | 17H | H_b | 0.129 |
| 5C | C_R | 0.058 | 18N | N_R | 0.079 |
| 6H | H_b | 0.205 | 19N | N_R | −0.010 |
| 7C | C_3 | 0.033 | 1B | B_3 | 0.828 |
| 8H | H_b | 0.089 | 2F | F_ | −0.457 |
| 9H | H_b | 0.089 | 3F | F_ | −0.457 |
| 10C | C_3 | −0.079 | 4F | F_ | −0.457 |
| 11H | H_b | 0.056 | 5F | F_ | −0.457 |
| 12H | H_b | 0.056 | - | - | - |
| 13H | H_b | 0.056 | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.; Dai, Q.; Wu, L.; Liu, X. Structure and Capacitance of Electrical Double Layers at the Graphene–Ionic Liquid Interface. Appl. Sci. 2017, 7, 939. https://doi.org/10.3390/app7090939
Lu P, Dai Q, Wu L, Liu X. Structure and Capacitance of Electrical Double Layers at the Graphene–Ionic Liquid Interface. Applied Sciences. 2017; 7(9):939. https://doi.org/10.3390/app7090939
Chicago/Turabian StyleLu, Pengfei, Qiaobo Dai, Liangyu Wu, and Xiangdong Liu. 2017. "Structure and Capacitance of Electrical Double Layers at the Graphene–Ionic Liquid Interface" Applied Sciences 7, no. 9: 939. https://doi.org/10.3390/app7090939
APA StyleLu, P., Dai, Q., Wu, L., & Liu, X. (2017). Structure and Capacitance of Electrical Double Layers at the Graphene–Ionic Liquid Interface. Applied Sciences, 7(9), 939. https://doi.org/10.3390/app7090939




