1. Introduction
Inorganic nanoparticles have opened new avenues of research in biomedicine, where a novel paradigm in therapy and diagnostics in the clinical practice is arising [
1]. Plasmonic nanoparticles typically composed of gold or silver appear as a highly promising building blocks due to their photothermal features, among other functionalities. Despite the fact that gold and silver are the most popular metals for fabricating the core of the plasmonic nanoparticles, efficient alternatives can be found using other metals [
2]. Photothermal therapy consists of the remote activation of the plasmon field of nanoparticles by irradiation with a resonant light wavelength, leading to a local increase in temperature [
3]. This localized heating can kill cancer cells and tissue in an effective manner, with no drugs or biochemical treatment required [
4,
5,
6,
7,
8,
9].
However, one of the most relevant barriers to be overcome by plasmonic nanoparticles that are to be used in actual clinical treatments is the complex biological media in which the nanoparticles must be dispersed [
10]. The ions and biological molecules present in biological media influence the aggregation state of the nanoparticles in most cases, leading to a significant modification of the plasmonic properties of the nanoparticles, which is highly sensitive to interparticle distance [
11].
Herein, we aim to determine a fundamental description of the effect of aggregation of gold nanoparticles induced by inorganic ions on the photothermal performance of such nanoparticles. A simplified scenario has been used: small spherical gold nanoparticles coated by citrate ions and sodium chloride as the aggregating-ion. Despite the simplicity of the model, we expect to obtain significant insights that might be used as the first step towards more sophisticated models of nanoparticles and biological media, possibly comprising anisotropic gold nanoparticles capped with different ligands and both inorganic ions and biological macromolecules in the media.
2. Materials and Methods
Materials. Milli-Q water (resistivity 18.2 MΩ·cm) produced by a Millipore Milli-Q unit, pretreated by a Millipore reverse osmosis system, was used in all experiments. Hydrogen tetrachloroaurate trihydrate (HAuCl4·3H2O, ≥99.9%), sodium citrate tribasic dihydrate (≥98%), sodium chloride (≥99.5%), and nitric acid (70%) were purchased from Sigma-Aldrich and used as received. Hydrochloric acid solution (37%) was purchased from Panreac and used as received. All glassware was washed with aqua regia, rinsed three times with Milli-Q water, and dried under a gentle stream of N2 before use.
Synthesis of Nanoparticles. Gold nanoparticle solution comprised an aqueous colloid (average diameter 9 ± 2 nm) that was prepared by adding 5 mL of 1 wt % sodium citrate solution to 95 mL of boiling 0.5 mM HAuCl4 under vigorous stirring. After 15 min of boiling, the solution was cooled down to room temperature and then stored at 4 °C for long-term storage.
Characterization. TEM images were collected with a JEOL JEM-1400PLUS transmission electron microscope operating at 120 kV, using carbon-coated 400 square mesh copper grids. UV–visible spectra of the gold nanoparticles as-synthesized and under different values of ionic strength were recorded in a 1 cm path length disposable cuvette using a Cary 100 Bio UV–visible spectrophotometer.
Photothermal heating experiments. The samples were irradiated using a Xe900 Xenon Arc Lamp (450 W ozone-free xenon arc lamp) from Edinburgh Photonics. The spot of the incoming beam was 1.1 cm, with a circular shape. The irradiation was performed using a constant power density of 1.64 mW/cm2. The temperature of the bulk solution were monitored along the heating process using an electronic thermometer. The probe of the thermometer was immersed in the bulk dispersion of the nanoparticles. The increase of temperature was found to be very reproducible. The standard deviation between measurements was in all cases equal to or lower than 5%.
3. Results and Discussion
3.1. Gold Nanoparticles Capped with Citrate Ions
Gold nanoparticles were synthesized by a modified protocol based on the Turkevich method [
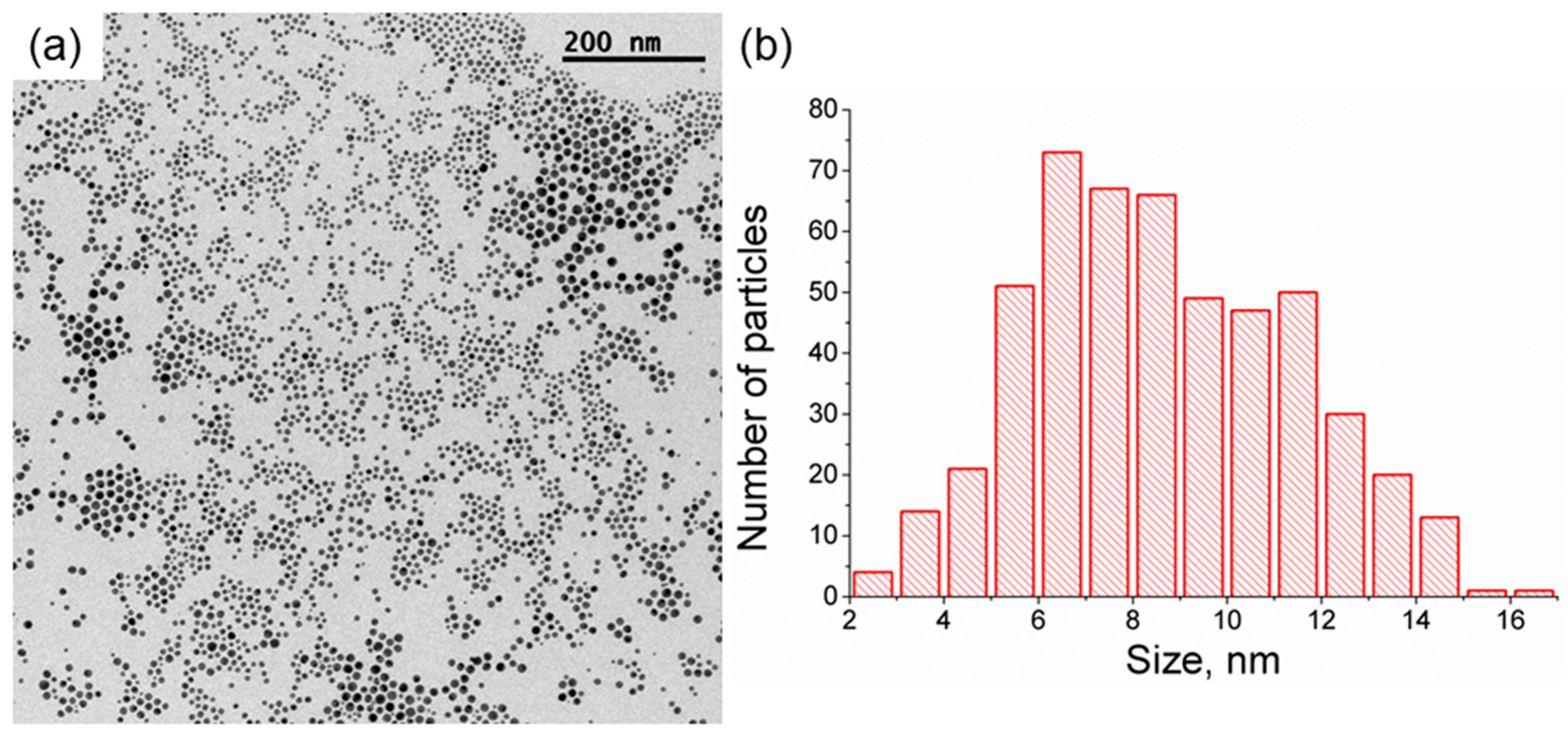
12]. A dispersion of citrate-capped spherical gold nanoparticles with a distibution of values of a diameter of 9 ± 2 nm and a polydispersity index (PDI) of 1.51 was obtained, see
Figure 1. The polydispersity of the obtained gold nanoparticles was slightly higher than expected from a typical synthesis based on the Turkevich method. We ascribe the higher polydispersity to the presence of impurities in the reaction mixture during the synthesis of the gold nanoparticles. Compared to physical deposition methods, this value of polydispersity is still good. On the other hand, physical deposition methods usually do not suffer from such sensitivity to impurities [
13,
14]. Note that our study focuses on the plasmonic features of the gold nanoparticle and its activation through incoming resonant light. According to the UV-vis spectra, the plasmon band of the gold nanoparticles is well defined, see
Figure 2. Therefore, we assume that the polydispersity of the gold nanoparticle does not play a role in the obtained results of photothermal heating.
During the synthesis of the nanoparticles, the citrate ions act both as reducing and capping agents. Therefore, the surface of the Au nanoparticles is coated by citrate ions attached by electrostatic and non-specific interactions. The gold nanoparticles display a net negative surface charge at the value of pH of the solution used herein. The gold nanoparticles are thus stabilized by charge repulsion [
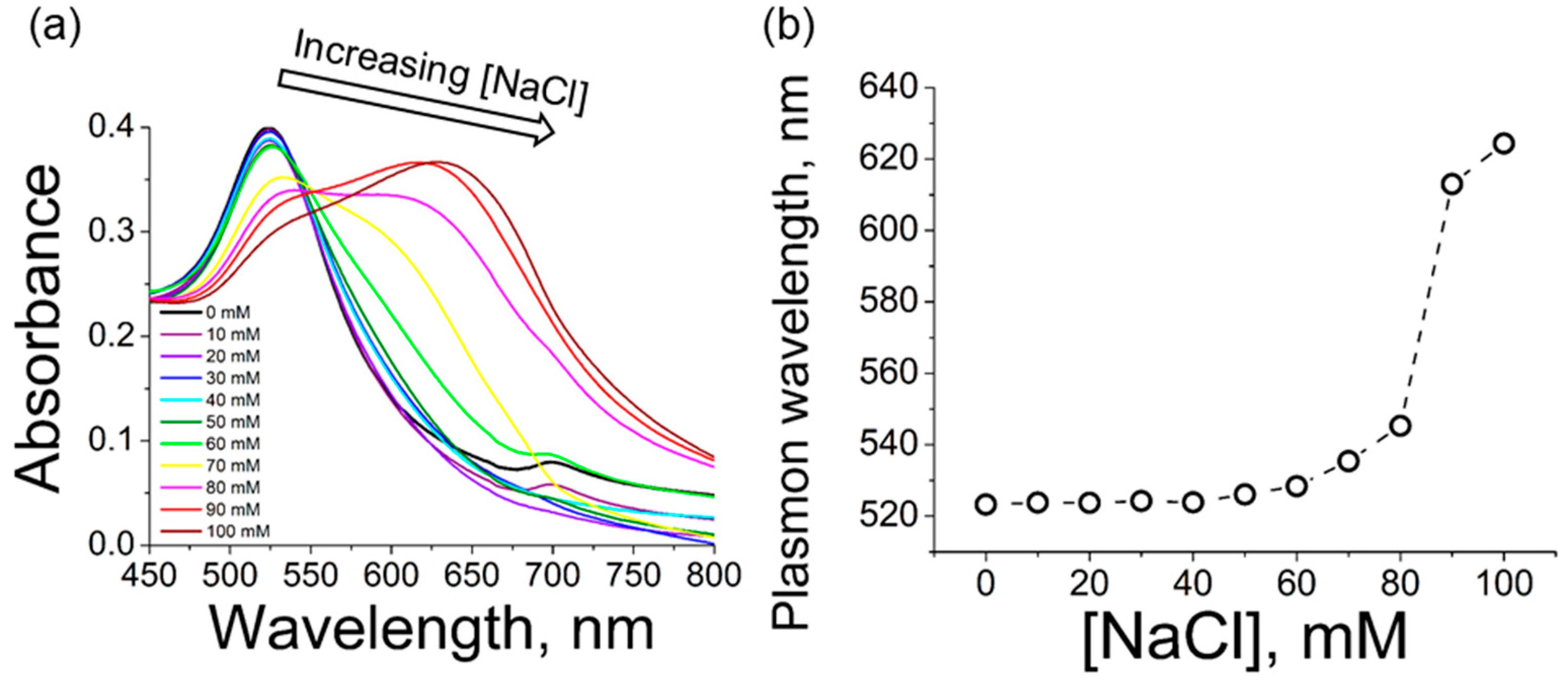
15]. The UV-vis spectrum shows the characteristic peak of small spherical gold nanoparticles at 524 nm, see
Figure 2. However, this wavelength value for the plasmon peak is obtained when the gold nanoparticles are dispersed in ultrapure water, a situation significantly different from biological media, which is typically enriched in ions and biological molecules [
10]. Therefore, we herein study the plasmonic photothermal features of the gold nanoparticles under different values of ionic strength by adding sodium chloride to the dispersion of gold nanoparticles. We note that the effect of the so-called “protein corona” formation on the gold nanoparticles will not be taken into account [
16]. Rather, we seek a fundamental observation on the effect of aggregation on the photothermal capability of the gold nanoparticles against ionic strength.
3.2. Effect of Ionic Strength on the Plasmon Field of the Gold Nanoparticles
With an increase of the ionic strength induced by including sodium chloride and increasing its concentration, the interparticle distance between the gold nanoparticles was effectively reduced [
17].
Figure 2 shows the UV-vis spectra for the different values of concentration of sodium chloride up to 100 mM. For higher values of concentration of sodium chloride, irreversible aggregation and loss of gold nanoparticles was obtained. The concentration of gold nanoparticles was estimated using the absorbance at 400 nm [
18]. The concentration of gold nanoparticles was ca. 2.7 × 10
18 nanoparticles/L in all cases.
There is almost no modification on the plasmon peak of the gold nanoparticles for a sodium chloride concentration up to 50 mM, with a slight shift to higher values of wavelength at 60 and 70 mM of sodium chloride. The plasmon band at 80 mM of sodium chloride displays a different shape with respect to the others, being the limit value for a a change in the tendency of shift of the plasmon band. This modification of the plasmon band, including a broad shoulder at ca. 640 nm, is ascribed to the appearance of large aggregates of nanoparticles. Such aggregation is mediated by the shielding of the repulsive electrostatic interactions of the citrate ions coating the nanoparticles by the sodium ions in solution. Yet, the more noticeable shift in the plasmon peak occurs at high values of ionic strength, as expected by the enhanced aggregation of the nanoparticles. In this case, a dispersion of gold nanoparticles in 90 and 100 mM of sodium chloride leads to a plasmon peak at ca. 630 nm, see
Table 1. Note that, in addition to the significant shift, the plasmon peaks becomes broader at high values of sodium chloride concentration.
3.3. Photothermal Experiments
The dispersion of gold nanoparticles was subjected to photothermal experiments by irradiation with a resonant wavelength of light entering the plasmon field of the nanoparticles. The temperature was monitored along the heating process. We note that the measured value of temperature in the bulk solution includes the systematic error of heat dissipation by the environment, especially by the solvent [
19]. Therefore, the increase of temperature is compared within the different values of ionic strength. Additional considerations in further studies comprising plasmonic nanoparticles of different sizes and shapes should be included [
20].
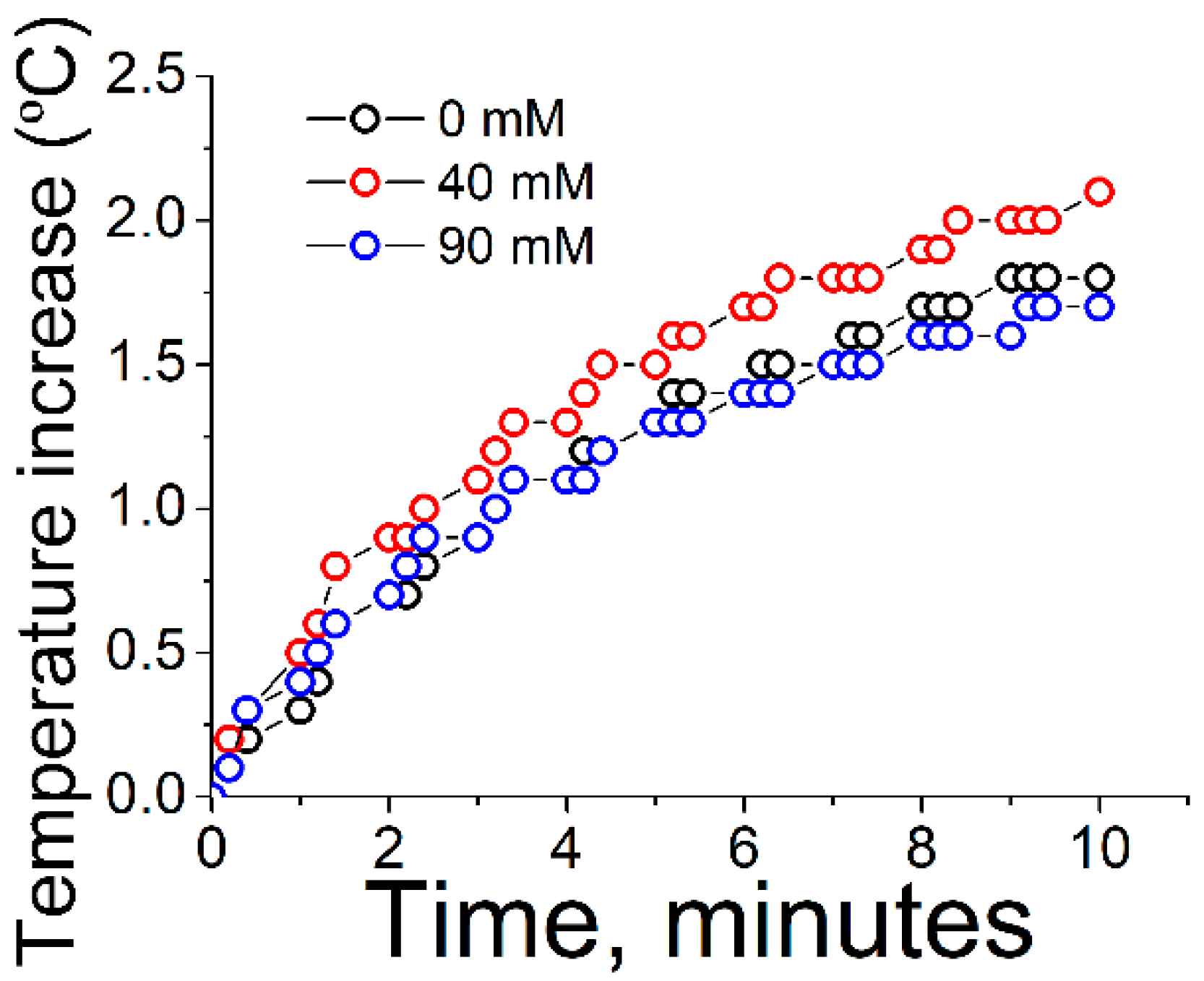
The wavelength of incoming light was adjusted to the maximum wavelength of the plasmon peak of the nanoparticles, see
Table 1. An increase of the temperature of the dispersion of nanoparticles was observed almost immediately after the irradiation begins, see
Figure 3. After an irradiation time of ca. 8 min the temperature was not increased further, instead reaching a constant value.
The kinetics of photothermal heating was similar in all cases. In a similar study published by Yslas et al., the kinetics of photothermal heating was similar, showing a plateau at ca. 10 min [
21]. In that study, the nanoparticles were coated by polyaniline (PANI) polymer. Therefore, we conclude that the kinetics of heating is not determined by the coating of the nanoparticles, but rather by the gold nanoparticles themselves. However, the final value of increase of temperature was slightly but significantly higher for the moderate values of ionic strength, i.e., 40 mM of sodium chloride. This comparatively superior result might be ascribed to a balance between the interplay of two opposite effects. At low values of ionic strength or in the absence of salt, the plasmonic nanoparticles are at a relatively large distance to each other due to the ionic repulsion between the citrate-coated surfaces of the nanoparticles. On the other hand, at high values of ionic strength, the gold nanoparticles are highly aggregated, therefore promoting scattering of the incoming light due to large aggregates, as well as an excessive broadening of the plasmon peak, with both effects contributing to a decrease in the photothermal efficiency.
The intermediate case of ionic strength appears to display a certain aggregation of the plasmonic nanoparticles that promotes the enhancement of the photothermal effect with no excessive aggregation of the nanoparticles. This limited aggregation of the nanoparticles allows for an effective plasmon coupling. Therefore, such an intermediate state of aggregation might be the optimum design when engineering plasmonic nanoparticles for in vivo application of photothermal therapy. The use of highly aggregated nanoparticles with a strongly shifted plasmon field might not yield optimum values in photothermal therapy, despite the interest of higher values of wavelength for biological applications [
22].
4. Conclusions
In summary, the photothermal performance of citrate-capped gold nanoparticles at different values of ionic strength has been studied. At low values of ionic strength, the effect of the inorganic ions is not noticeable in the position of the plasmon peak and the photothermal effect. High values of ionic strength lead to strong aggregation of the nanoparticles and large shift in the plasmon position with no net enhancement on the photothermal effect. The optimum scenario for photothermal therapy has been found to be an intermediate value of ionic strength, which allows for a certain aggregation of the plasmonic nanoparticles with no enhanced scattering. The state of aggregation of the plasmonic nanoparticles in the biological media when pursuing applications in photothermal therapy is thus a highly relevant factor in the efficiency of the nanoparticle-based therapy.
Acknowledgments
Support from the Ministry of Economy and Competitiveness is acknowledged through the following projects: CTQ2014-56422-P and CTQ2014-57515-C2. J.J.G.-C. acknowledges the Ministry of Economy and Competitiveness for a Ramon y Cajal contract (#RyC-2014-14956). Alexander D. Jodlowski and Gustavo de Miguel are acknowledged for the assistance and great help with the irradiation experiments.
Author Contributions
D.A.-M., M.T.M.-R., L.C. and J.J.G.-C. conceived and designed the experiments; D.A.-M.and J.J.G.-C. performed the experiments¸ the paper was written with contributions from all authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Inorganic nanoparticles for biomedicine: Where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Lalisse, A.; Tessier, G.; Plain, J.; Baffou, G. Quantifying the Efficiency of Plasmonic Materials for Near-Field Enhancement and Photothermal Conversion. J. Phys. Chem. C 2015, 119, 25518–25528. [Google Scholar] [CrossRef]
- Garcia, M.A. Surface plasmons in metallic nanoparticles: Fundamentals and applications. J. Phys. D Appl. Phys. 2011, 44, 283001. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Patino, T.; Mahajan, U.; Palankar, R.; Medvedev, N.; Walowski, J.; Münzenberg, M.; Mayerle, J.; Delcea, M. Multifunctional gold nanorods for selective plasmonic photothermal therapy in pancreatic cancer cells using ultra-short pulse near-infrared laser irradiation. Nanoscale 2015, 7, 5328–5337. [Google Scholar] [CrossRef] [PubMed]
- Khantamat, O.; Li, C.-H.; Yu, F.; Jamison, A.C.; Shih, W.-C.; Cai, C.; Lee, T.R. Gold Nanoshell-Decorated Silicone Surfaces for the Near-Infrared (NIR) Photothermal Destruction of the Pathogenic Bacterium E. faecalis. ACS Appl. Mater. Interfaces 2015, 7, 3981–3993. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-K.; Yu, X.-F.; Wang, J.-H.; Li, Z.-B.; Li, P.-H.; Wang, H.; Song, L.; Chu, P.K.; Li, C. Gold-nanorods-siRNA nanoplex for improved photothermal therapy by gene silencing. Biomaterials 2016, 78, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Baffou, G.; Quidant, R. Thermo-plasmonics: Using metallic nanostructures as nano-sources of heat. Laser Photonics Rev. 2013, 7, 171–187. [Google Scholar] [CrossRef]
- De Sio, L.; Caracciolo, G.; Annesi, F.; Placido, T.; Pozzi, D.; Comparelli, R.; Pane, A.; Curri, M.; Agostiano, A.; Bartolino, R. Plasmonics Meets Biology through Optics. Nanomaterials 2015, 5, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Henriksen-Lacey, M.; Carregal-Romero, S.; Liz-Marzán, L.M. Current Challenges toward In Vitro Cellular Validation of Inorganic Nanoparticles. Bioconjug. Chem. 2017, 28, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, N.; Bordley, J.A.; El-Sayed, M.A. The Sensitivity of the Distance Dependent Plasmonic Coupling between Two Nanocubes to their Orientation: Edge-to-Edge versus Face-to-Face. J. Phys. Chem. C. 2016, 120, 4564–4570. [Google Scholar] [CrossRef]
- Enüstün, B.V.; Turkevich, J. Coagulation of Colloidal Gold. J. Am. Chem. Soc. 1963, 85, 3317–3328. [Google Scholar] [CrossRef]
- Ruffino, F.; Crupi, I.; Irrera, A.; Grimaldi, M.G. Pd/Au/SiC Nanostructured Diodes for Nanoelectronics: Room Temperature Electrical Properties. IEEE Trans. Nanotechnol. 2010, 9, 414–421. [Google Scholar] [CrossRef]
- Ruffino, F.; Grimaldi, M.G.; Bongiorno, C.; Giannazzo, F.; Roccaforte, F.; Raineri, V. Microstructure of Au nanoclusters formed in and on SiO2. Superlattices Microstruct. 2008, 44, 588–598. [Google Scholar] [CrossRef]
- Grzelczak, M.; Liz-Marzán, L.M. Colloidal Nanoplasmonics: From Building Blocks to Sensing Devices. Langmuir 2013, 29, 4652–4663. [Google Scholar] [CrossRef] [PubMed]
- Hadjidemetriou, M.; Al-Ahmady, Z.; Kostarelos, K. Time-evolution of in vivo protein corona onto blood-circulating PEGylated liposomal doxorubicin (DOXIL) nanoparticles. Nanoscale 2016, 8, 6948–6957. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; El-Sayed, M.A. Plasmonic coupling in noble metal nanostructures. Chem. Phys. Lett. 2010, 487, 153–164. [Google Scholar] [CrossRef]
- Scarabelli, L.; Grzelczak, M.; Liz-Marzán, L.M. Tuning Gold Nanorod Synthesis through Prereduction with Salicylic Acid. Chem. Mater. 2013, 25, 4232–4238. [Google Scholar] [CrossRef]
- Pallavicini, P.; Basile, S.; Chirico, G.; Dacarro, G.; D’Alfonso, L.; Donà, A.; Patrini, M.; Falqui, A.; Sironi, L.; Taglietti, A. Monolayers of gold nanostars with two near-IR LSPRs capable of additive photothermal response. Chem. Commun. 2015, 51, 12928–12930. [Google Scholar] [CrossRef] [PubMed]
- Maestro, L.M.; Haro-González, P.; Sánchez-Iglesias, A.; Liz-Marzán, L.M.; García Solé, J.; Jaque, D. Quantum Dot Thermometry Evaluation of Geometry Dependent Heating Efficiency in Gold Nanoparticles. Langmuir 2014, 30, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Yslas, E.I.; Ibarra, L.E.; Molina, M.A.; Rivarola, C.; Barbero, C.A.; Bertuzzi, M.L.; Rivarola, V.A. Polyaniline nanoparticles for near-infrared photothermal destruction of cancer cells. J. Nanopart. Res. 2015, 17, 389. [Google Scholar] [CrossRef]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; García, I.; Liz-Marzán, L.M. Plasmonic Surfaces for Cell Growth and Retrieval Triggered by Near-Infrared Light. Angew. Chem. Int. Ed. 2016, 55, 974–978. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).