Removal of Escherichia coli by Intermittent Operation of Saturated Sand Columns Supplemented with Hydrochar Derived from Sewage Sludge
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Escherichia coli Suspension
2.2. Hydrochar
2.3. Material Characterization
2.3.1. Zeta Potential
2.3.2. Elemental Composition
2.3.3. Surface Functional Groups
2.3.4. Specific Surface Area and Pore Size Distribution
2.3.5. Surface Morphology
2.3.6. Hydrophobicity
2.4. Column Experiments
2.4.1. Experimental Setup
2.4.2. Small Column Experiments
2.4.3. Large Column Experiments
3. Results
3.1. Material Characterization
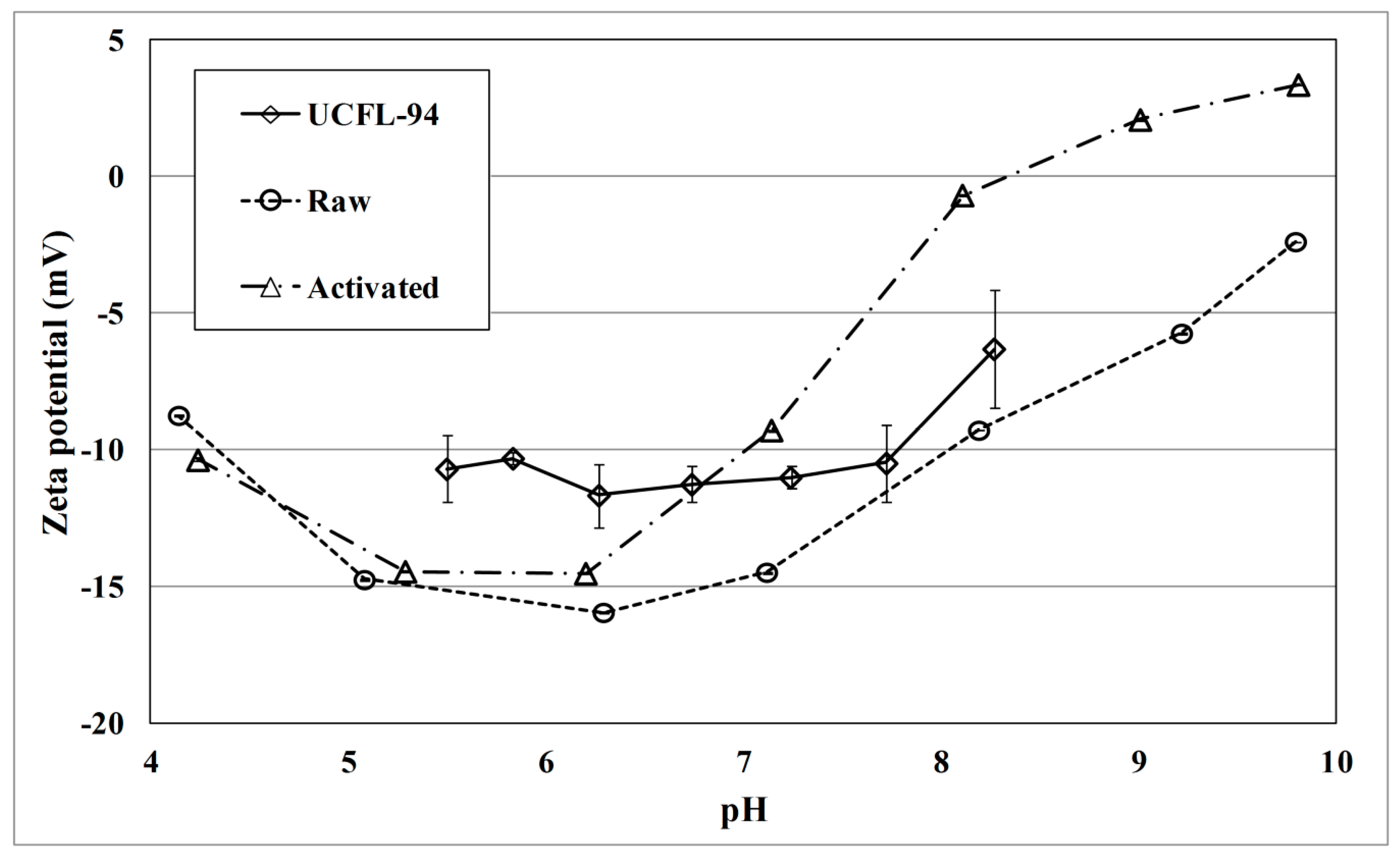
3.1.1. Zeta Potential
3.1.2. Elemental Composition
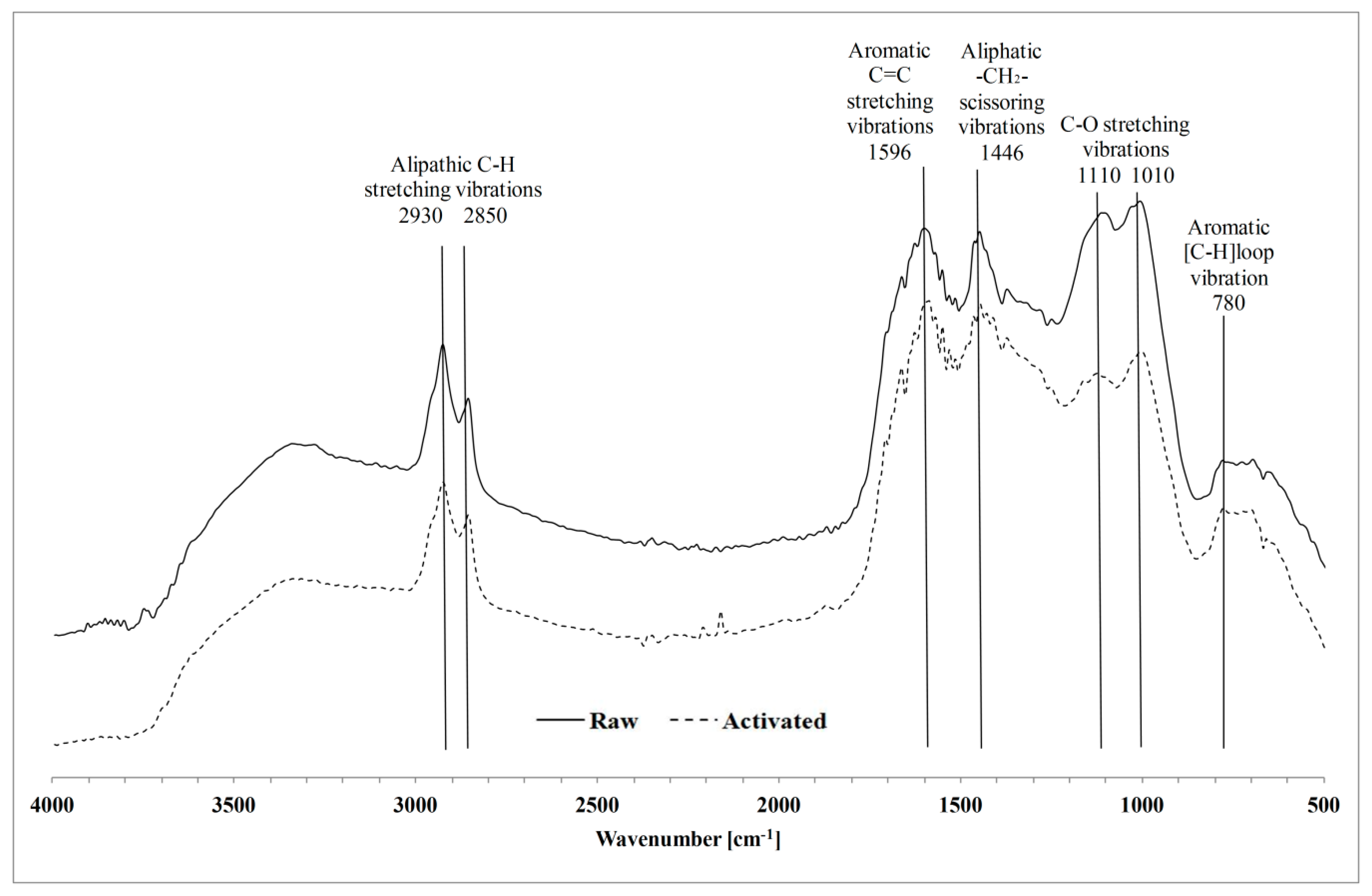
3.1.3. Surface Functional Groups
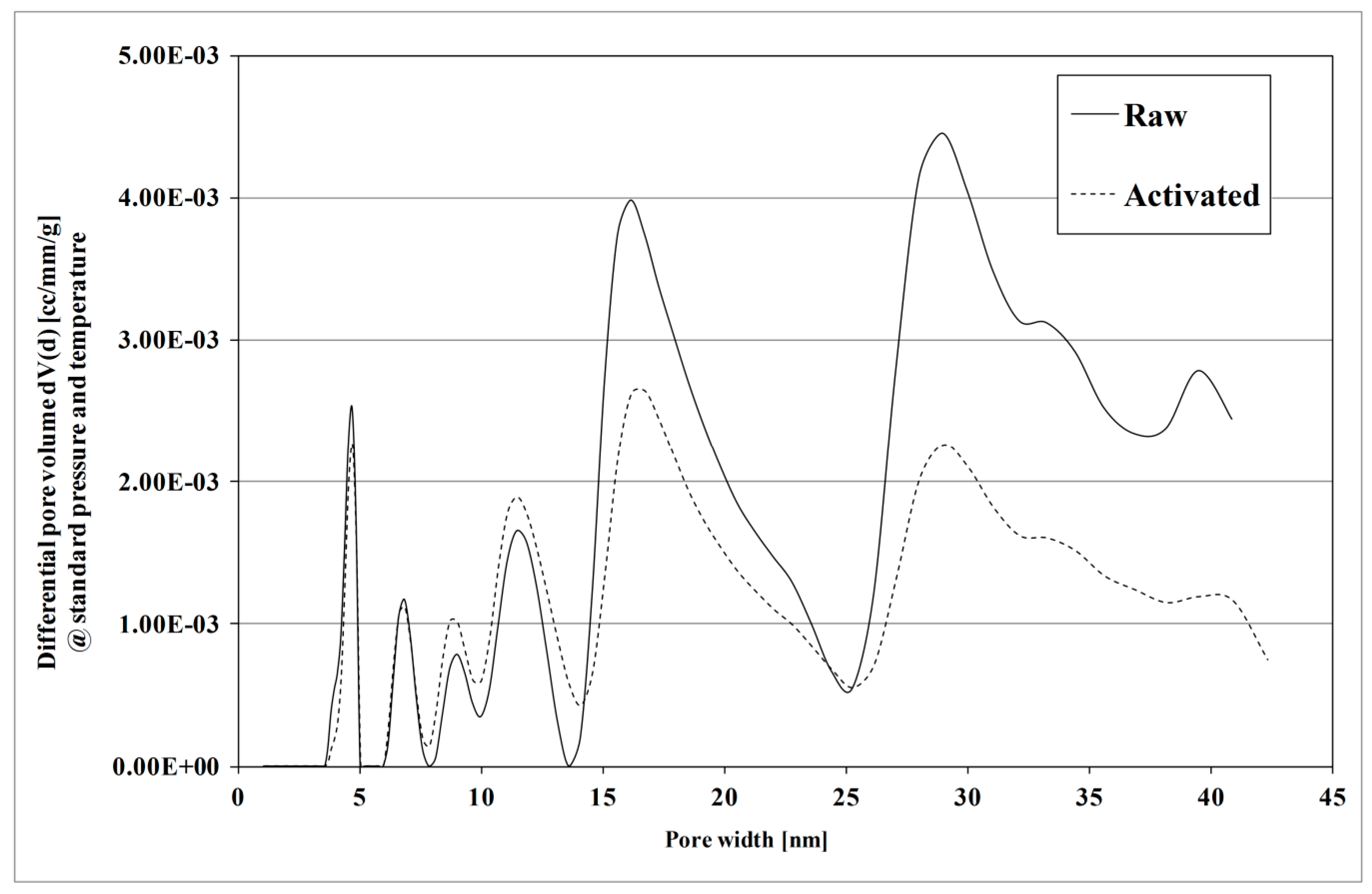
3.1.4. Specific Surface Area and Pore Size Distribution
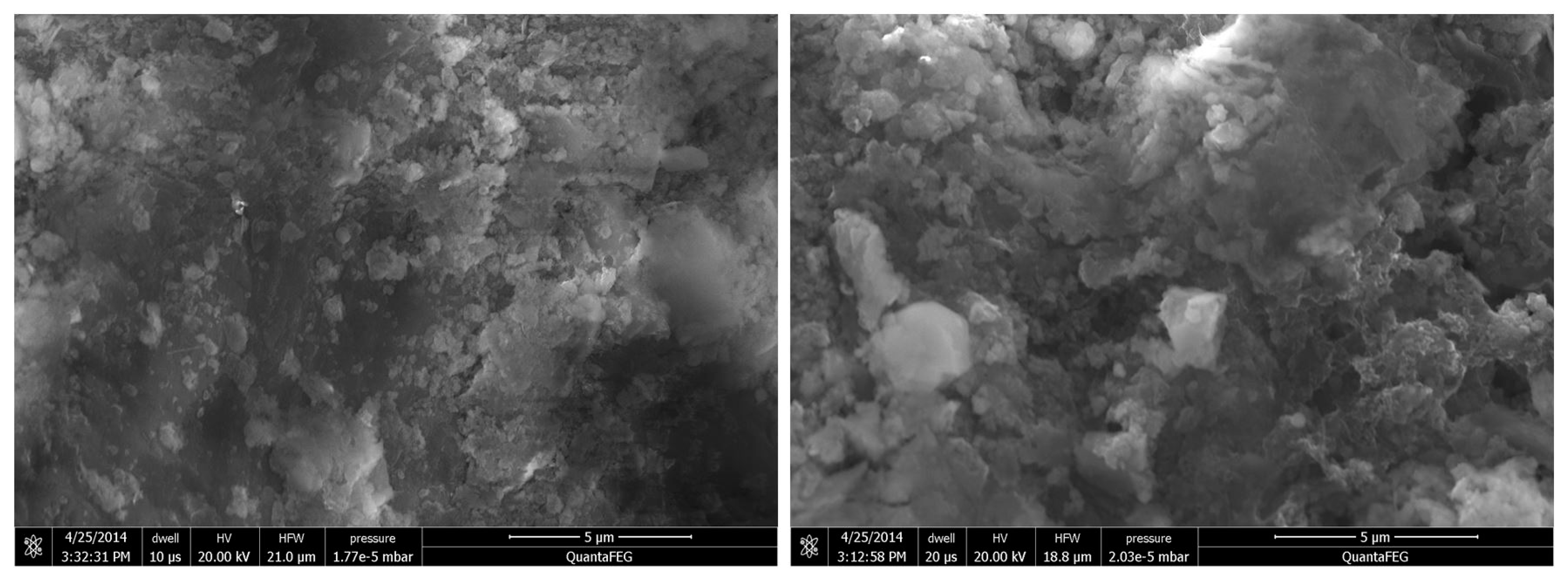
3.1.5. Surface Morphology
3.1.6. Hydrophobicity
3.2. E. coli Flushing Test
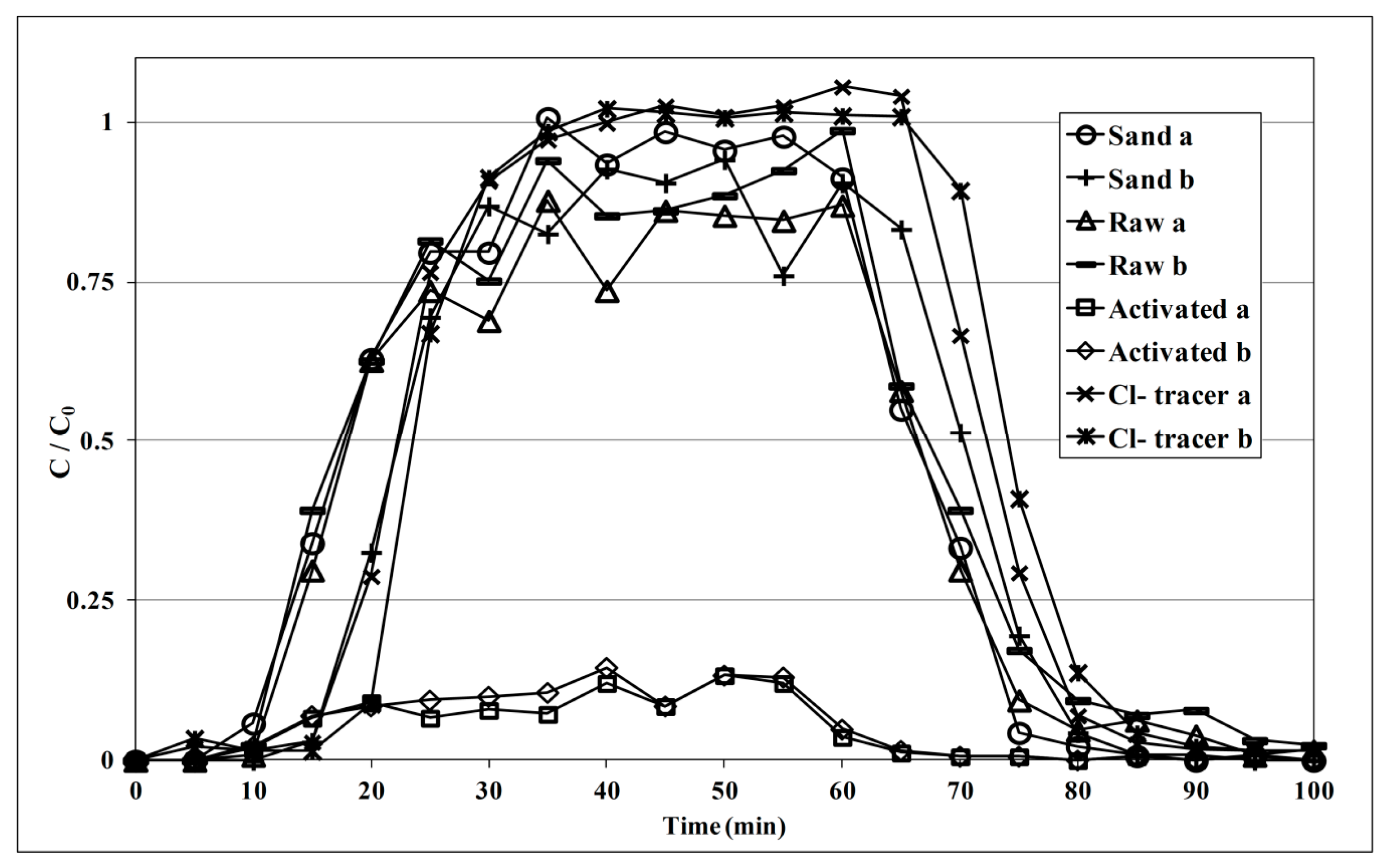
3.2.1. Breakthrough Analysis in Small Column Experiments
3.2.2. E. coli Removal Efficiency in Large Column Experiments
4. Discussion
4.1. Effect of KOH Activation of Hydrochar on E. coli Removal
4.2. Effect of Idling Time on E. coli Removal Efficiency
4.3. Potential of HTC-Sand Filters for Pathogen Removal
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Falco, C.; Sevilla, M. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796–6822. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Lu, X.; Jordan, B.; Berge, N.D. Thermal conversion of municipal solid waste via hydrothermal carbonization: Comparison of carbonization products to products from current waste management techniques. Waste Manag. 2012, 32, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Nizamuddin, S.; Jayakumar, N.S.; Sahu, J.N.; Ganesan, P.; Bhutto, A.W.; Mubarak, N.M. Hydrothermal carbonization of oil palm shell. Korean J. Chem. Eng. 2015, 32, 1789–1797. [Google Scholar] [CrossRef]
- Erdogan, E.; Atila, B.; Mumme, J.; Reza, M.T.; Toptas, A.; Elibol, M.; Yanik, J. Characterization of products from hydrothermal carbonization of orange pomace including anaerobic digestibility of process liquor. Bioresour. Technol. 2015, 196, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Tan, I.A.W.; Benhouria, A.; Asif, M.; Hameed, B.H. Mesoporous and adsorptive properties of palm date seed activated carbon prepared via sequential hydrothermal carbonization and sodium hydroxide activation. Chem. Eng. J. 2015, 270, 187–195. [Google Scholar] [CrossRef]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in eu application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Escala, M.; Zumbuhl, T.; Koller, C.; Junge, R.; Krebs, R. Hydrothermal carbonization as an energy-efficient alternative to established drying technologies for sewage sludge: A feasibility study on a laboratory scale. Energy Fuels 2013, 27, 454–460. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal carbonisation of sewage sludge: Effect of process conditions on product characteristics and methane production. Bioresour. Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Wirth, B.; Reza, T.; Mumme, J. Influence of digestion temperature and organic loading rate on the continuous anaerobic treatment of process liquor from hydrothermal carbonization of sewage sludge. Bioresour. Technol. 2015, 198, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Fowler, G.D.; Pullket, S.; Graham, N.J.D. Sewage sludge-based adsorbents: A review of their production, properties and use in water treatment applications. Water Res. 2009, 43, 2569–2594. [Google Scholar] [CrossRef] [PubMed]
- Alatalo, S.M.; Repo, E.; Makila, E.; Salonen, J.; Vakkilainen, E.; Sillanpaa, M. Adsorption behavior of hydrothermally treated municipal sludge & pulp and paper industry sludge. Bioresour. Technol. 2013, 147, 71–76. [Google Scholar] [PubMed]
- Spataru, A. The Use of Hydrochar as a Low Cost Adsorbent for Heavy Metal and Phosphate Removal from Wastewater. Master’s Thesis, UNESCO-IHE, Delft, The Netherlands, 2014. [Google Scholar]
- Chung, J.W.; Foppen, J.W.; Gerner, G.; Krebs, R.; Lens, P.N.L. Removal of rotavirus and adenovirus from artificial ground water using hydrochar derived from sewage sludge. J. Appl. Microbiol. 2015, 119, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Lutterodt, G.; Basnet, M.; Foppen, J.W.A.; Uhlenbrook, S. Determining minimum sticking efficiencies of six environmental Escherichia coli isolates. J. Contam. Hydrol. 2009, 110, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Foppen, J.W.; Izquierdo, M.; Lens, P.N.L. Removal of Escherichia coli from saturated sand columns supplemented with hydrochar produced from maize. J. Environ. Qual. 2014, 43, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Regmi, P.; Moscoso, J.L.G.; Kumar, S.; Cao, X.Y.; Mao, J.D.; Schafran, G. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J. Environ. Manag. 2012, 109, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.J.; Tang, J.C.; Gong, Y.Y.; Zhang, H.R. Characterization of potassium hydroxide (koh) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water. Environ. Sci. Pollut. Res. 2015, 22, 16640–16651. [Google Scholar] [CrossRef] [PubMed]
- Neimark, A.V.; Lin, Y.; Ravikovitch, P.I.; Thommes, M. Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon 2009, 47, 1617–1628. [Google Scholar] [CrossRef]
- Jeong, S.-B.; Yang, Y.-C.; Chae, Y.-B.; Kim, B.-G. Characteristics of the treated ground calcium carbonate powder with stearic acid using the dry process coating system. Mater. Trans. 2009, 50, 409–414. [Google Scholar] [CrossRef]
- Matthess, G.; Bedbur, E.; Gundermann, K.O.; Loof, M.; Peters, D. Investigation on filtration mechanisms of bacteria and organic particles in porous-media. I. Background and methods. Zentralblatt Fuer Hygiene Und Umweltmed. 1991, 191, 53–97. [Google Scholar]
- Baccile, N.; Weber, J.; Falco, C.; Titirici, M.-M. Characterization of hydrothermal carbonization materials. In Sustainable Carbon Materials from Hydrothermal Processes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 151–211. [Google Scholar]
- Parshetti, G.K.; Liu, Z.G.; Jain, A.; Srinivasan, M.P.; Balasubramanian, R. Hydrothermal carbonization of sewage sludge for energy production with coal. Fuel 2013, 111, 201–210. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem. Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Loganathan, V.A.; Gupta, R.B.; Barnett, M.O. An assessment of u(vi) removal from groundwater using biochar produced from hydrothermal carbonization. J. Environ. Manag. 2011, 92, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2005; Volume 33487-2742, p. 33. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 07030-577, pp. 82–88. [Google Scholar]
- Unur, E. Functional nanoporous carbons from hydrothermally treated biomass for environmental purification. Microporous Mesoporous Mater. 2013, 168, 92–101. [Google Scholar] [CrossRef]
- Foppen, J.W.A.; Schijven, J.F. Evaluation of data from the literature on the transport and survival of Escherichia coli and thermotolerant coliforms in aquifers under saturated conditions. Water Res. 2006, 40, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Jarrah, N.; van Ommen, J.G.; Lefferts, L. Development of monolith with a carbon-nanofiber-washcoat as a structured catalyst support in liquid phase. Catal. Today 2003, 79, 29–33. [Google Scholar] [CrossRef]
- Elliott, M.; Stauber, C.; Koksal, F.; DiGiano, F.; Sobsey, M. Reductions of E. Coli, echovirus type 12 and bacteriophages in an intermittently operated household-scale slow sand filter. Water Res. 2008, 42, 2662–2670. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.A.; DiGiano, F.A.; Sobsey, M.D. Virus attenuation by microbial mechanisms during the idle time of a household slow sand filter. Water Res. 2011, 45, 4092–4102. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Imae, Y.; Shioi, J.I.; Oosawa, F. Effect of temperature on motility and chemotaxis of Escherichia coli. J. Bacteriol. 1976, 127, 1039–1046. [Google Scholar] [PubMed]
- Li, G.; Tam, L.K.; Tang, J.X. Amplified effect of brownian motion in bacterial near-surface swimming. Proc. Natl. Acad. Sci. USA 2008, 105, 18355–18359. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P.; Schaiberger, G.E. Effect of particulates on virus survival in seawater. J. Water Pollut. Control Federation 1975, 47, 93–103. [Google Scholar]
- Burton, G.A., Jr.; Gunnison, D.; Lanza, G.R. Survival of pathogenic bacteria in various freshwater sediments. Appl. Environ. Microbiol. 1987, 53, 633–638. [Google Scholar] [PubMed]
- Sherer, B.M.; Miner, J.R.; Moore, J.A.; Buckhouse, J.C. Indicator bacterial survival in stream sediments. J. Environ. Qual. 1992, 21, 591–595. [Google Scholar] [CrossRef]
- Howell, J.M.; Coyne, M.S.; Cornelius, P.L. Effect of sediment particle size and temperature on fecal bacteria mortality rates and the fecal coliform/fecal streptococci ratio. J. Environ. Qual. 1996, 25, 1216–1220. [Google Scholar] [CrossRef]
- Sobsey, M.D.; Stauber, C.E.; Casanova, L.M.; Brown, J.M.; Elliott, M.A. Point of use household drinking water filtration: A practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ. Sci. Technol. 2008, 42, 4261–4267. [Google Scholar] [CrossRef] [PubMed]
- Stauber, C.E.; Elliott, M.A.; Koksal, F.; Ortiz, G.M.; DiGiano, F.A.; Sobsey, M.D. Characterisation of the biosand filter for E. coli reductions from household drinking water under controlled laboratory and field use conditions. Water Sci. Technol. 2006, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.V.; Neufeld, K.; Cusworth, D.; Gavrilovic, S.; Ngai, T. Investigation of the effect of grain size, flow rate and diffuser design on the cawst biosand filter performance. Int. J. Serv. Learn. Eng. Humanit. Eng. Soc. Entrep. 2015, 10, 1–23. [Google Scholar]
- Buzunis, B.J. Intermittently operated slow sand filtration: A new water treatment process. In Civil Engineering; University of Calgary: Calgary, AB, Canada, 1995. [Google Scholar]
- Huang, H.J.; Yuan, X.Z. The migration and transformation behaviors of heavy metals during the hydrothermal treatment of sewage sludge. Bioresour. Technol. 2016, 200, 991–998. [Google Scholar] [CrossRef] [PubMed]
- vom Eyser, C.; Palmu, K.; Schmidt, T.C.; Tuerk, J. Pharmaceutical load in sewage sludge and biochar produced by hydrothermal carbonization. Sci. Total Environ. 2015, 537, 180–186. [Google Scholar] [CrossRef] [PubMed]

| C | H | N | O | Ca | Mg | Al | K | Fe | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Raw | 28.6 | 3.6 | 2.0 | 22.3 | 5.1 | 0.9 | 2.6 | 0.5 | 5.0 | 4.5 |
| Activated | 29.4 | 3.7 | 1.9 | 21.1 | 5.8 | 1.0 | 2.0 | 0.9 | 5.5 | 2.6 |
| Content | Pore Volume | Total | ||
|---|---|---|---|---|
| First | Second | |||
| 24 h idle | 72 h idle | |||
| Sand | 52.1 ± 13.3 (n = 46) | 66.9 ± 14.7 (n = 12) | 17.2 ± 8.64 § (n = 60) | 36.5 ± 10.1 (n = 60) |
| Raw | 23.0 ± 17.3 † (n = 44) | 35.1 ± 12.9 (n = 10) | 20.0 ± 12.5 § † (n = 56) | 24.4 ± 10.5 † (n = 56) |
| Activated | 92.8 ± 5.0 ‡ (n = 46) | 92.6 ± 8.0 ‡ (n = 12) | 90.0 ± 9.1 ‡ (n = 60) | ± 7.5 ‡ (n = 60) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, J.W.; Edewi, O.C.; Foppen, J.W.; Gerner, G.; Krebs, R.; Lens, P.N.L. Removal of Escherichia coli by Intermittent Operation of Saturated Sand Columns Supplemented with Hydrochar Derived from Sewage Sludge. Appl. Sci. 2017, 7, 839. https://doi.org/10.3390/app7080839
Chung JW, Edewi OC, Foppen JW, Gerner G, Krebs R, Lens PNL. Removal of Escherichia coli by Intermittent Operation of Saturated Sand Columns Supplemented with Hydrochar Derived from Sewage Sludge. Applied Sciences. 2017; 7(8):839. https://doi.org/10.3390/app7080839
Chicago/Turabian StyleChung, Jae Wook, Oghosa Charles Edewi, Jan Willem Foppen, Gabriel Gerner, Rolf Krebs, and Piet Nicolaas Luc Lens. 2017. "Removal of Escherichia coli by Intermittent Operation of Saturated Sand Columns Supplemented with Hydrochar Derived from Sewage Sludge" Applied Sciences 7, no. 8: 839. https://doi.org/10.3390/app7080839
APA StyleChung, J. W., Edewi, O. C., Foppen, J. W., Gerner, G., Krebs, R., & Lens, P. N. L. (2017). Removal of Escherichia coli by Intermittent Operation of Saturated Sand Columns Supplemented with Hydrochar Derived from Sewage Sludge. Applied Sciences, 7(8), 839. https://doi.org/10.3390/app7080839




