Synthesis, Characterization, and Antibacterial Activities of High-Valence Silver Propamidine Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
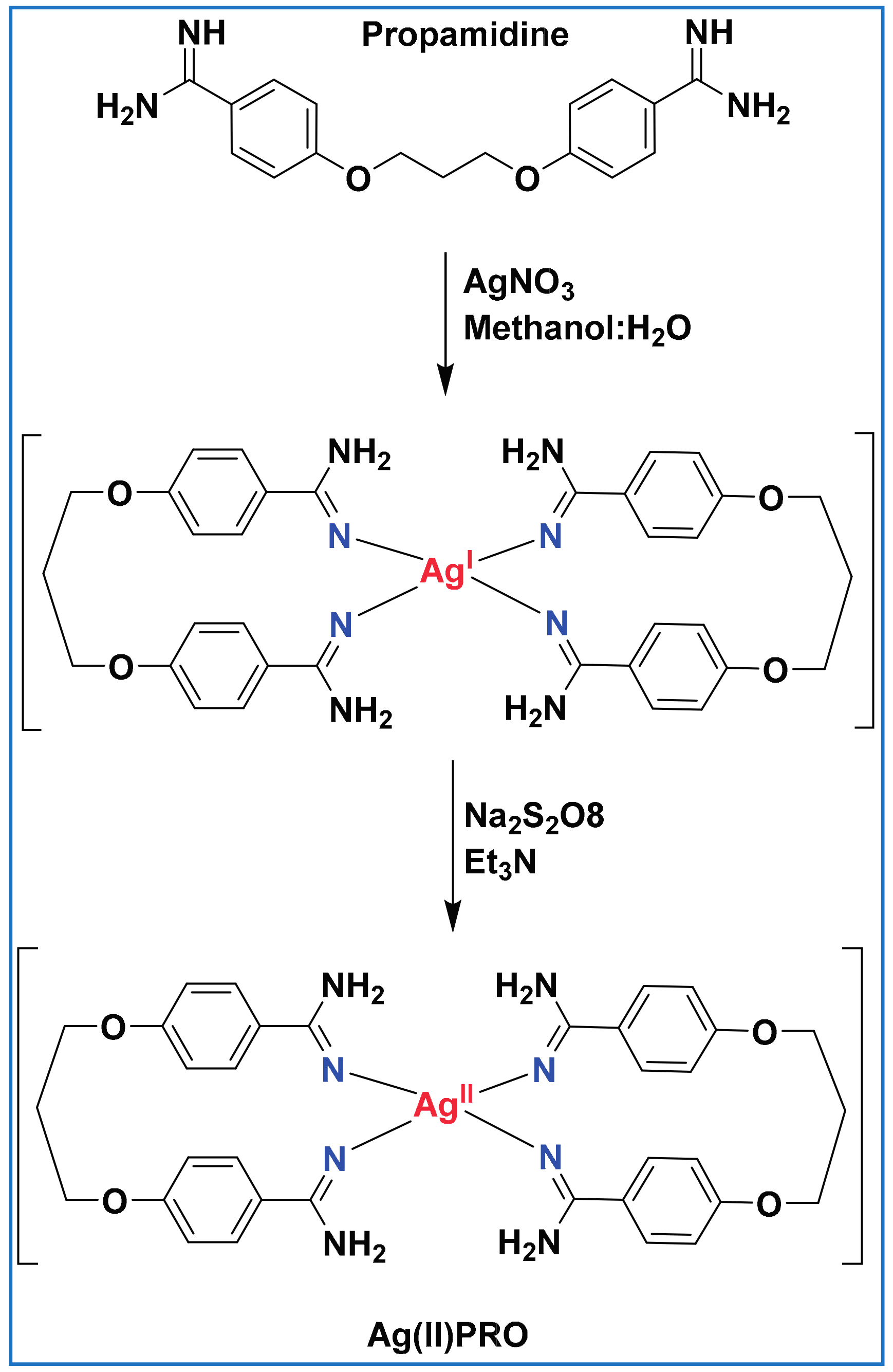
2.2. Synthesis of Ag(II)PRO Complex
2.3. Characterization of Ag(II)PRO Complex
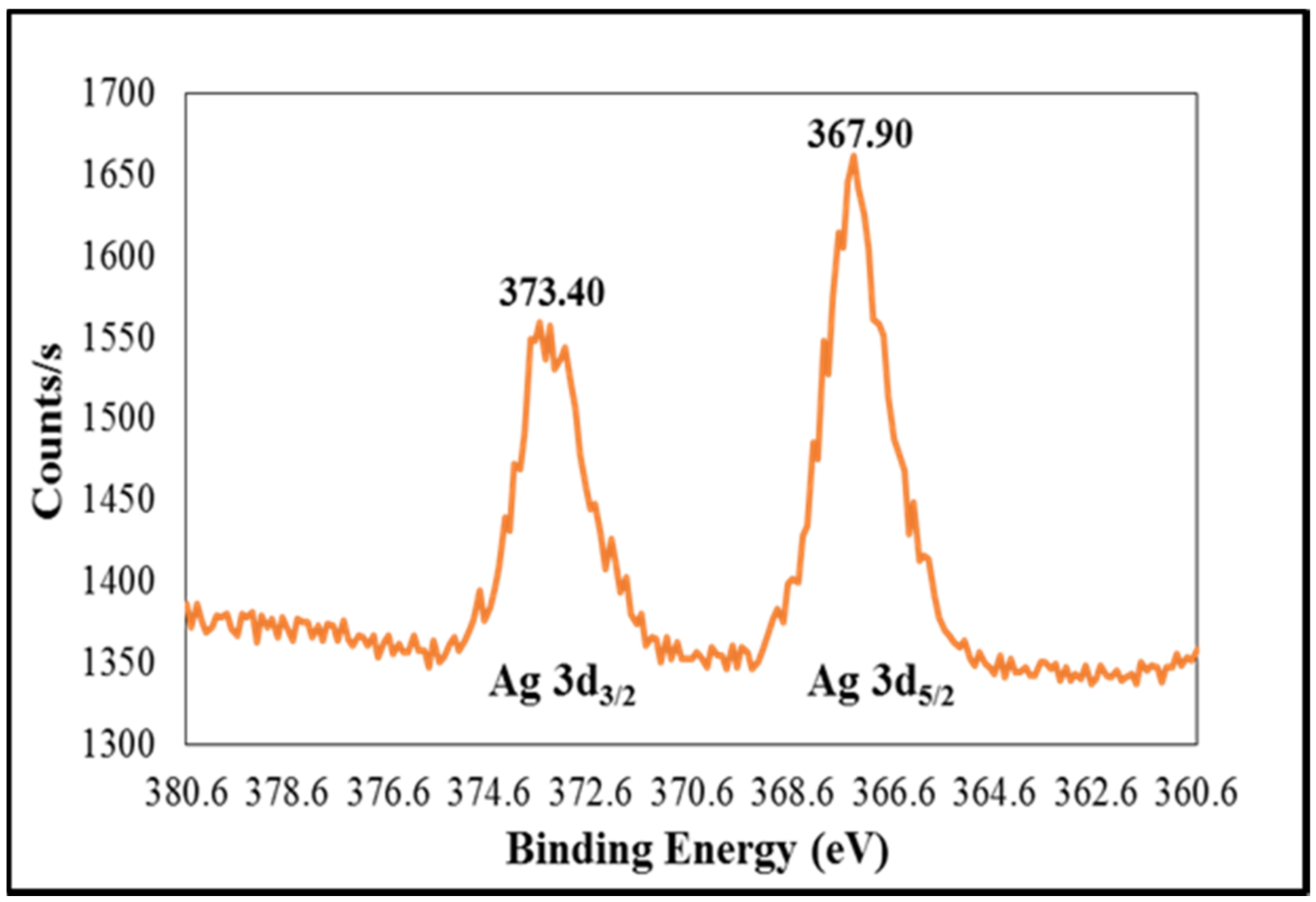
2.4. Characterization of Ag(II)PRO Complex by X-ray Photoelectron Spectroscopy (XPS)
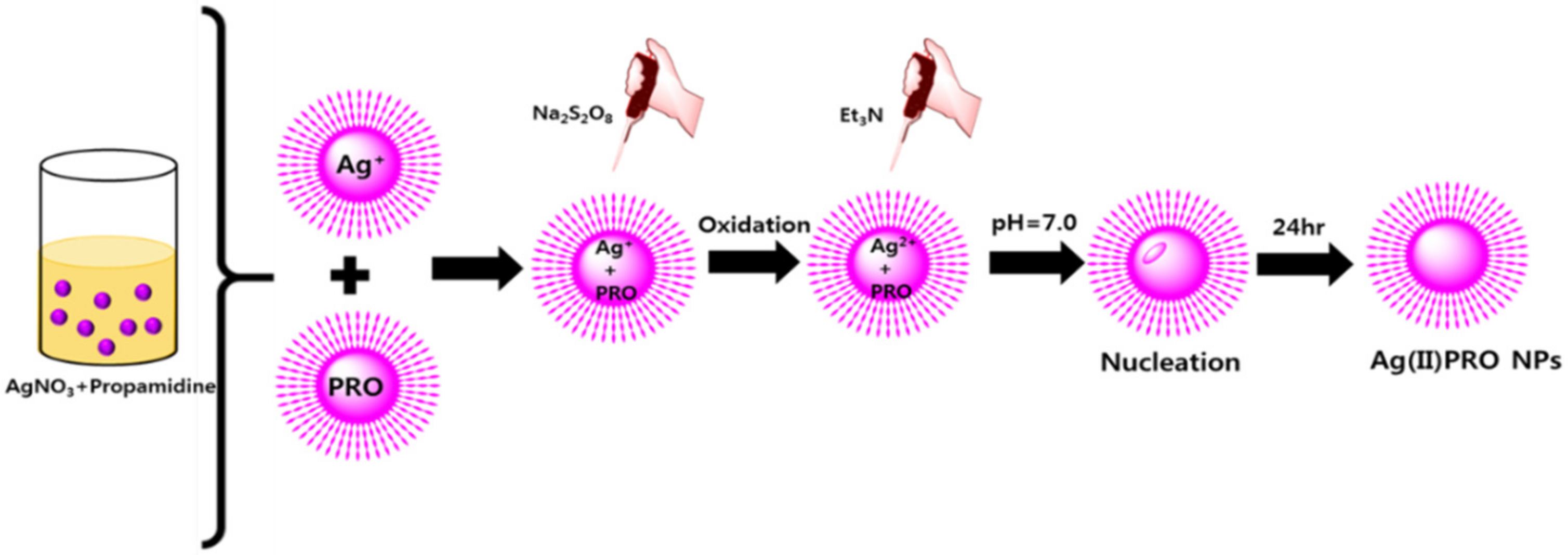
2.5. Synthesis of High-Valence Ag(II)PRO Nanoparticles
2.6. Synthesis of AgNPs
2.7. Transmission Electron Microscope (TEM) Analysis
2.8. Bacterial Strain and Culture Conditions
2.9. Determinations of MICs and MBCs
3. Results and Discussion
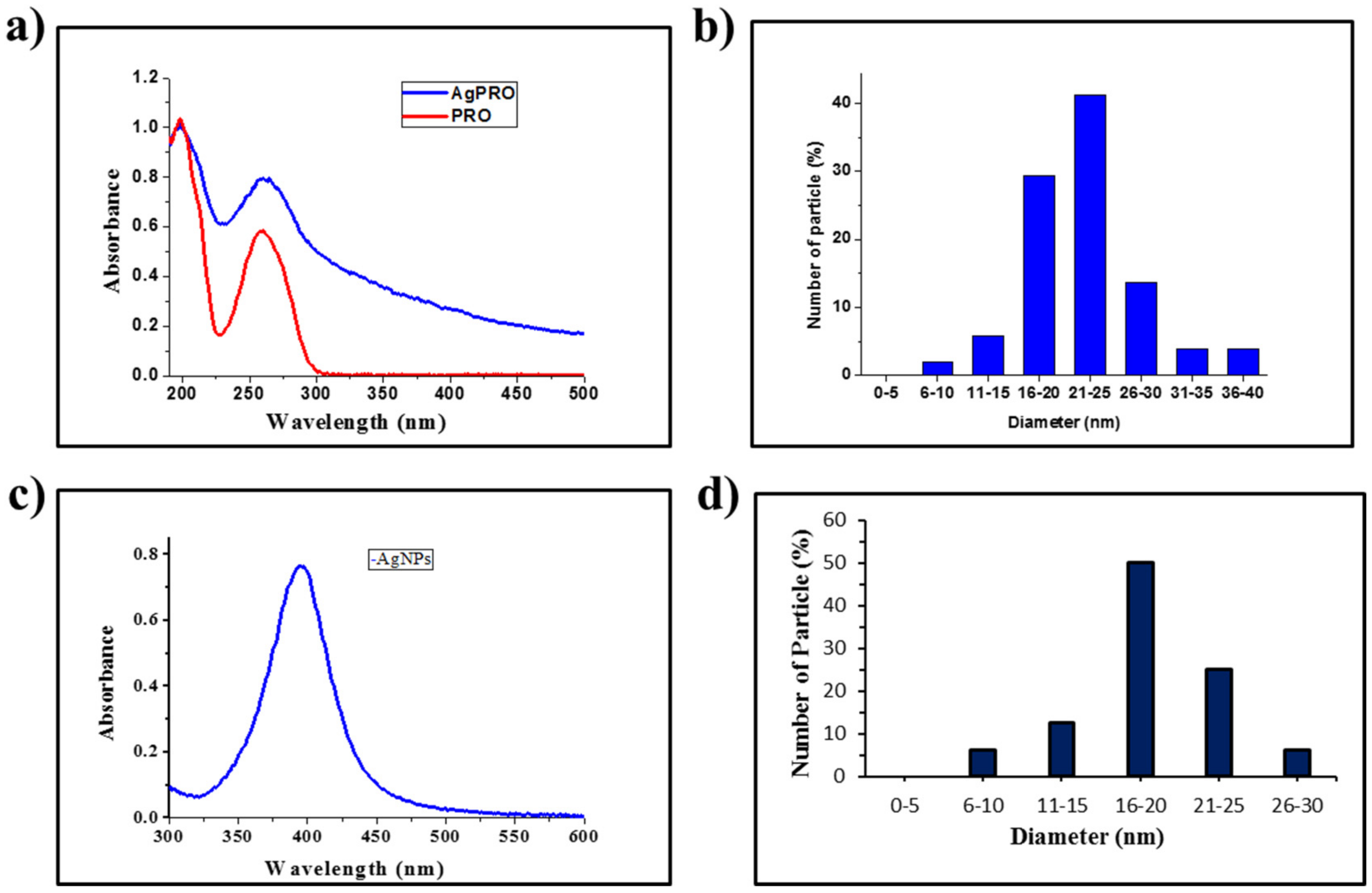
3.1. Synthesis of AgNPs and Ag(II)PRO Nanoparticles
3.2. Antibacterial Activities of Ag(II)PRO Nanoparticles and AgNPs (Comparative Study)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gariani, K.; Ugkay, I.; Lipsky, B.A. Managing diabetic foot infections: A review of the new guidelines. Acta Chir. Belg. 2014, 114, 7–16. [Google Scholar] [PubMed]
- Sinwar, P.D. The diabetic foot management—Recent advance. Int. J. Surg. 2015, 15, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. Infectious diseases society of america clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2012, 54, 132–173. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A. Treating diabetic foot osteomyelitis primarily with surgery or antibiotics: Have we answered the question? Diabetes Care 2014, 37, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Citron, D.M.; Goldstein, E.J.; Merriam, C.V.; Lipsky, B.A.; Abramson, M.A. Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J. Clin. Microbiol. 2007, 45, 2819–2828. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Sukumar, S.; Hajela, A.; Mukherjee, S.; Dutta, P.; Bhadada, S.K.; Bhansali, A. The microbiology of diabetic foot infections in patients recently treated with antibiotic therapy: A prospective study from India. J. Diabetes Its Complicat. 2017, 31, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Shankar, E.M.; Mohan, V.; Premalatha, G.; Srinivasan, R.S.; Usha, A.R. Bacterial etiology of diabetic foot infections in South India. Eur. J. Intern. Med. 2005, 16, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.; Jasmine, J.J.; Snehalatha, C.; Ramachandran, A. Prevalence of pathogens in diabetic foot infection in South Indian type 2 diabetic patients. J. Assoc. Physicians India 2002, 50, 1013–1016. [Google Scholar] [PubMed]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Marx, D.E.; Barillo, D.J. Silver in medicine: The basic science. Burns 2014, 40, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 8, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Pham, X.H.; Shim, S.; Kim, T.H.; Hahm, E.; Kim, H.M.; Rho, W.Y.; Jeong, D.H.; Lee, Y.S.; Jun, B.H. Glucose detection using 4-mercaptophenyl boronic acid-incorporated silver nanoparticles-embedded silica-coated graphene oxide as a SERS substrate. BioChip J. 2017, 11, 46–56. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, D.; Sun, L.; Huang, Z.; Zhang, X.; Ma, W.; Wu, J.; Xiao, L.; Zhao, Y.; Gu, N. Activation of autophagy by elevated reactive oxygen species rather than released silver ions promotes cytotoxicity of polyvinylpyrrolidone-coated silver nanoparticles in hematopoietic cells. Nanoscale 2017, 9, 5489–5498. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.K.; Kumar, A. Isolation and genetic analysis of multidrug resistant bacteria from diabetic foot ulcers. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, J.Y.; Kwon, S.Y.; Yang, H.J.; Choi, E.K.; Shin, M.H.; Ahn, K.S.; Um, J.Y.; Lee, J.H.; Jang, H.J. Comparative transcriptomic analysis of the multi-targeted effects of the herbal extracts against Escherichia coli O157:H7. BioChip J. 2012, 6, 379–390. [Google Scholar] [CrossRef]
- Yang, K.; Liu, J.; Shi, H.G.; Zhang, W.; Qu, W.; Wang, G.X.; Wang, P.L.; Ji, J.H. Electron transfer driven highly valent silver for chronic wound treatment. J. Mater. Chem. B 2016, 4, 5729–5736. [Google Scholar] [CrossRef]
- Dellasega, D.; Facibeni, A.; Di Fonzo, F.; Bogana, M.; Polissi, A.; Conti, C.; Ducati, C.; Casari, C.S.; Bassi, A.L.; Bottani, C.E. Nanostructured Ag4O4 films with enhanced antibacterial activity. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, W.; Wang, G. Inorganic Antibacterial Agents Containing High Valent Silver and Preparation Method Thereof. US Patent Application US 10/578,532, 27 October 2004. [Google Scholar]
- Pal, S.; Yoon, E.J.; Tak, Y.K.; Choi, E.C.; Song, J.M. Synthesis of highly antibacterial nanocrystalline trivalent silver polydiguanide. J. Am. Chem. Soc. 2009, 131, 16147–16155. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.; Tak, Y.K.; Kim, S.; Paul, A.; Song, J.M. Bifunctional therapeutic high-valence silver-pyridoxine nanoparticles with proliferative and antibacterial wound-healing activities. J. Biomed. Nanotechnol. 2016, 12, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Thrower, W.R.; Valentine, F.C. Propamidine in chronic wound sepsis: An experimental and clinical study. Lancet 1943, 241, 133–136. [Google Scholar] [CrossRef]
- Wien, R.; Harrison, J.; Freeman, W.A. Diamidines as antibacterial compounds. Br. J. Pharmacol. Chemother. 1948, 3, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.A.; Hall, J.E.; Kyle, D.E.; Grogl, M.; Ohemeng, K.A.; Allen, M.A.; Tidwell, R.R. Structure-activity relationships of analogs of pentamidine against plasmodium falciparum and leishmania mexicana amazonensis. Antimicrob. Agents Chemother. 1990, 34, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Donkor, I.O.; Clark, A.M. In vitro antimicrobial activity of aromatic diamidines and diimidazolines related to pentamidine. Eur. J. Med. Chem. 1999, 34, 639–643. [Google Scholar] [CrossRef]
- Werbovetz, K. Diamidines as antitrypanosomal, antileishmanial and antimalarial agents. Curr. Opin. Investig. Drugs 2006, 2, 147–157. [Google Scholar]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Strains | AgNPs | Ag(II)PRO NPs | ||
|---|---|---|---|---|
| MIC (μM) | MBC (μM) | MIC (μM) | MBC (μM) | |
| Pseudomonas aeruginosa | 20 | 60 | 6.25 | 12.5 |
| Staphylococcus aureus | 40 | 80 | 6.25 | 6.25 |
| Acinetobacter calcoaceticus | 40 | 80 | 6.25 | 6.25 |
| Enterococcus faecalis | 20 | 60 | 6.25 | 6.25 |
| Klebsiella pneumonia | 20 | 60 | 6.25 | 6.25 |
| Streptococcus agalactiae | 40 | 60 | 6.25 | 6.25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Purushothaman, B.; Li, Z.; Kulsi, G.; Song, J.M. Synthesis, Characterization, and Antibacterial Activities of High-Valence Silver Propamidine Nanoparticles. Appl. Sci. 2017, 7, 736. https://doi.org/10.3390/app7070736
Lee J, Purushothaman B, Li Z, Kulsi G, Song JM. Synthesis, Characterization, and Antibacterial Activities of High-Valence Silver Propamidine Nanoparticles. Applied Sciences. 2017; 7(7):736. https://doi.org/10.3390/app7070736
Chicago/Turabian StyleLee, Jinran, Baskaran Purushothaman, Zhao Li, Goutam Kulsi, and Joon Myong Song. 2017. "Synthesis, Characterization, and Antibacterial Activities of High-Valence Silver Propamidine Nanoparticles" Applied Sciences 7, no. 7: 736. https://doi.org/10.3390/app7070736
APA StyleLee, J., Purushothaman, B., Li, Z., Kulsi, G., & Song, J. M. (2017). Synthesis, Characterization, and Antibacterial Activities of High-Valence Silver Propamidine Nanoparticles. Applied Sciences, 7(7), 736. https://doi.org/10.3390/app7070736






